Abstract
Brain-derived neurotrophic factor (BDNF) is a secreted protein important for development and function of neocortical circuitry. Although it is well established that BDNF contributes to the sculpting of dendrite structure and modulation of synapse strength, the range and directionality of BDNF signaling underlying these functions are incompletely understood. To gain insights into the role of BDNF at the level of individual neurons, we tested the cell-autonomous requirements for Bdnf in visual cortical layer 2/3 neurons. We found that the number of functional Bdnf alleles a neuron carries relative to the prevailing genotype determines its density of dendritic spines, the structures at which most excitatory synapses are made. This requirement for Bdnf exists both during postnatal development and in adulthood, suggesting that the amount of BDNF a neuron is capable of producing determines its success in ongoing competition in the environment of the neocortex. Our results suggest that BDNF may perform a long-sought function for a secreted growth factor in mediating the competitive events that shape individual neurons and their circuits.
Keywords: Cre recombinase, conditional knockout
Although metazoans require cooperation between cells to succeed, competition between cells underlies certain developmental processes. In one compelling example, motor neurons compete for innervation of skeletal muscle fibers in an activity-dependent process, resulting in a precise connectivity relationship (1–3). Similarly, in the visual cortex, where activity-dependent competitive mechanisms also shape neural circuitry, perturbation of sensory input can have lasting functional and anatomical consequences (4, 5). For instance, visual deprivation can lead to structural changes including shrinkage of axonal arbors and the loss of dendritic spines, the structures where most excitatory synapses occur (6, 7).
In addition to neural activity, many proteins are known to be essential for competitive reorganization of synaptic circuitry (8). However, it is not known if the competitive process requires the exchange of protein signals between pre- and postsynaptic partners. Neurotrophic factors, initially identified as neuronal survival factors in the peripheral nervous system, were subsequently recognized for their role in determining the density of peripheral tissue innervation (9). As their actions in the central nervous system became apparent, neurotrophins emerged as logical candidates to mediate competitive interactions during development and refinement of CNS circuitry, perhaps regulating innervation at the level of individual synapses (10, 11).
In particular, BDNF exhibits many properties consistent with such functions in the cerebral cortex (12, 13). Neocortical BDNF expression and release are regulated by neural activity (14, 15) and can influence synapse strength and dendrite morphology (11, 16). Manipulation of BDNF signaling by infusion of either BDNF or a scavenger for BDNF perturbs the structural development of ocular dominance columns, eye-specific columns of thalamic axons in the primary visual cortex (17, 18). Bdnf overexpression accelerates the development of neocortical inhibitory neurons and leads to a precocious critical period (19, 20). However, heterozygosity for Bdnf does not perturb the timing of the critical period for ocular dominance plasticity (21), and signaling by the TrkB receptor for BDNF is required for recovery of vision after monocular deprivation but not for ocular dominance shifts after such deprivations (22). Thus, BDNF-TrkB signaling appears to have selective roles in the structural and functional development and plasticity of visual cortical circuitry but its function in competitive structural reorganizations is unclear.
Using conditional knockout mice, we previously found that Bdnf is essential for stabilization of visual cortical pyramidal neuron dendritic trees during postnatal development (23). Whether this function reflects release of BDNF from axons or dendrites is uncertain and evidence has been presented supporting both pathways (15). Genetic manipulation of signaling pathways in individual cells can lead to insights into the range and directionality of signaling. Overexpression of Bdnf by individual cortical neurons in organotypic slices enhanced dendritic branching but reduced dendritic spine stability and suggested that the effects of the overexpressed BDNF on neighboring neurons are limited to a range of ∼4.5 µm (24, 25). Also in organotypic slices, isolated Bdnf mutant layer 2/3 pyramidal neurons develop reduced inhibitory synapse density (26). Transgenic overexpression of TrkB.T1-EGFP, a fusion protein predicted to interfere with TrkB signaling, in scattered layer 2/3 neurons led to reduced dendritic spine density (27). However, whether BDNF must be produced by individual neurons, in vivo, for their development and maintenance of dendritic spines has been unclear. Thus, we genetically tested whether there is a cell-autonomous requirement for Bdnf in layer 2/3 pyramidal neurons in vivo. We found that individual layer 2/3 pyramidal neurons require Bdnf to display normal dendritic spine density both during postnatal development and in the adult. Furthermore, we describe genetic evidence that this cell-autonomous function, determining neuronal morphology related to synaptic connectivity, reflects a competitive mechanism.
Results
Most Neurons in Layer 2/3 of the Visual Cortex Express BDNF.
Although BDNF is the most abundantly expressed neurotrophin in the cerebral cortex, its mRNA is nonetheless rare (28). This rarity has made it difficult to establish the fraction of neurons expressing Bdnf. If just a subset of excitatory neurons normally express Bdnf, these neurons might be the only cells affected by Bdnf deletion. Therefore, we sought to determine the fraction of Bdnf-expressing neurons in layer 2/3 of primary visual cortex (V1). Following Cre-mediated recombination of the Bdnflox allele, lacZ is expressed under control of the Bdnf promoters (Fig. 1A) (23). Using Emx1IREScre to direct Cre-lox recombination in excitatory neurons and glia (29), we scored the fraction of β-gal actosidase (β-Gal) immunopositive neurons in layer 2/3 using NeuN as a panneuronal marker (30). With a range of β-Gal staining intensities, 84% of neurons were β-Gal+ (Fig. 1 B and C, and see Fig. 5D). The β-Gal− neurons are likely inhibitory neurons, estimated as 13% of visual cortical neurons (31), which do not express Bdnf (32). Thus, either all or nearly all visual cortical layer 2/3 excitatory neurons express Bdnf at some level.
Fig. 1.
Bdnflox and Thy1-stop-YFP enable the study of BDNF in layer 2/3 cortical neurons. (A, Top) Alternate 5′ exons are joined with the exon containing the BDNF protein coding region (BDNF) using a splice acceptor (SA) site. Alternative polyadenylation signals are present in the 3′-untranslated region (3′-UTR). (Middle) Bdnflox was created by inserting loxP sites surrounding the BDNF protein coding region and a trimerized polyadenylation signal followed by lacZ between the two endogenous polyadenylation signals. (Bottom) Following Cre-mediated recombination, the BDNF coding sequence is deleted and lacZ is brought under control of the BDNF promoters. (B) In V1 of Emx1IREScre; Bdnflox/+ mice, β-Gal immunoreactivity is detected at higher levels in layers 2, 3, and 6 and at lower levels in layers 4 and 5. (C) Although some cells in layer 2/3 display faint and/or punctate β-Gal immunoreactivity, the majority of NeuN+ cells also display β-Gal (white arrowheads, β-Gal− cells). (D) Sample Golgi-stained layer 2/3 pyramidal neuron basal dendrite segments from Bdnflox/lox and wild-type mice. (E) YFP expression in layer 2/3 of V1 in an Emx1IREScre; Thy1-stop-YFP mouse. In this representative field, the single YFP− NeuN+ neuron is also GAD67+ (arrowhead).
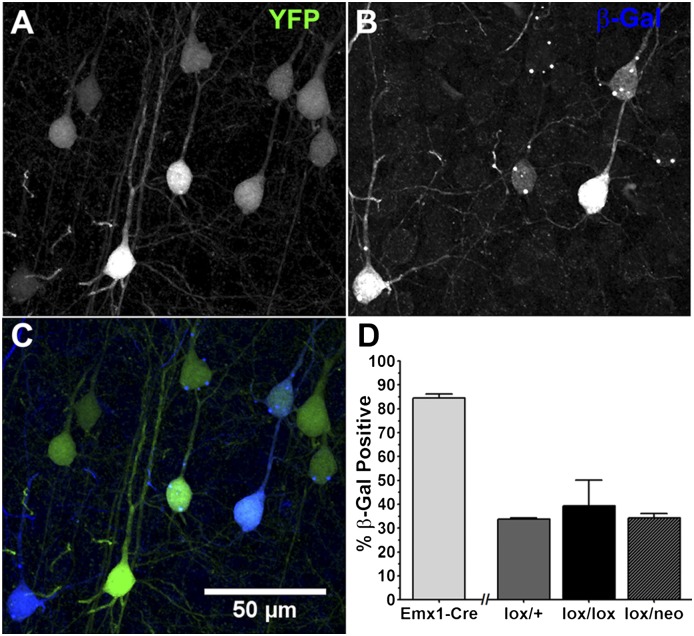
Fig. 5.
The influence of Bdnf genotype on expression at the Bdnf locus. In AAV-Cre injected Thy1-stop-YFP; Bdnflox/+ mice, only YFP+ neurons (A) display β-Gal (B) and just a subset are β-Gal+ (C). (D) Percentage of NeuN+ neurons also expressing β-Gal in the fully Bdnf heterozygous cortex of Emx1IREScre; Bdnflox/+ mice (n = 3 mice, 563 cells) is much higher than in isolated YFP+ Bdnf mutant neurons generated by viral injection of the indicated Bdnf genotypes (n = 3 mice each; Bdnflox/+ 130 cells, Bdnflox/lox 111 cells, and Bdnflox/neo 51 cells; error bars = SD).
Floxed Mouse Strains Enable a Test of Cell Autonomy.
The Bdnf gene has a complex structure, including the use of alternative polyadenylation signals resulting in a short or a long 3′-untranslated region (33). The Bdnflox allele used here does not produce BDNF mRNAs including the long 3′-untranslated (UTR) region due to the insertion of polyadenylation signals and the lacZ reporter (Fig. 1A). Bdnflox/lox mice display a modest increase in apical dendrite spine density on layer 2/3 neurons at 4 mo but not at 3 wk of age, apparently due to a lack of distal dendritic transport of the long 3′-UTR BDNF mRNAs (34). Although these mice have reduced protein in whole cortex at 5–6 wk of age (34), we did not observe reduced BDNF protein in the visual cortex at 5 wk of age (Fig. S1). This is consistent with an increasing relative contribution of the long 3′-UTR mRNA to cortical BDNF as the cortex matures, perhaps due to increased activity-regulated BDNF translation requiring the long 3′-UTR mRNA (35), and with the rostral–caudal and ventral–dorsal gradients of cortical maturation (36). We analyzed visual cortical layer 2/3 basal dendrite spine density in postnatal day 84 (P84) Bdnflox/lox mice using Golgi staining and found that the spine density was not significantly different from wild type (Fig. 1D) [Bdnf+/+ 1.24 ± 0.036 spines per micrometer (n = 3 mice, 21 neurons, 51 segments, and 2,742 spines); Bdnflox/lox 1.27 ± 0.028 spines per micrometer (n = 3 mice, 23 neurons, 44 segments, and 2,421 spines)].
We focused our analyses here on basal dendrites of layer 2/3 pyramidal neurons in V1 for several reasons. The branching complexity of these dendrites is affected in forebrain-specific Bdnf knockouts (23). These dendrites receive the majority of their excitatory afferents from layer 4 and other layer 2/3 neurons (37), layer 4–layer 2/3 long-term potentiation is BDNF dependent (21), and altered visual experience can rapidly reorganize layer 2/3 horizontal projections (38). Finally, Bdnf mRNA expression reductions in response to visual deprivation are particularly pronounced in layer 2/3 (39–41), and either dark rearing or prolonged monocular deprivation leads to reduced spine density on layer 2/3 basal dendrites (42, 43). These observations, combined, suggested that BDNF-dependent spine changes might occur on layer 2/3 pyramidal basal dendrites.
To analyze the phenotype of sparsely distributed Bdnf mutant neurons, we sought a Cre-recombinase–dependent reporter having robust expression in the vast majority of layer 2/3 cortical neurons. Nearly all layer 5 neurons in Thy1-stop-YFP mice express YFP following Cre-mediated recombination (44). We determined the proportion of layer 2/3 visual cortical neurons expressing YFP following Emx1IREScre-mediated recombination, finding that 92% of NeuN+ cells were YFP+, 7% were GAD67+ and YFP−, 1% were double positive, and 0.2% double negative (n = 630 cells; Fig. 1E). The double positive (1%) cells are likely inhibitory neurons derived from the Emx1-expressing lineage (29). Thus, the vast majority of excitatory neurons within layer 2/3 of the visual cortex can be visualized after Cre-mediated recombination with the Thy1-stop-YFP reporter.
Cell-Autonomous Requirement for BDNF Exists During Postnatal Development.
To investigate the cell-autonomous role of Bdnf in the cortex, we injected adeno-associated virus 2/2-Cre (AAV-Cre) into the ventricles of neonatal mice having various Bdnf genotypes and the Thy1-stop-YFP transgene. By adjusting the virus amount and allowing sufficient time for YFP accumulation, we obtained sparsely distributed Cre-lox recombined cells exhibiting Golgi-like YFP staining (Fig. 2 B and C). At P35, the basal dendrites of recombined layer 2/3 pyramidal neurons in Bdnflox/lox mice had a 26% lower spine density than in wild-type mice (Fig. 2D), demonstrating a cell-autonomous requirement for Bdnf during postnatal development.
Fig. 2.
The Bdnf genotype of individual cortical neurons determines dendritic spine density during postnatal development. (A) Experimental timeline. (B) Visual cortex of a Thy1-stop-YFP; Bdnf+/+ mouse injected neonatally with AAV-Cre displays mosaic YFP expression at P35. (C) Representative layer 2 pyramidal neuron. (D) Mean dendritic spine densities determined from Thy1-stop-YFP mice having the indicated Bdnf genotypes (error bars = SD; **P < 0.01, ***P < 0.001). Schematic below each bar in the histogram depicts the amount of BDNF (arrows) a Cre-recombined YFP-labeled cell (open circle) is expected to produce compared with its neighbors (closed circles) for each Bdnf genotype. Bdnf+/+ (n = 5 mice, 25 neurons, 47 segments, and 3,840 spines), Bdnfneo/+ (n = 3 mice, 19 neurons, 36 segments, and 2,904 spines), Bdnflox/+ (n = 5 mice, 29 neurons, 59 segments, and 4,726 spines), and Bdnflox/lox (n = 3 mice, 20 neurons, 36 segments, and 2,980 spines).
We next used mice with different Bdnf genotypes to analyze the phenotype of Bdnf heterozygous cells in different Bdnf environments. Bdnfneo/+ heterozygous mutant mice produce half normal BDNF levels, indicating that the level of BDNF expression depends directly upon the number of functional Bdnf alleles (23, 45). YFP-labeled neurons in Bdnfneo/+ mice (in which all neurons have a single functional Bdnf allele) had spine densities similar to wild-type (Fig. 2D), similar to other observations of wild-type or close to wild-type cortical dendrite morphology and synapse density in Bdnf null heterozygous mice (23, 34, 46, 47). In contrast, isolated recombined neurons in Bdnflox/+ mice had a 25% lower spine density than in wild-type mice, similar to that found in Bdnflox/lox mice (Fig. 2D). Thus, we found that the phenotype of Bdnf heterozygous cortical neurons is determined by the Bdnf genotype of the animal. A simple interpretation is that the relative ability of individual cortical neurons to synthesize BDNF determines their phenotype through competitive interactions during postnatal cortical development.
Ongoing Expression of BDNF Is Required.
We next tested whether the Bdnf requirement that pyramidal neurons display during postnatal development extends to maintenance of dendritic spine density in adulthood. Young adult (P35) mice were injected with AAV-Cre and analyzed at P84. The spine density of isolated recombined neurons in both Bdnflox/lox and Bdnfneo/lox mice was 30% lower than wild type (Fig. 3). Therefore, Bdnf is also required cell autonomously to maintain dendritic spines in layer 2/3 neurons of adult mice. Notably, P35 is near the end of the critical period defined for mouse visual cortical development (48). If BDNF is no longer required for competitive interactions in adulthood, it would be predicted that the Bdnf requirement existing between birth and P35 (Fig. 2) would not persist later in adulthood. However, layer 2/3 neurons in Bdnflox/+ mice injected with AAV-Cre at P35 and analyzed at P84 had 26% lower spine density than wild-type mice and 24% lower than Bdnfneo/+ mice, which were similar to wild type (Fig. 3 D and E). Thus, individual layer 2/3 neurons must be able to produce BDNF on an ongoing basis to maintain dendritic spines. In addition, we found that both soma volume and the number of primary dendrites are reduced in isolated Cre-recombined layer 2/3 pyramidal neurons of Bdnflox/lox and Bdnflox/+ mice compared with Bdnfneo/+ and wild type (Fig. 4), indicating that the losses of dendritic spines are accompanied by additional morphological declines. Like the requirement for Bdnf during postnatal development, the dependency of the phenotype of heterozygous cells on the genotype of the animal suggests ongoing BDNF-dependent competition in the adult.
Fig. 3.
The Bdnf genotype of individual cortical neurons determines whether dendritic spine density is maintained in adulthood. (A) Experimental timeline. (B) Visual cortex of a Thy1-stop-YFP; Bdnf+/+ mouse injected at P35 with AAV-Cre displays mosaic YFP expression in V1 at P84. (C) Representative layer 3 pyramidal neuron. (D) Representative layer 2/3 basal dendrite segments for each of the genotypes analyzed. (E) Mean dendritic spine densities determined from Thy1-stop-YFP mice having the Bdnf genotypes indicated (error bars = SD; ***P < 0.001). Schematic below each bar in the histogram depicts the amount of BDNF (number of arrows proportional to number of functional Bdnf alleles) a Cre-recombined YFP-labeled cell (open circle) is expected to produce compared with its neighbors (closed circles) for each Bdnf genotype. Bdnf+/+ (n = 10 mice, 44 neurons, 89 segments, and 9,541 spines), Bdnfneo/+ (n = 5 mice, 33 neurons, 57 segments, and 4,722 spines), Bdnflox/+ (n = 10 mice, 60 neurons, 157 segments, and 11,150 spines), Bdnflox/lox (n = 10 mice, 58 neurons, 127 segments, and 7,892 spines), and Bdnfneo/lox (n = 4 mice, 25 neurons, 53 segments, and 3,034 spines).
Fig. 4.
Bdnf is needed by individual cortical neurons to maintain primary dendrites and soma size in adulthood. (A) Representative layer 2/3 pyramidal neurons for each of the genotypes analyzed. (B) Mean primary dendrite number determined from Thy1-stop-YFP mice having the BDNF genotypes indicated (error bars = SD; *P < 0.05, **P < 0.01, ***P < 0.001). (C) Mean layer 2/3 neuron soma volume determined from Thy1-stop-YFP mice having the BDNF genotypes indicated (error bars = SD; *P < 0.05). BDNF+/+ (n = 3 mice, 21 neurons, and 163 dendrites), BDNFneo/+ (n = 3 mice, 23 neurons, and 179 dendrites), BDNFlox/+ (n = 3 mice, 21 neurons, and 130 dendrites), and BDNFlox/lox (n = 3 mice, 21 neurons, and 136 dendrites).
BDNF Gene Expression Is Reduced in Isolated Mutant Neurons.
As described above, the vast majority of layer 2/3 excitatory neurons in visual cortex express β-Gal in Emx1IREScre; Bdnflox/+ mice or YFP in Emx1IREScre; Thy1-stop-YFP mice that have undergone widespread Cre-lox recombination in the dorsal telencephalon (Fig. 1). Expression of the Bdnf gene is regulated in part by neural activity (14). We were curious how Bdnf expression is affected in the isolated mutant neurons, as assessed using the lacZ reporter. In Bdnflox/+, Bdnflox/lox, and Bdnfneo/lox mice, all β-Gal+ layer 2/3 neurons also expressed YFP, but the converse was not true; only 34–39% of the YFP+ cells were β-Gal+ (Fig. 5). The slightly higher proportion of β-Gal+ neurons seen in Bdnflox/lox mice compared with Bdnfneo/lox and Bdnflox/+ is likely due to the presence of two recombined Bdnflox transgenes. This contrasts strikingly with the vast majority of excitatory neurons expressing lacZ from the Bdnflox allele when the Bdnf genotypes of cortical excitatory neurons are all heterozygous (Fig. 1; Emx1-Cre in Fig. 5D). Thus, expression at the Bdnf locus in the isolated mutant neurons is reduced compared with a genetically uniform cortex.
Discussion
Our results lead to several conclusions. First, cortical pyramidal neurons have a cell-autonomous requirement for Bdnf. Second, the genetics of this requirement suggest a competitive function for BDNF at the level of individual neurons. Third, this requirement exists during both postnatal development and in adulthood. In sum, our results suggest that individual pyramidal neurons must synthesize BDNF to compete in the environment of the cerebral cortex.
Competition between peripheral nervous system axons for a limited supply of target-derived neurotrophic factors is believed to determine innervation density (9). Because BDNF enhances cortical axon branching (49), it is possible that BDNF released from dendrites acts as a classic retrograde trophic signal that stabilizes presynaptic terminals. In this model, the loss of presynaptic axon terminals causes the loss of the corresponding postsynaptic dendritic spines from isolated mutant neurons.
Alternatively, the dendritic spine defects we observed could reflect anterograde signaling. Pyramidal neurons unable to release BDNF from their axons might compete less successfully for postsynaptic targets and thus fail to receive reciprocal retrograde trophic support, leading to retrograde degeneration similar to that occurring after innervation target ablation (50). Anterograde transport of BDNF by corticostriatal pyramidal neurons is well documented (51, 52) and in the hippocampus BDNF is found in presynaptic terminals but not at postsynaptic sites (53), although there is evidence for both axonal and dendritic BDNF release from cultured cortical neurons (54, 55).
In addition to retrograde and anterograde signaling, other signaling modes could explain our results. Paracrine BDNF signaling through the p75NTR receptor is believed to mediate competitive interactions by olfactory and sympathetic peripheral nervous system neurons (56–58). Importantly, as the p75NTR receptor is either undetectable or expressed at very low levels in the adult neocortex (59–61), cortical pyramidal neurons are more likely to use TrkB to relay the relevant signals. Interestingly, transgenic overexpression of TrkB.T1-EGFP, a fusion protein of EGFP with the TrkB.T1 truncated form of the TrkB receptor, led to a 24% reduction in dendritic spine density on layer 2/3 visual cortical pyramidal neuron basal dendrites (27), similar to the phenotype we observed in Bdnf mutant neurons. The similarity between the phenotypes could be due to BDNF internalization and sequestration by TrkB.T1-EGFP (62), preventing anterograde or retrograde signaling. Alternatively, the similarity could indicate autocrine BDNF-TrkB signaling, in which case TrkB.T1-EGFP overexpression cell-autonomously inhibits TrkB signaling (63). Autocrine BDNF signaling supports sensory neuron survival (64) and stimulates the formation of cultured hippocampal neuron axons (65).
However, a pure autocrine mechanism cannot explain our results, specifically, the finding that the dendritic spine phenotype of heterozygous Bdnf neurons depends upon the Bdnf genotype of their environment. At least one additional signal would be required to explain the competitive aspect of an autocrine Bdnf requirement. For example, autocrine BDNF signaling might enhance pyramidal neuron activity and neurotransmitter release, enhancing competitive ability. BDNF addition is known to enhance the activity of cultured cortical neurons (66). Analysis of single cell TrkB mutants will be useful in distinguishing among the possible BDNF-TrkB signaling modes.
In adult-onset forebrain-specific Bdnf conditional knockouts, in which BDNF is lost throughout the cortex, we have found reduced dendritic spine density in V1 layer 2/3 basal dendrites similar to that described here (47). This could be viewed as surprising in the context of a competitive requirement for Bdnf. All neurons are equally handicapped in a homogeneously Bdnf mutant cortex, so they might be predicted to compete normally. However, a parsimonious interpretation of our combined results is that a subset of excitatory circuitry requires BDNF for development and ongoing stabilization. If cells are able to make BDNF they compete in forming and maintaining this circuitry, but if they are either unable to produce BDNF or are disadvantaged in the level of BDNF production, the circuitry does not form or is lost.
The effects of BDNF on cortical dendritic spines appear to be context dependent. BDNF addition can either increase or reduce spine density on deep layer pyramidal neurons in cultured cortical slices (67, 68). Overexpression of a BDNF cDNA in individual layer 2/3 pyramidal neurons in cultured slices destabilizes dendritic spines (25), which contrasts with our results. Notably, the relative timing of BDNF addition and synaptic activity determines whether BDNF-dependent spine growth occurs in cultured hippocampal neurons (69). Thus, BDNF augmentation through direct addition or cDNA overexpression, which lack aspects of normally intricate alternative mRNA processing (33), translation regulation (35), and activity-regulated control of release (15), might interact variably with a timing-dependent mechanism. Additionally, the phenotypes resulting from either overexpression or loss of BDNF may partly reflect homeostatic mechanisms (70), and altered patterns of activity, which determine dendritic spine density in layer 5 pyramidal neurons (71).
The relationship between the competitive model for Bdnf we describe here and mechanisms of cortical plasticity is uncertain. Recent longitudinal observations of layer 2/3 neurons following visual deprivation, and over time frames in which physiological changes in pyramidal neuron responsiveness occur, have not detected changes in dendritic spine density (72, 73). Perhaps BDNF modulates dendritic spine density following prolonged alterations in experience and activity, such as with dark rearing, which reduces both Bdnf expression and spine density on visual cortical layer 2/3 basal dendrites (39, 43).
Although the Bdnf mutant cortical neurons survived for several weeks in our experiments, the long-term fate of such cells is unknown. Disadvantages in BDNF production among subpopulations of neurons could contribute to their demise and to the spread of neural network deterioration in neurodegenerative diseases.
Materials and Methods
Experimental Animals.
The Bdnflox (23), Bdnfneo (a null allele) (74), Emx1IREScre (29), and Thy1-stop-YFP (3) mouse strains used were backcrossed over 10 generations to C57BL/6J (Jackson Laboratories). Animal experiments were approved by and carried out according to the guidelines of the University of Colorado Institutional Animal Care and Use Committee. To determine BDNF protein levels, brains were cut into thirds in the coronal plane, the region encompassing visual cortex excised with a scalpel, the ventral half of cortex separated, extracts prepared, and BDNF protein assayed as described previously (23).
Golgi Stain Analysis of Dendritic Spines.
Golgi staining was performed with FD Rapid GolgiStain kit (FD Neurotechnologies) using the included protocol. Brains were cryosectioned at 100 µm and sections mounted in Permount (Fisher). Basal dendrite segments at least 45 µm from the cell soma were imaged in 0.2-µm Z steps using Metamorph (Molecular Devices) to control a Nikon Eclipse TE2000-U with a 100× objective (1.40 NA; Nikon) and a Cascade II 16bit EMCCD camera (Photometrics) (pixel size = 0.09 µm/pixel). Z stacks were transferred to ImageJ [National Institutes of Health (NIH), http://rsb.info.nih.gov/ij] and protrusions over 0.5 μm counted as dendritic spines. The Z stack was collapsed into a maximum intensity projection and a line drawn freehand along the segment between the most proximal and distal spines counted to obtain segment length. To generate the images in Fig. 1D, masks corresponding to the in-focus portion of dendrites were created from Z stack images, subtracted from each image, and the resulting stacks projected to single images using the Stack Focuser plug-in in ImageJ.
Virus Injections.
CMV-AAV2/2-Cre virus was purchased from the University of Iowa’s Gene Transfer Vector Core. One-day-old pups were cryoanesthetized with wet ice, the ventricles visualized by illumination with a fiber optic, and 2 µL of viral stock (1.0 × 109 vg/mL) containing 0.05% trypan blue was injected into the third ventricle using a Hamilton RN 33 gauge needle (75). P35 mice were anesthetized with isoflurane and secured in a stereotaxic frame fitted with a model 5000 microinjection unit and model 5002 syringe holder (David Kopf Instruments). A 1-mm diameter burr hole was drilled into the skull, and 1-µL of viral stock was injected into the parenchyma at a rate of 0.5 µL/min. The needle was left in place for 5 min after the injection to minimize outflow of virus (76). The stereotaxic coordinates used were 3.0, 2.0, and 2.0 (caudal to bregma, left of midline, and ventral to pial surface) (77).
Fluorescent Imaging and Analysis
At P35 for the P1 injections and P84 for the P35 injections, mice were processed for vibratome sectioning as previously described (23). Sections (50 µm thick) were mounted in 20% (vol/vol) 0.1 M sodium phosphate, pH 8.5, 80% glycerol. Confocal Z stacks (step size = 0.25 µm) of basal dendrites on layer 2/3 visual cortical pyramidal neuron segments located 45–100 µm from the soma were collected using the Nikon microscope described above with a spinning disk confocal system (Solamere Technology Group) for P35 injected mice, or using Zeiss’s Zen software to control a Zeiss LSM 510 confocal system with a 100× objective, 1.40 NA (Carl Zeiss) for neonatally injected mice. The pixel size in both image sets was 0.09 µm per pixel. Dendritic spines exceeding 0.25 µm in length were counted using ImageJ. To determine primary dendrite number and soma volume, confocal Z stacks (step size = 1.00 µm) of individual neurons from the P84 brain sections were acquired using a Zeiss LSM 510 confocal system with a 40× objective (1.40 NA; 0.44 µm per pixel). Z stacks were transferred to ImageJ and processes greater than 5 μm in length emanating directly from the soma were counted as primary dendrites. Then, Z stacks were thresholded to the point where the entire soma was saturated and the AB-Snakes plug-in was used to trace the soma boundary in 3D, generating a binary mask. Soma volume was calculated by multiplying the number of pixels in the resulting mask by 0.44.
Immunocytochemistry.
Vibratome sections (50 μm each) were processed as previously described (23). Primary antibodies were mouse anti-NeuN (MAB377; Chemicon), rabbit anti–β-gal (55976; MP Biomedicals), and rabbit anti-GAD67 (AB108; Chemicon). Secondary antibodies were goat antimouse Alexa 647 (A21244; Molecular Probes) and goat antirabbit Alexa 555 (A21429; Molecular Probes). Sections were mounted with Fluoromount-G (Southern Biotech).
Statistics.
Statistical significance was determined for experiments having two distributions using a two-tailed unpaired Student t test, and for three or more distributions by one-way ANOVA with Tukey's post hoc test, using Graphpad Prism and with n = the number of animals. All dendritic spine counts were performed with the experimenter blinded to genotype.
Supplementary Material
Acknowledgments
We thank Jessica Gorski and Susan Tamowski for BDNF quantification and Josh Sanes for providing Thy1-stop-YFP mice. Funding was provided by NIH Grant R01 EY014998 and the Department of Molecular, Cellular, and Developmental Biology, University of Colorado.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206492109/-/DCSupplemental.
References
- 1.Wyatt RM, Balice-Gordon RJ. Activity-dependent elimination of neuromuscular synapses. J Neurocytol. 2003;32(5–8):777–794. doi: 10.1023/B:NEUR.0000020623.62043.33. [DOI] [PubMed] [Google Scholar]
- 2.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 3.Buffelli M, et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424(6947):430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 4.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 5.Hooks BM, Chen C. Critical periods in the visual system: Changing views for a model of experience-dependent plasticity. Neuron. 2007;56(2):312–326. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 7.Dahlhaus M, Levelt CN. Structure and function relationships during ocular dominance plasticity in the visual cortex. Rev Neurosci. 2010;21(3):223–237. doi: 10.1515/revneuro.2010.21.3.223. [DOI] [PubMed] [Google Scholar]
- 8.Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 10.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270(5236):593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 11.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 12.Tropea D, Van Wart A, Sur M. Molecular mechanisms of experience-dependent plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364(1515):341–355. doi: 10.1098/rstb.2008.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol. 1996;6(1):119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuczewski N, Porcher C, Gaiarsa J-L. Activity-dependent dendritic secretion of brain-derived neurotrophic factor modulates synaptic plasticity. Eur J Neurosci. 2010;32(8):1239–1244. doi: 10.1111/j.1460-9568.2010.07378.x. [DOI] [PubMed] [Google Scholar]
- 16.Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70(5):271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabelli RJ, Hohn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267(5204):1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 18.Cabelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19(1):63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 19.Huang ZJ, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98(6):739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 20.Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19(22):RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartoletti A, et al. Heterozygous knock-out mice for brain-derived neurotrophic factor show a pathway-specific impairment of long-term potentiation but normal critical period for monocular deprivation. J Neurosci. 2002;22(23):10072–10077. doi: 10.1523/JNEUROSCI.22-23-10072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko M, Hanover JL, England PM, Stryker MP. TrkB kinase is required for recovery, but not loss, of cortical responses following monocular deprivation. Nat Neurosci. 2008;11(4):497–504. doi: 10.1038/nn2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23(17):6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5(11):1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- 25.Horch HW, Krüttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23(2):353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- 26.Kohara K, et al. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci. 2007;27(27):7234–7244. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakravarthy S, et al. Postsynaptic TrkB signaling has distinct roles in spine maintenance in adult visual cortex and hippocampus. Proc Natl Acad Sci USA. 2006;103(4):1071–1076. doi: 10.1073/pnas.0506305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maisonpierre PC, et al. NT-3, BDNF, and NGF in the developing rat nervous system: Parallel as well as reciprocal patterns of expression. Neuron. 1990;5(4):501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 29.Gorski JA, et al. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22(15):6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116(1):201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 31.Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993;609(1–2):284–292. doi: 10.1016/0006-8993(93)90884-p. [DOI] [PubMed] [Google Scholar]
- 32.Cellerino A, Maffei L, Domenici L. The distribution of brain-derived neurotrophic factor and its receptor trkB in parvalbumin-containing neurons of the rat visual cortex. Eur J Neurosci. 1996;8(6):1190–1197. doi: 10.1111/j.1460-9568.1996.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu QR, et al. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006;1067(1):1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko M, Xie Y, An JJ, Stryker MP, Xu B. Dendritic BDNF synthesis is required for late-phase spine maturation and recovery of cortical responses following sensory deprivation. J Neurosci. 2012;32(14):4790–4802. doi: 10.1523/JNEUROSCI.4462-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau AG, et al. Distinct 3′UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF) Proc Natl Acad Sci USA. 2010;107(36):15945–15950. doi: 10.1073/pnas.1002929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bayer SA, Altman J. Neocortical Development. New York: Raven; 1991. [Google Scholar]
- 37.Dantzker JL, Callaway EM. Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat Neurosci. 2000;3(7):701–707. doi: 10.1038/76656. [DOI] [PubMed] [Google Scholar]
- 38.Trachtenberg JT, Stryker MP. Rapid anatomical plasticity of horizontal connections in the developing visual cortex. J Neurosci. 2001;21(10):3476–3482. doi: 10.1523/JNEUROSCI.21-10-03476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castrén E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci USA. 1992;89(20):9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lein ES, Shatz CJ. Rapid regulation of brain-derived neurotrophic factor mRNA within eye-specific circuits during ocular dominance column formation. J Neurosci. 2000;20(4):1470–1483. doi: 10.1523/JNEUROSCI.20-04-01470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bozzi Y, et al. Monocular deprivation decreases the expression of messenger RNA for brain-derived neurotrophic factor in the rat visual cortex. Neuroscience. 1995;69(4):1133–1144. doi: 10.1016/0306-4522(95)00321-9. [DOI] [PubMed] [Google Scholar]
- 42.Pizzorusso T, et al. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci USA. 2006;103(22):8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace W, Bear MF. A morphological correlate of synaptic scaling in visual cortex. J Neurosci. 2004;24(31):6928–6938. doi: 10.1523/JNEUROSCI.1110-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med. 2005;11(12):1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- 45.Kolbeck R, Bartke I, Eberle W, Barde YA. Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. J Neurochem. 1999;72(5):1930–1938. doi: 10.1046/j.1471-4159.1999.0721930.x. [DOI] [PubMed] [Google Scholar]
- 46.Genoud C, Knott GW, Sakata K, Lu B, Welker E. Altered synapse formation in the adult somatosensory cortex of brain-derived neurotrophic factor heterozygote mice. J Neurosci. 2004;24(10):2394–2400. doi: 10.1523/JNEUROSCI.4040-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vigers AJ, et al. Sustained expression of brain-derived neurotrophic factor is required for maintenance of dendritic spines and normal behavior. Neuroscience. 2012;212:1–18. doi: 10.1016/j.neuroscience.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16(10):3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeanneteau F, Deinhardt K, Miyoshi G, Bennett AM, Chao MV. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat Neurosci. 2010;13(11):1373–1379. doi: 10.1038/nn.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cowan WM. Anterograde and Retrograde Transneuronal Degeneration on the Central and Peripheral Nervous System. New York: Springer; 1970. [Google Scholar]
- 51.Altar CA, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389(6653):856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 52.Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24(17):4250–4258. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dieni S, et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196(6):775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haubensak W, Narz F, Heumann R, Lessmann V. BDNF-GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. J Cell Sci. 1998;111(Pt 11):1483–1493. doi: 10.1242/jcs.111.11.1483. [DOI] [PubMed] [Google Scholar]
- 55.Adachi N, Kohara K, Tsumoto T. Difference in trafficking of brain-derived neurotrophic factor between axons and dendrites of cortical neurons, revealed by live-cell imaging. BMC Neurosci. 2005;6:42. doi: 10.1186/1471-2202-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deppmann CD, et al. A model for neuronal competition during development. Science. 2008;320(5874):369–373. doi: 10.1126/science.1152677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh KK, et al. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci. 2008;11(6):649–658. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- 58.Cao L, et al. Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo. Curr Biol. 2007;17(11):911–921. doi: 10.1016/j.cub.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan Q, Johnson EM., Jr An immunohistochemical study of the nerve growth factor receptor in developing rats. J Neurosci. 1988;8(9):3481–3498. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiss J, McGovern J, Patel AJ. Immunohistochemical localization of cells containing nerve growth factor receptors in the different regions of the adult rat forebrain. Neuroscience. 1988;27(3):731–748. doi: 10.1016/0306-4522(88)90179-0. [DOI] [PubMed] [Google Scholar]
- 61.Koh S, Oyler GA, Higgins GA. Localization of nerve growth factor receptor messenger RNA and protein in the adult rat brain. Exp Neurol. 1989;106(3):209–221. doi: 10.1016/0014-4886(89)90154-4. [DOI] [PubMed] [Google Scholar]
- 62.Biffo S, Offenhäuser N, Carter BD, Barde YA. Selective binding and internalisation by truncated receptors restrict the availability of BDNF during development. Development. 1995;121(8):2461–2470. doi: 10.1242/dev.121.8.2461. [DOI] [PubMed] [Google Scholar]
- 63.Eide FF, et al. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16(10):3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Acheson A, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374(6521):450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 65.Cheng PL, et al. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci USA. 2011;108(45):18430–18435. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desai NS, Rutherford LC, Turrigiano GG. BDNF regulates the intrinsic excitability of cortical neurons. Learn Mem. 1999;6(3):284–291. [PMC free article] [PubMed] [Google Scholar]
- 67.McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15(4):791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 68.McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18(5):767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka J, et al. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319(5870):1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- 71.Wyatt RM, Tring E, Trachtenberg JT. Pattern and not magnitude of neural activity determines dendritic spine stability in awake mice. Nat Neurosci. 2012;15(7):949–951. doi: 10.1038/nn.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457(7227):313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen JL, et al. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 2012;74(2):361–373. doi: 10.1016/j.neuron.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones KR, Fariñas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76(6):989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shen JS, Watabe K, Ohashi T, Eto Y. Intraventricular administration of recombinant adenovirus to neonatal twitcher mouse leads to clinicopathological improvements. Gene Ther. 2001;8(14):1081–1087. doi: 10.1038/sj.gt.3301495. [DOI] [PubMed] [Google Scholar]
- 76.Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. Stereotaxic gene delivery in the rodent brain. Nat Protoc. 2006;1(6):3166–3173. doi: 10.1038/nprot.2006.450. [DOI] [PubMed] [Google Scholar]
- 77.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.