Abstract
We tested the hypothesis that light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) is inhibited by moderately elevated temperature through an effect on Rubisco activase. When cotton (Gossypium hirsutum L.) or wheat (Triticum aestivum L.) leaf tissue was exposed to increasing temperatures in the light, activation of Rubisco was inhibited above 35 and 30°C, respectively, and the relative inhibition was greater for wheat than for cotton. The temperature-induced inhibition of Rubisco activation was fully reversible at temperatures below 40°C. In contrast to activation state, total Rubisco activity was not affected by temperatures as high as 45°C. Nonphotochemical fluorescence quenching increased at temperatures that inhibited Rubisco activation, consistent with inhibition of Calvin cycle activity. Initial and maximal chlorophyll fluorescence were not significantly altered until temperatures exceeded 40°C. Thus, electron transport, as measured by Chl fluorescence, appeared to be more stable to moderately elevated temperatures than Rubisco activation. Western-blot analysis revealed the formation of high-molecular-weight aggregates of activase at temperatures above 40°C for both wheat and cotton when inhibition of Rubisco activation was irreversible. Physical perturbation of other soluble stromal enzymes, including Rubisco, phosphoribulokinase, and glutamine synthetase, was not detected at the elevated temperatures. Our evidence indicates that moderately elevated temperatures inhibit light activation of Rubisco via a direct effect on Rubisco activase.
It is well documented that high temperatures inhibit photosynthetic CO2 fixation (Berry and Björkman, 1980) and damage photosynthetic electron transport, particularly at the site of PSII (Havaux and Tardy, 1996; Havaux et al., 1996). High temperatures also inhibit assimilate export from leaves (Jiao and Grodzinski, 1996). More than a decade ago, Weis (1981b) reported that light-dependent activation of Rubisco in spinach (Spinacia oleracea L.) chloroplasts was inhibited by moderately elevated temperatures and that inhibition was closely correlated with reversible inhibition of CO2 fixation. A similar effect of temperature on Rubisco activation and CO2 fixation was reported for wheat (Triticum aestivum L.) leaves (Kobza and Edwards, 1987). In this latter study, inhibition of carbon assimilation at high temperature occurred under both photorespiratory and nonphotorespiratory conditions. Thus, the temperature-induced inhibition of carbon assimilation cannot be explained solely by increased photorespiration via the enhanced specificity of Rubisco for oxygen at higher temperatures (Jordan and Ogren, 1984).
Activation of Rubisco in the light is regulated by a stromal enzyme named Rubisco activase (for review, see Portis, 1992; Andrews et al., 1995; Salvucci and Ogren, 1996). This nuclear-encoded protein was first reported by Salvucci et al. (1985) as the biochemical lesion in a high-CO2-requiring mutant of Arabidopsis (Somerville et al., 1982). These and subsequent studies (Portis et al., 1986; Salvucci et al., 1986; Mate et al., 1993; Eckhardt et al., 1997) have firmly established an essential role for activase in maintaining the activation state of Rubisco in the light at levels that are adequate for photosynthesis. Several studies have shown that isolated activase is particularly sensitive to inactivation by elevated temperature (Robinson and Portis, 1989; Holbrook et al., 1991; Crafts-Brandner et al., 1997; Eckhardt and Portis, 1997). Thus, inactivation of activase provides a potential biochemical explanation for the inactivation of Rubisco at elevated temperatures reported by Weis (1981a, 1981b) and Kobza and Edwards (1987).
In the present study we examined the effect of moderately elevated temperature on the activation state of Rubisco in the light. We compared wheat and cotton (Gossypium hirsutum L.), species generally adapted to different thermal environments. Rubisco activation assays, Chl fluo-rescence analysis, and western-blot analysis confirmed that Rubisco activation is rapidly and reversibly inhibited by high temperature and suggested that inhibition is caused by changes in the structural properties of activase.
MATERIALS AND METHODS
Wheat (Triticum aestivum L. cv Arina) caryopses and cotton (Gossypium hirsutum L. cv Delta Pine 5415) seeds were planted in 15- × 15-cm pots containing a commercial potting mixture (Grow More, Inc., Gardena, CA2). Plants were grown in a growth chamber (E-15, Conviron Products Co., Winnipeg, Manitoba, Canada) under 14 h of light (350 μmol photons m−2 s−1 PAR) at 25°C and 10 h of darkness at 23°C/day. Plants were fertilized weekly by adding approximately 0.2 g of Grow More 20–20–20 fertilizer (Grow More, Inc.) to each pot. Wheat leaves that were recently fully elongated (first, second, and third leaves) or fully expanded cotton leaves (third and fourth true leaves) were used as experimental material. For the various experiments, the middle 5 cm of the wheat leaves and 1.13-cm2 leaf discs cut from between the major veins of the cotton leaves were sampled.
Determination of Light-Dependent Activation of Rubisco
Leaf tissue harvested from dark-adapted plants was floated on water for 20 min under the conditions described in Results and then immediately extracted using a glass homogenizer in 2 mL of extraction buffer containing 100 mm Tricine, pH 8.0, 5 mm MgCl2, 0.1 mm EDTA, 5 mm DTT, 1% (w/v) PVP, 1% (w/v) casein, 0.05% (v/v) Triton X-100, 1 mm PMSF, and 20 μm leupeptin. The resuspended extract (50 μL) was assayed at 30°C either immediately, to determine the Rubisco activation state (initial Rubisco activity), or after incubation for 10 min at 30°C in an assay mixture containing 10 mm NaHCO3 but lacking ribulose-1,5-bisphosphate, to determine the activity of fully carbamylated Rubisco (total Rubisco activity). Initial and total Rubisco assays were conducted as described in detail by Salvucci and Anderson (1987), except that Triton X-100 and casein were not included in the assay medium. Assays were terminated after 30 s and incorporation of 14CO2 into acid-stable products was determined as described by Salvucci and Anderson (1987).
Two replications were used for each temperature treatment. For wheat, replicates consisted of one 5-cm leaf section from two independent plants. For cotton, each replicate consisted of two 1.13-cm2 leaf discs sampled from each of two different plants.
Determination of Chl Fluorescence
Modulated Chl fluorescence was determined at 25°C from the upper surface of leaf tissue using a fluorometer (PAM-2000, Walz, Effeltrich, Germany). Fluorescence induction and quenching analysis of dark-adapted leaf tissue were determined as described by Schreiber et al. (1986). A preprogrammed protocol (Standard Run 3) was used after minimal adjustment of the measuring light parameters. Fo was determined using a weak, modulated red light. Fm was determined after a 0.8-s pulse of strong white light (>4000 μmol photons m−2 s−1 PAR). After a 20-s lag, a 5-min quenching analysis was initiated using continuous actinic light (125 μmol photons m−2 s−1 emitted at 665 nm) and saturating pulses of 0.8 s every 20 s.
Before fluorescence analysis, plants were irradiated in the growth chambers for at least 1 h to ensure complete metabolism of any carboxyarabinitol 1-phosphate in the cotton leaves (Salvucci and Anderson, 1987). Leaf tissue (1.13 cm2 for cotton; 1-cm segment for wheat) was removed and floated on water in the dark at 25°C for 15 min before fluorescence analysis. Preliminary experiments showed that 15 min of dark adaptation led to Fo and Fm values that were comparable to those of leaf tissue that was kept in the dark for longer times (data not shown). For the heat treatments, leaf tissue was floated on water equilibrated at the higher temperature for the final 5 min of the 15-min dark-adaptation period. Immediately after the heat treatment, the leaf tissue was placed in a dark leaf clip (Walz) at 25°C with the tissue oriented at a right angle to the light source. Three measurements, each from a different leaf, were made for each temperature treatment.
To test the effect of nigericin and DTT on qN, wheat leaves were excised under water and placed in a 50-mL flask containing 100 μm nigericin or 3 mm DTT. Control leaves were excised under water and placed in flasks containing water. After 2.5 to 3 h in the light (350 μmol photons m−2 s−1 PAR), leaf tissue was removed and incubated in the dark for 15 min at 25°C or for 10 min at 25°C followed by 5 min at 37.5°C. Chl fluorescence-quenching analysis was immediately conducted at 25°C.
Relaxation kinetics of qN were analyzed to ascertain the effect of high temperature on leaf tissue incubated under saturating levels of light. Initially, detached leaf tissue was dark adapted at 25°C for 15 min, after which Fm and Fo were determined as described above. The leaf tissue was then incubated under 1800 μmol photons m−2 s−1 PAR for 15 min at 25°C or for 10 min at 25°C followed by 5 min at a higher temperature. Immediately after the incubation in the light, qN relaxation kinetics were analyzed at 25°C using a preprogrammed protocol (Standard Run 5) of the PAM-2000 fluorometer (Walz) after minimal adjustment of the measuring light parameters. After approximately 1 min of continuous actinic light (125 μmol photons m−2 s−1 emitted at 665 nm), the actinic light was turned off and 1.2-s pulses of strong white light (>4000 μmol photons m−2 s−1 PAR) were applied at exponentially increasing time intervals over a 16-min period.
Purification of Rubisco Activase
Escherichia coli BLR(DE3)pLysS cells transformed with the pET 23d plasmid (Novagen) harboring a cDNA encoding tobacco activase were used for expression of recombinant activase (van de Loo and Salvucci, 1996). Recombinant activase was purified from the cells as described by van de Loo and Salvucci (1996). Activase protein concentration was determined by the method of Bradford (1976).
Antibody Production and Western-Blot Analysis
Monospecific polyclonal antibodies against purified recombinant tobacco activase were produced commercially in New Zealand White rabbits (Cocalico Biologicals, Inc., Reamstown, PA). Antibody specificity was verified by western-blot analysis of purified recombinant proteins and crude leaf extracts of cotton, wheat, and several other species (data not shown).
Antibody production against purified phosphoribulokinase has been described (Crafts-Brandner et al., 1990). Antiserum for chloroplastic Gln synthetase was kindly provided by G. Ochs and A. Wild (Johannes-Guttenberg Universitat, Mainz, Germany), and antiserum for the N-terminal 25 amino acids of the Rubisco large subunit were kindly provided by R. Houtz (University of Kentucky, Lexington) and M. Mulligan (University of California, Riverside).
For western-blot analysis, samples consisting of a 5-cm segment from each of four different wheat leaves or a 1.13-cm2 leaf disc from each of four different cotton leaves were extracted in 1.5 mL of buffer containing 20 mm sodium phosphate, pH 7.5, 1% (w/v) polyvinylpolypyrrolidone, and 0.1% (v/v) β-mercaptoethanol. One milliliter of resuspended extract was centrifuged for 2 min at 13,000g, supernatants were removed, and the pellets were resuspended in 1 mL of extraction buffer minus polyvinylpolypyrrolidone. Aliquots of the supernatant and pellet fractions were added to a solution containing 5% (w/v) SDS, 30% (w/v) Suc, 100 mm DTT, and 0.001% (w/v) bromphenol blue, boiled for 3 min, and stored at −20°C before western-blot analysis. The samples were electrophoresed in 12% SDS-PAGE gels according to the method of Laemmli (1970). The proteins were electrophoretically transferred to nitrocellulose (Tijssen, 1985). The blots were probed overnight with the appropriate primary antibody and further developed according to the work of Mitsuhashi and Feller (1992).
Heat treatments consisted of floating intact leaf tissue in beakers of water pre-equilibrated at a given temperature under the conditions described in Results. At least two replicate samples were analyzed for each temperature/time treatment.
Data Analysis
For Rubisco activation assays and fluorescence analysis, all results are reported as the mean ± se. For western-blot analysis, results of one replicate for each temperature treatment are presented.
RESULTS
Because of the large amount of endogenous Rubisco, it is difficult to directly determine the activity of Rubisco activase in leaf extracts. Instead, activase activity is usually determined by measuring the activation state (initial activity) of Rubisco. This measurement provides a valid estimate of the activity of activase in the leaf because the activation state of Rubisco in the light is a direct consequence of the activity of activase (Portis et al., 1986; Salvucci et al., 1986; Andrews et al., 1995; Eckhardt et al., 1997).
Irradiating intact leaf tissue of cotton or wheat with 1800 μmol photons m−2 s−1 PAR for 20 min at 22.5°C promoted full activation of Rubisco (data not shown). Full activation was indicated by the fact that the level of Rubisco activity measured immediately after extraction (initial activity) was the same as the activity measured after extracts were incubated with saturating CO2 to fully carbamylate the enzyme (total activity). Increasing the leaf temperature above 22.5°C for wheat or 30°C for cotton during the last 5 min of the irradiation period caused progressive inhibition of Rubisco activation (Fig. 1). At a given treatment temperature, the inhibition of Rubisco activation was more pronounced for wheat than for cotton.
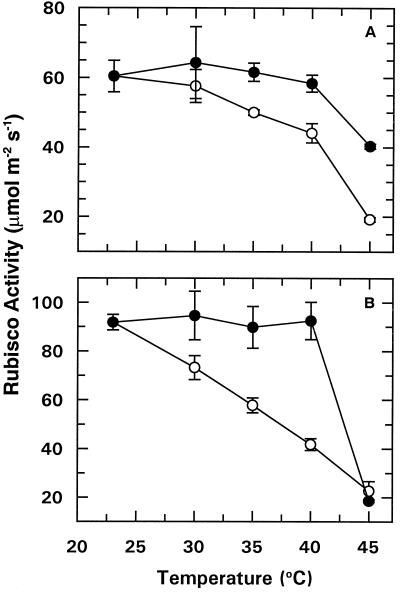
Figure 1.
The effect of temperature on light activation of Rubisco. Intact cotton (A) and wheat (B) leaf tissue was irradiated with 1800 μmol photons m−2 s−1 PAR either for 15 min at 22.5°C followed by an additional 5 min at the indicated temperature (○), or for 5 min at the indicated temperature followed by a 15-min incubation at 22.5°C (•). After the 20-min irradiation period, leaf tissue was immediately homogenized to determine Rubisco activity. Each point represents the mean ± se of two replications.
In preliminary experiments we established that incubating leaf tissue at elevated temperatures during the first 5 min in the light inhibited Rubisco activation to an equal or greater extent than exposure to high temperature during the last 5 min of a 20-min irradiation period (data not shown). However, when a 40°C temperature exposure during the initial 5 min of light was followed with an additional 15 min of irradiation at 22.5°C, Rubisco activation was restored to control levels (Fig. 1). Thus, inhibition of Rubisco activation by moderately elevated temperature was fully reversible at temperatures up to 40°C.
Incubation of leaf tissue at 45°C led to an irreversible inhibition of light-dependent Rubisco activation, especially for wheat (Fig. 1). For example, heat treatment during the initial 5 min of the 20-min incubation caused a 78% decrease in Rubisco activation (initial activity) for wheat, but only a 34% decrease for cotton. In contrast, exposure of the leaf tissue to 45°C had no effect on total Rubisco activity (data not shown). Therefore, temperature-induced inhibition of light-dependent activation of Rubisco was attributable to decreased activity of Rubisco activase rather than to direct inhibition of Rubisco.
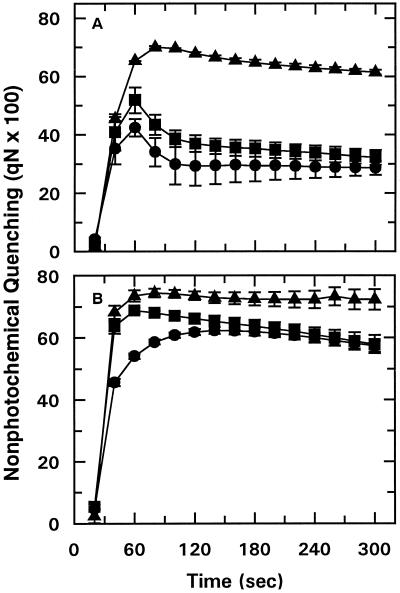
Rubisco activation in cotton and wheat was inhibited by lower temperatures than those that are generally reported to affect electron transport activity (Bilger et al., 1987; Havaux and Tardy, 1996). To determine if Rubisco activation was inhibited at a lower temperature than electron transport in our system, Chl fluorescence-quenching analysis was performed using dark-adapted leaves (Fig. 2). Exposing leaf tissue to 35 or 40°C during the final 5 min of a 15-min dark-adaptation period led to a significant increase in qN for both cotton and wheat, particularly during the first 90 s of the time course (Fig. 2). For wheat and cotton, the temperature-induced increase in qN was first observed at 32.5 and 35°C, respectively (data not shown), and the effect was progressively greater as the treatment temperature was increased. There was no effect of temperature on qN when the high-temperature treatment, up to 40°C, was applied during the first 5 min of the 15-min dark-adaptation period (data not shown). These results indicated that the high-temperature-induced increase in qN was reversible up to 40°C.
Figure 2.
Effect of temperature on the time course of qN of dark-adapted cotton (A) and wheat (B) leaf tissue. The time course of Chl fluorescence was measured at 25°C after leaf tissue was incubated in the dark for 10 min at 25°C followed by an additional 5 min at 25°C (•), 35°C (▪), or 40°C (▴). Data points represent the mean ± se of three replications.
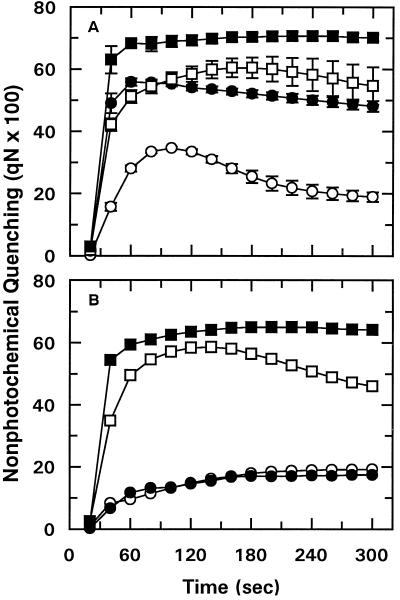
It has been reported that qN is increased under photooxidative conditions because of a stimulation of the conversion of violaxanthin to zeaxanthin (Bilger et al., 1989; Demmig-Adams et al., 1989). More recently, xanthophyll metabolism has been reported to be altered at high temperature (Havaux and Tardy, 1996; Havaux et al., 1996). To determine if the temperature-induced increase in qN was associated with xanthophyll metabolism, detached leaves were allowed to accumulate DTT via the transpiration stream. This treatment blocks conversion of violaxanthin to zeaxanthin, eliminating the increase in qN normally observed under photooxidative conditions (Bilger et al., 1989; Demmig-Adams et al., 1989). Treatment with DTT significantly decreased qN for leaf tissue that was exposed to either 25 or 37.5°C (Fig. 3A). However, the relative effect of increased temperature on qN was not markedly affected. In the presence of DTT the magnitude of the high-temperature-induced increase in qN was noticeably enhanced (Fig. 3A). Thus, under our experimental conditions, the increase in qN that accompanied moderately elevated temperatures was apparently not associated with changes in the conversion of violaxanthin to zeaxanthin.
Figure 3.
Effect of DTT and nigericin on the time course of qN in wheat leaf tissue. Detached leaves were supplied with 3 mm DTT (○, •) (A) or 100 μm nigericin (○, •) (B) via the transpiration stream. Detached control leaves (□, ▪) were allowed to transpire in water. Subsequently, leaf tissue was incubated in the dark for 15 min at 25°C (○, □) or for 10 min at 25°C followed by 5 min at 37.5°C (•, ▪). After incubation, the time course of Chl fluorescence was measured at 25°C. Data points represent the mean ± se of two replications.
In a second control experiment, detached wheat leaves were allowed to accumulate nigericin via the transpiration stream to collapse the trans-thylakoid pH gradient. This treatment had the expected effect of decreasing qN equally for leaf tissue that was exposed to either 25 or 37.5°C before analysis (Fig. 3B).
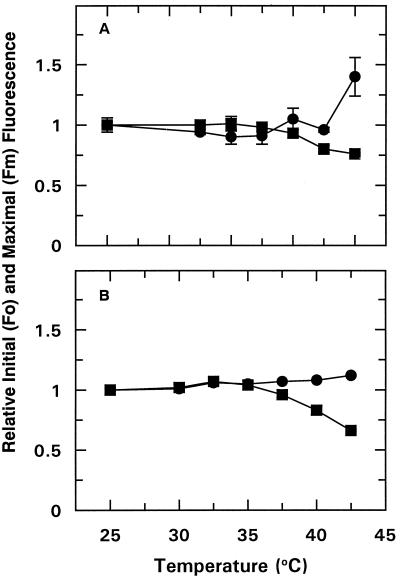
Measurements of Fo and Fm provided an indication of the effect of increased temperature on electron transport. Temperatures at or below 40°C had a minimal effect on Fo or Fm for either species (Fig. 4). Similarly, photochemical quenching did not decrease at these temperatures; in fact, it increased with temperature up to 42.5°C (data not shown).
Figure 4.
Effect of temperature on the Fo (•) and Fm (▪) Chl fluorescence of dark-adapted cotton (A) and wheat (B) leaf tissue. Leaf tissue was treated as described in Figure 2. Data points represent the mean ± se of three replications.
Results from the Chl fluorescence quenching analysis confirmed the earlier work of Bilger et al. (1987), indicating that qN, measured during photosynthetic induction, was very sensitive to high temperature. Because of the requirement to dark-adapt leaf tissue before conducting quenching analysis, the results are not directly comparable to those of the Rubisco activation assays reported in Figure 1. We therefore determined the effect of high temperature during illumination with 1800 μmol photons m−2 s−1 PAR on Chl fluorescence by analyzing the relaxation kinetics of qN. The results (data not shown) indicated that the relaxation of qN was inhibited by elevated temperatures in a manner that was completely analogous to that seen in the Rubisco activation assays (Fig. 1) and the Chl fluorescence quenching analysis (Fig. 2).
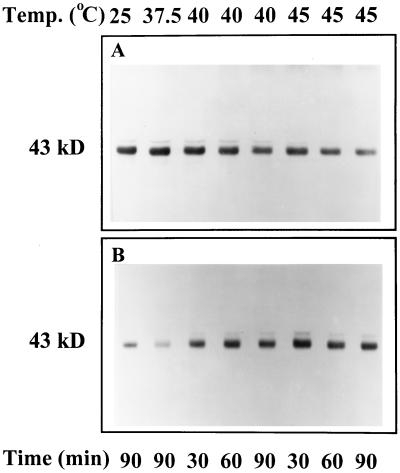
Western-blot analysis was used to examine the effect of moderately elevated temperatures on the distribution and form of activase and other stromal components. Similar results were obtained for leaf tissue that was incubated in the dark or under strong illumination (data not shown). At temperatures above 37.5°C, there was a shift in the distribution of activase from the soluble to the insoluble phase of wheat leaf extracts (Fig. 5). This “insoluble” activase was not solubilized by treatment with 0.1 to 1% Triton X-100 (data not shown). In contrast to the effects on wheat, temperatures as high as 45°C did not alter the relative distribution of activase in leaf extracts of cotton (data not shown).
Figure 5.
Effect of temperature on the distribution of activase in the soluble and insoluble fractions of extracts of wheat leaves. Detached leaf tissue was incubated for the indicated times and at the indicated temperatures and then was immediately homogenized and separated into soluble (A) and insoluble (B) fractions by centrifugation. Polypeptides in the two fractions were separated by SDS-PAGE and analyzed for the presence of activase by western-blot analysis. Lanes were loaded with extract corresponding to equal amounts of leaf area. The activase subunits are indicated by the 43-kD label.
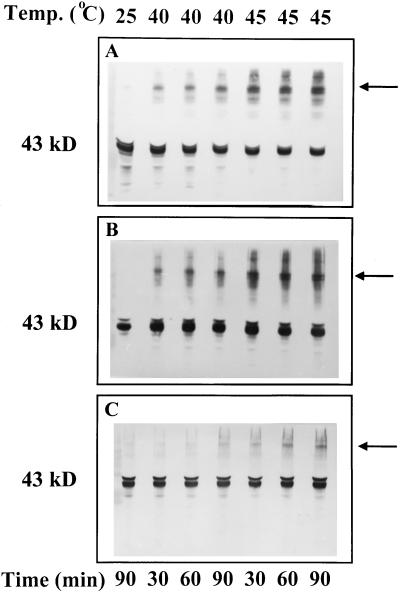
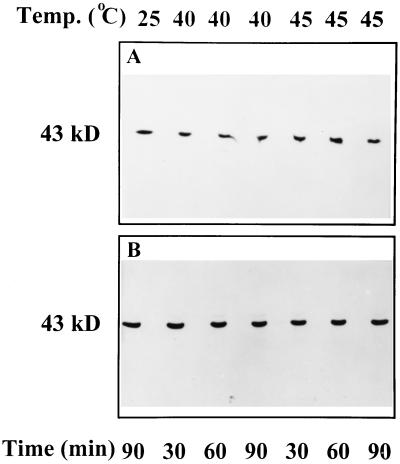
Western blots of gels loaded with higher-than-usual amounts of protein revealed the presence of high-molecular-weight aggregates of activase in both the soluble and insoluble phases of wheat leaf extracts after exposure to temperatures of 37.5 (data not shown), 40, and 45°C (Fig. 6, A and B). More aggregated activase was visible as the treatment temperature was increased. At 45°C, both the solubility and the aggregation of activase from wheat leaves were perturbed within 10 min of exposure time (data not shown). After treatment at temperatures above 40°C, high-molecular-weight aggregates of activase were also visible in the insoluble phase of cotton leaf extracts (Fig. 6C). Exposure of leaf tissue to temperatures as high as 45°C for up to 90 min did not alter the relative solubilities or cause formation of high-molecular-weight aggregates of several other stromal proteins, including phosphoribulokinase (Fig. 7), Rubisco, and Gln synthetase (data not shown).
Figure 6.
Effect of temperature on the formation of high-molecular-weight aggregates of Rubisco activase in the supernatant (A) and pellet (B) fractions of wheat leaf extracts and in the pellet (C) fraction of cotton leaf extracts. Detached leaf tissue was incubated for the indicated times and at the indicated temperatures and then processed and analyzed as described in Figure 5. Lanes were loaded with extract corresponding to equal amounts of leaf area. To better visualize large-molecular-weight aggregates, the gels were overloaded with protein. The activase subunits are indicated by the 43-kD label and the predominant high-molecular-weight aggregates of activase are indicated by the arrows.
Figure 7.
Effect of temperature on the form of phosphoribulokinase in the soluble leaf extracts of wheat (A) and cotton (B). Detached leaf tissue was incubated for the indicated times and at the indicated temperatures and then processed and analyzed as described in Figure 5. Lanes were loaded with extract corresponding to equal amounts of leaf area. Only the supernatant fraction is shown because there was no phosphoribulokinase visible on western blots of proteins from the pellet fraction.
DISCUSSION
Weis (1981a, 1981b) provided clear evidence that light-dependent activation of Rubisco in situ is sensitive to moderately elevated temperatures. In those reports the loss of Rubisco activation that occurred at moderately elevated temperature was accompanied by a corresponding decrease in the rate of photosynthetic CO2 fixation and an increase in light scattering and the electrochromic shift at 534 nm, which are measures of the trans-thylakoid pH gradient. However, there was no context in which to evaluate these results because the biochemical mechanism for Rubisco activation was unknown at that time. In addition to confirming the earlier findings of Weis (1981a, 1981b) and Kobza and Edwards (1987) of a temperature effect on Rubisco activation, our results also show that moderately elevated temperatures have a direct effect on the distribution and form of activase, the biochemical component controlling Rubisco activation. A sensitivity of activase to inactivation at moderately elevated temperatures is consistent with the poor thermal stability of the isolated enzyme (Robinson and Portis, 1989; Holbrook et al., 1991; Crafts-Brandner et al., 1997; Eckhardt and Portis, 1997).
For both cotton and wheat, a 5-min exposure of leaf tissue to temperatures of 30 to 35°C had a rapid and readily reversible effect on light activation of Rubisco. Weis (1981a) also observed that the inhibition of photosynthesis caused by moderately high temperatures was reversible, a result that we explain by reversible temperature inhibition of activase. It is clear from our measurements that Rubisco per se was not affected by moderately high temperatures because incubation of leaf extracts with saturating levels of CO2 for 10 min restored Rubisco activity to the levels determined for the 25°C controls. There was no effect on Rubisco even at 45°C when the in situ activity was irreversibly inhibited in both cotton and wheat leaf tissue (Fig. 1). This result is consistent with measurements with isolated Rubisco, showing that enzyme activity is stable at temperatures above 50°C (Eckhardt and Portis, 1997).
The increase in qN of leaf tissues after exposure to elevated temperature indicated that there was decreased use of ATP and NADPH caused by inhibition of Calvin cycle activity (Schreiber et al., 1986). Both Weis (1981a, 1981b) and Kobza and Edwards (1987) observed a close relationship between loss of Rubisco activation and inhibition of CO2 fixation in response to increasing temperature. Based on these findings and our understanding of Rubisco activation in vivo (for review, see Salvucci and Ogren, 1996), it is likely that increases in qN that were observed upon exposure of cotton and wheat to elevated temperatures were attributable to the consequent effects of activase inhibition on Rubisco activation and CO2 fixation. It is interesting to note that Weis (1981a) reported an increase in thylakoid energization (a major component of qN) in response to loss of Rubisco activation at elevated temperature. Similarly, Salvucci et al. (1987) showed that qN and light scattering were both increased under ambient conditions in a mutant of Arabidopsis that is unable to activate Rubisco because of a lack of activase.
Evidence that inactivation of Rubisco is an early event in the inhibition of photosynthesis by elevated temperature (Weis, 1981a, 1981b; Kobza and Edwards, 1987) conflicts with recent reports that concluded that the primary site of high-temperature damage is associated with a component(s) of the thylakoid membranes (Havaux and Tardy, 1996; Havaux et al., 1996). Using Chl fluorescence parameters, we were unable to detect any inhibition of the electron transport system at temperatures that significantly inhibited Rubisco activation (Figs. 1 and 4). Our results for cotton and wheat are in total agreement with the fluorescence quenching analysis of Arbutus unedo L. (Bilger et al., 1987). It should be noted that Bilger et al. (1987) reported that qN was increased at lower temperatures than those that inhibited assimilation rate. However, these researchers measured CO2 assimilation by determining O2 evolution at 1% CO2. This concentration of CO2 alters the relationship between Rubisco activation and photosynthesis by causing spontaneous carbamylation of Rubisco (Salvucci et al., 1986). However, at air levels of CO2, there is a close correlation between assimilation rate and Rubisco activation (Salvucci et al., 1986; Seemann et al., 1990; Portis, 1992; Mate et al., 1993; Eckhardt et al., 1997), and a parallel effect of temperature on the two processes (Weis, 1981a, 1981b; Kobza and Edwards, 1987).
The formation of high-molecular-weight aggregates of activase after exposure of intact leaf tissue to high temperature provided direct evidence that the physical structure of the enzyme was perturbed by the temperature treatment. Activase from wheat was much more susceptible to structural damage compared with cotton (Fig. 6), similar to the results for Rubisco activation (Fig. 1) and Chl fluorescence (Fig. 2). The temperature required to perturb the distribution and form of activase was much higher than the temperature that caused reversible inhibition of Rubisco activation, occurring at temperatures that caused irreversible damage.
The precise physical nature of insoluble and aggregated activase is not known. The lack of effect of Triton X-100 on the insoluble and/or aggregated activase indicated that the insolubilization was not caused by adherence of activase to membranes. Also, the effect of high temperature on solubility and aggregation of activase is apparently unique to in situ conditions. The isolated protein remains soluble and does not form high-molecular-weight aggregates when incubated at temperatures as high as 60°C (Crafts-Brandner et al., 1997). Crafts-Brandner et al. (1997) also showed that the two forms of spinach activase differ markedly in their thermal stabilities. Differences in thermal stability between the two forms of activase were related to differing abilities to maintain functional subunit associations. Based on these findings, it is likely that functional activase subunit associations also break down at elevated temperatures in vivo. If so, we believe that dissociation of activase subunits is at first reversible. However, if the process is prolonged, dissociation may be extensive and become irreversible if the activase subunits aggregate into nonfunctional units or bind to other components of the leaf extract. Nonspecific aggregation of dissociated activase could account for the formation of the insoluble and aggregated forms of activase, and the irreversible loss of light-dependent Rubisco activation, observed after exposure of leaves to elevated temperatures.
In conclusion, our previous (Crafts-Brandner et al., 1997) and present results provide evidence that high temperature physically perturbs activase, leading to an inhibition of enzyme activity and the consequent effect on light activation of Rubisco. These results serve to unify earlier reports of high-temperature effects on photosynthetic CO2 fixation (Weis, 1981a, 1981b; Bilger et al., 1987; Kobza and Edwards, 1987), and indicate that the inhibition of Rubisco activase may be a key regulatory process affected by high-temperature stress. We suspect that the genetic differences in the primary structure of activase, or the expression of the different activase polypeptide forms (Jiménez et al., 1995; Crafts-Brandner et al., 1997), account for the greater thermal sensitivity of Rubisco activation in wheat compared with cotton.
ACKNOWLEDGMENTS
The authors acknowledge the excellent technical support provided by Jason A. Jones. The authors also acknowledge the reviewer who suggested that we determine the high-temperature effects on qN for light-adapted leaf tissue.
Abbreviations:
- Chl
chlorophyll
- Fm
maximal Chl fluorescence
- Fo
initial Chl fluorescence
- qN
nonphotochemical fluorescence quenching
Footnotes
This work was supported in part by a grant from the Swiss National Science Foundation (project no. 3100-043174.95).
Mention of a trade name does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval over other products that may also be suitable.
LITERATURE CITED
- Andrews TJ, Hudson GS, Mate CJ, von Caemmerer S, Evans JR, Avridsson YBC. Rubisco, consequences of altering its expression and activation in transgenic plants. J Exp Bot. 1995;46:1293–1300. [Google Scholar]
- Berry JA, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol. 1980;31:491–543. [Google Scholar]
- Bilger W, Björkman O, Thayer SS. Light-induced spectral absorbance changes in relation to photosynthesis and the epoxidation state of xanthophyll cycle components in cotton leaves. Plant Physiol. 1989;91:542–551. doi: 10.1104/pp.91.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger W, Schreiber U, Lange OL. Chlorophyll fluorescence as an indicator of heat induced limitation of photosynthesis in Arbutus unedo L. In: Tenhunen JD, Catarino FM, Lange OL, editors. Plant Response to Stress. Berlin: Springer; 1987. pp. 391–399. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–259. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME, Egli DB. Changes in ribulosebisphosphate carboxylase/oxygenase and ribulose 5-phosphate kinase abundances and photosynthetic capacity during leaf senescence. Photosynth Res. 1990;23:223–230. doi: 10.1007/BF00035013. [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, van de Loo FJ, Salvucci ME. The two forms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiol. 1997;114:439–444. doi: 10.1104/pp.114.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW, III, Heber U, Neimanis S, Winter K, Krüger A, Czygan F-C, Bilger W, Björkman O. Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol. 1989;92:293–301. doi: 10.1104/pp.92.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt NA, Portis AR., Jr Heat denaturation profiles of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and Rubisco activase and the inability of Rubisco activase to restore activity of heat-denatured Rubisco. Plant Physiol. 1997;113:243–248. doi: 10.1104/pp.113.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt NA, Snyder GW, Portis AR, Jr, Ogren WL. Growth and photosynthesis under high and low irradiance of Arabidopsis thaliana antisense mutants with reduced ribulose-1,5-bisphosphate carboxylase/oxygenase activase content. Plant Physiol. 1997;113:575–586. doi: 10.1104/pp.113.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Tardy F. Temperature-dependent adjustment of the thermal stability of photosystem II in vivo: possible involvement of xanthophyll-cycle pigments. Planta. 1996;198:324–333. [Google Scholar]
- Havaux M, Tardy F, Ravenel J, Chanu D, Parot P. Thylakoid membrane stability to heat stress studied by flash spectroscopic measurements of the electrochromic shift in intact potato leaves: influence of the xanthophyll content. Plant Cell Environ. 1996;19:1359–1368. [Google Scholar]
- Holbrook GP, Galasinski SC, Salvucci ME. Regulation of 2-carboxyarabinitol 1-phosphatase. Plant Physiol. 1991;97:894–899. doi: 10.1104/pp.97.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Grodzinski B. The effect of leaf temperature and photorespiratory conditions on export of sugars during steady-state photosynthesis in Salvia splendons. Plant Physiol. 1996;111:169–178. doi: 10.1104/pp.111.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez ES, Medrano L, Martínez-Barajas E. Rubisco activase, a possible new member of the molecular chaperone family. Biochemistry. 1995;34:2826–2831. doi: 10.1021/bi00009a012. [DOI] [PubMed] [Google Scholar]
- Jordan DB, Ogren WL. The CO2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase: dependence on ribulosebisphosphate concentration, pH and temperature. Planta. 1984;161:308–313. doi: 10.1007/BF00398720. [DOI] [PubMed] [Google Scholar]
- Kobza J, Edwards GE. Influences of leaf temperature on photosynthetic carbon metabolism in wheat. Plant Physiol. 1987;83:69–74. doi: 10.1104/pp.83.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mate CJ, Hudson GS, von Caemmerer S, Evans JR, Andrews TJ. Reduction of ribulose bisphosphate carboxylase activase levels in tobacco (Nicotianatabacum) by antisense RNA reduces ribulose bisphosphate carboxylase carbamylation and impairs photosynthesis. Plant Physiol. 1993;102:1119–1128. doi: 10.1104/pp.102.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi W, Feller U. Effects of light and external solutes on the catabolism of nuclear-encoded stromal proteins in intact chloroplasts isolated from pea leaves. Plant Physiol. 1992;100:2100–2105. doi: 10.1104/pp.100.4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis AR., Jr Regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase activity. Annu Rev Plant Physiol. 1992;43:415–437. [Google Scholar]
- Portis AR, Jr, Salvucci ME, Ogren WL. Activation of ribulosebisphosphate carboxylase/oxygenase at physiological CO2 and ribulosebisphosphate concentrations by Rubisco activase. Plant Physiol. 1986;82:967–971. doi: 10.1104/pp.82.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SP, Portis AR., Jr Adenosine triphosphate hydrolysis by purified Rubisco activase. Arch Biochem Biophys. 1989;268:93–99. doi: 10.1016/0003-9861(89)90568-7. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Anderson JC. Factors affecting the activation state and the level of total activity of ribulose bisphosphate carboxylase in tobacco protoplasts. Plant Physiol. 1987;85:66–71. doi: 10.1104/pp.85.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Ogren WL. The mechanism of Rubisco activase: insights from studies of the properties and structure of the enzyme. Photosynth Res. 1996;47:1–11. doi: 10.1007/BF00017748. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Portis AR, Jr, Heber U, Ogren WL. Stimulation of thylakoid energization and ribulose-bisphosphate carboxylase/oxygenase activation in Arabidopsis leaves by methyl viologen. FEBS Lett. 1987;221:215–230. [Google Scholar]
- Salvucci ME, Portis AR, Jr, Ogren WL. A soluble chloroplast protein catalyzes ribulosebisphosphate carboxylase/oxygenase activation in vivo. Photosynth Res. 1985;7:193–201. doi: 10.1007/BF00037012. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Portis AR, Jr, Ogren WL. Light and CO2 response of ribulose-1,5-bisphosphate carboxylase/oxygenase activation in Arabidopsis leaves. Plant Physiol. 1986;80:655–659. doi: 10.1104/pp.80.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- Seemann J, Kobza J, Moore B. Metabolism of 2-carboxyarabinitol 1-phosphate and regulation of ribulose-1,5-bisphosphate carboxylase activity. Photosynth Res. 1990;23:119–130. doi: 10.1007/BF00035005. [DOI] [PubMed] [Google Scholar]
- Somerville CR, Portis AR, Jr, Ogren WL. A mutant of Arabidopsis thaliana which lacks activation of RuBP carboxylase in vivo. Plant Physiol. 1982;70:381–387. doi: 10.1104/pp.70.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijssen P. Practice and Theory of Immunoassays. Amsterdam: Elsevier Science Publishers; 1985. [Google Scholar]
- van de Loo FJ, Salvucci ME. Activation of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) involves Rubisco activase trp 16. Biochemistry. 1996;35:8143–8148. doi: 10.1021/bi9604901. [DOI] [PubMed] [Google Scholar]
- Weis E. Reversible heat-inactivation of the Calvin cycle: a possible mechanism of the temperature regulation of photosynthesis. Planta. 1981a;151:33–39. doi: 10.1007/BF00384234. [DOI] [PubMed] [Google Scholar]
- Weis E. The temperature sensitivity of dark-inactivation and light-activation of the ribulose-1,5-bisphosphate carboxylase in spinach chloroplasts. FEBS Lett. 1981b;129:197–200. [Google Scholar]