Abstract
Most ectothermic organisms mature at smaller body sizes when reared in warmer conditions. This phenotypically plastic response, known as the “temperature-size rule” (TSR), is one of the most taxonomically widespread patterns in biology. However, the TSR remains a longstanding life-history puzzle for which no dominant driver has been found. We propose that oxygen supply plays a central role in explaining the magnitude of ectothermic temperature-size responses. Given the much lower oxygen availability and greater effort required to increase uptake in water vs. air, we predict that the TSR in aquatic organisms, especially larger species with lower surface area–body mass ratios, will be stronger than in terrestrial organisms. We performed a meta-analysis of 1,890 body mass responses to temperature in controlled experiments on 169 terrestrial, freshwater, and marine species. This reveals that the strength of the temperature-size response is greater in aquatic than terrestrial species. In animal species of ∼100 mg dry mass, the temperature-size response of aquatic organisms is 10 times greater than in terrestrial organisms (−5.0% °C−1 vs. −0.5% °C−1). Moreover, although the size response of small (<0.1 mg dry mass) aquatic and terrestrial species is similar, increases in species size cause the response to become increasingly negative in aquatic species, as predicted, but on average less negative in terrestrial species. These results support oxygen as a major driver of temperature-size responses in aquatic organisms. Further, the environment-dependent differences parallel latitudinal body size clines, and will influence predicted impacts of climate warming on food production, community structure, and food-web dynamics.
Keywords: physiology, aerobic scope, scaling, plasticity
Ectothermic organisms, which comprise over 99% of species, usually mature at a smaller body size when reared in warmer conditions (1–3). This response, called the “temperature-size rule” (TSR) (1, 2), is one of the most widespread patterns in biology (4, 5) and is found in organisms as diverse as bacteria, protists, invertebrates, plants, and ectothermic vertebrates (1, 6). The TSR contributes to reduced crop yields in warm years (7), and accords with the recently described ecological response of declining body size associated with global warming (8, 9). Despite the widespread importance of both temperature and body size in ecosystem functioning (10), the effect of temperature on organism size remains poorly understood (5), with no dominant driver having been identified (4, 5, 11).
To reveal the major influences on temperature-size responses across the whole of the ectotherms, analysis of the quantitative variation in body size responses among all taxa and environments is required. So far, many size- and temperature-dependent influences on growth, reproduction, and survival have been proposed to explain the variation in size responses to temperature, but no dominant cause or mechanism has been confirmed (1–6). No systematic differences in the strength of the TSR, for example, have been found among taxonomic or ecological (e.g., trophic) groups in aquatic protists (6). However, it is not known whether such differences might occur in metazoans, whose larger size and fundamentally different body plans might limit such responses.
Oxygen supply has been proposed as an important driver of the TSR in animals (12). The rate of diffusional uptake of oxygen is less sensitive to warming than is aerobic metabolism, and later in ontogeny, respiratory surface area for oxygen uptake is expected to face greater challenges in supplying the requirements of a large body (12). These oxygen-mediated pressures of warming to reduce body size at later stages of ontogeny are especially likely in aquatic environments (12, 13), as oxygen is far less available in water than in air, and the energetic costs of moving the much denser and more viscous water also are greater (13). The Oxygen Supply Index (14), which measures the rate of molecular diffusion at the respiratory surface based on Fick’s diffusion equation, is more than five orders of magnitude greater in air than in freshwater and seawater (14). These pressures to reduce body size in the warm may increase not only during ontogeny, but also at maturity between smaller and larger species. However, such predicted differences in aquatic vs. terrestrial and large vs. small species have yet to be tested. If oxygen is an important component of the TSR, we may expect temperature-size responses to be more negative in aquatic than terrestrial organisms and in larger than smaller species. Here, we aim to assess the importance of oxygen in driving the TSR in metazoans by testing the following two hypotheses: (i) The TSR is more negative in aquatic than terrestrial species. (ii) The TSR is more negative in larger than smaller species.
Results
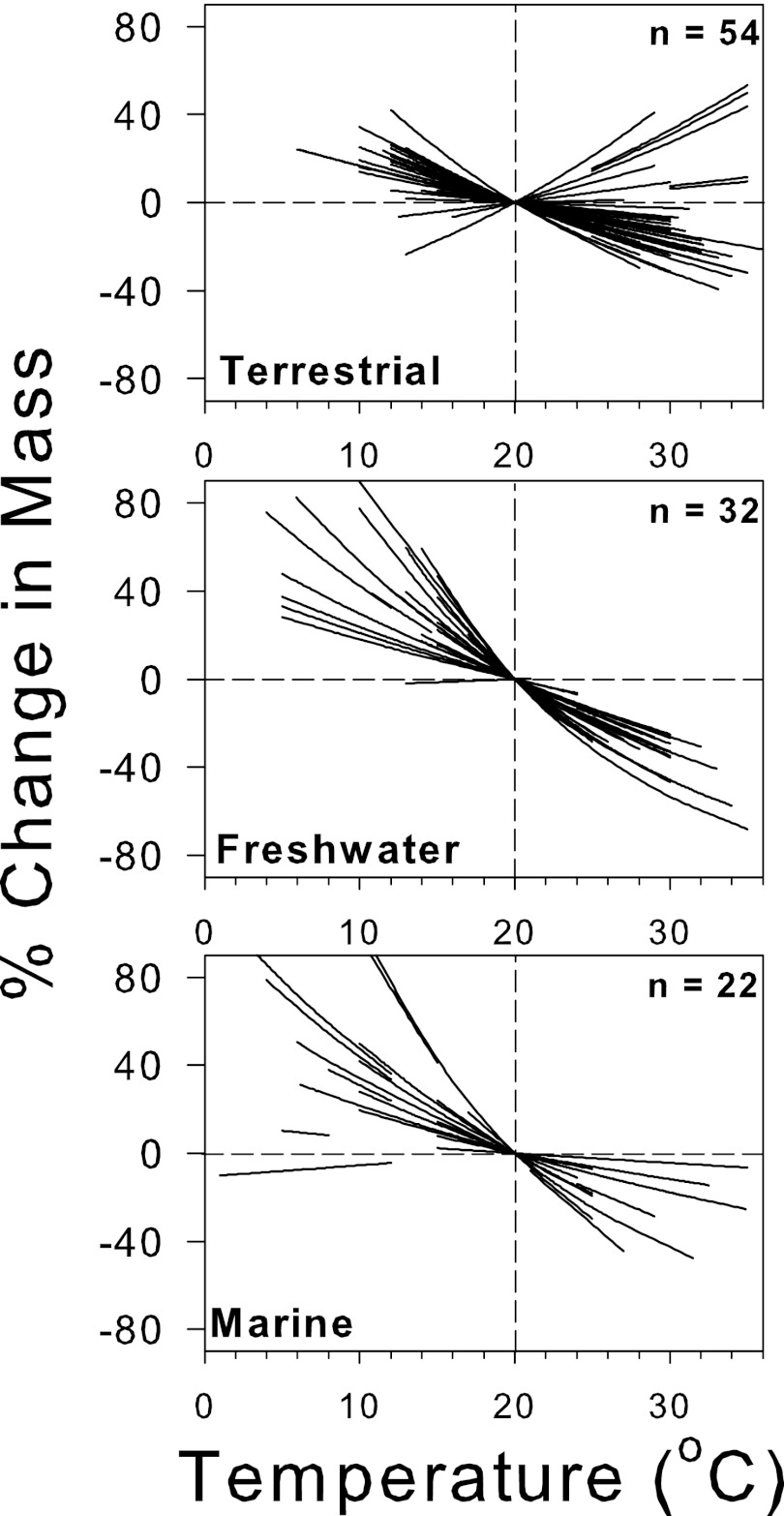
To investigate the differences between ectothermic metazoans by environment and size, we performed a meta-analysis to determine the temperature-size response [% change in dry mass (DM) °C−1] for a wide range of species. In general, our meta-analysis of metazoan temperature-size responses reveals that slopes are negative, hence supporting the TSR, in 90% of cases (99 of 110 metazoan species; Fig. 1).
Fig. 1.
Temperature-size response of species in terrestrial, freshwater, and marine environments. Size changes are expressed as a percentage change from that at 20 °C; each line represents a single species. n, number of species.
Using a general linear model (GLM), we found that the environment inhabited (marine, freshwater, terrestrial) and the interaction between environment type and species mean DM have significant effects on species-specific percentage mass changes with warming (% °C−1). The best-fit model shows a significant difference in temperature-size responses between freshwater and terrestrial species (P < 0.0001), but not between marine and freshwater species; therefore, the latter two are grouped together as “aquatic species.” The GLM shows significant differences not only in the absolute percentage mass change between aquatic and terrestrial environments, but also between the mass dependence of the temperature-size response in these groups (Fig. S1). Although terrestrial and aquatic metazoans both have a response of around −2.5% °C−1 at body sizes between 0.01 and 0.1 mg DM, the responses of metazoans from these two environments diverge with increasing species size.
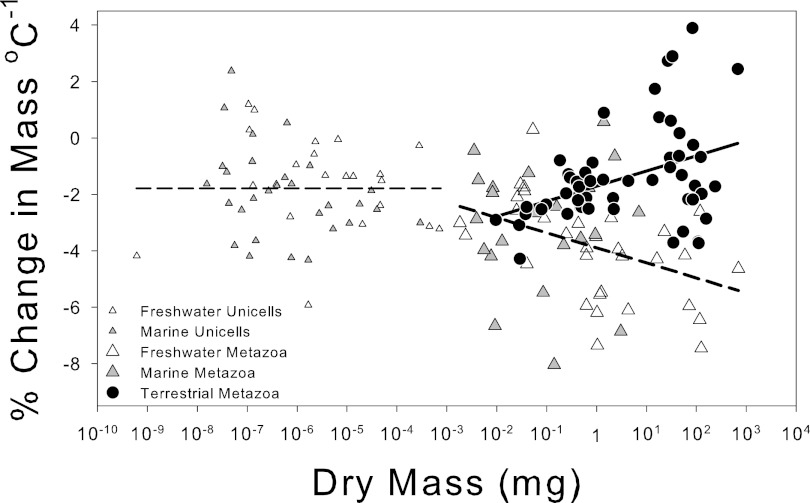
As the mass of aquatic species increases, the temperature-size response becomes increasingly negative, reaching around −5% °C−1 when mean species dry body mass is 100 mg. However, in terrestrial species the average temperature-size response progressively reduces, reaching around −0.5% °C−1 when dry body mass is 100 mg, resulting in a 10-fold difference in the size of the response between aquatic and terrestrial species. Perhaps more important than this average response in terrestrial species, however, is the increased variability as species size increases. Thus, small species show a relatively consistent size response, whereas the responses of larger terrestrial species range from a magnitude similar to that of smaller species to a sizeable converse TSR (Fig. 2). The dependence of the response on species size in both aquatic and terrestrial organisms applies only to multicellular organisms; the much smaller unicellular organisms show no significant species mass dependence on their temperature-size response (Fig. 2).
Fig. 2.
Species-specific temperature-size responses (% change in mass per °C) expressed as a function of the organism size (dry mass) in aquatic (marine and freshwater) and terrestrial environments, including both uni- and multicellular organisms. Terrestrial species have a significant positive regression (PCM = −1.72 + 0.54 × log10DM, R2 = 0.15, df = 53, P < 0.01, solid line); aquatic species have a significant negative regression (PCM = −3.90 – 0.53 × log10DM, R2 = 0.14, df = 53, P < 0.01, thick dashed line). Because there is no significant change in the temperature-size response with mass in unicellular species, the mean response is given by the thin dashed horizontal line (−1.80%°C−1).
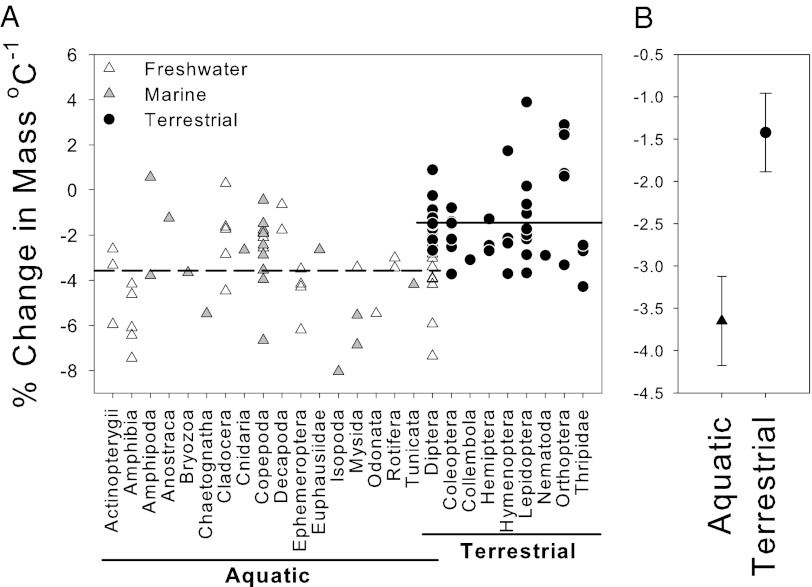
Notably, both the taxonomic group and the midexperimental temperature at which each species was examined were not significant parameters in the GLM. These findings are important because they indicate that the environment and its interaction with species size overrides any effect of taxon (Fig. 3) and temperature range on variation in the temperature-size response, even though the terrestrial species typically are from higher temperatures than the aquatic species (Fig. 1). The more negative size response in aquatic vs. terrestrial species is illustrated in the Diptera, the only order represented in both environments (Fig. 3): species with aquatic larvae had a nearly threefold stronger size response to temperature [−4.54 ± 1.03 (95% CI)] than those that are terrestrial [−1.63 ± 0.44 (95% CI); two-sample t test, t = 5.98, df = 21, P < 0.0001]. Our analysis also reveals that the differences in adult temperature-size responses between environments are not the result of differences in the size responses of their progeny, which show no significant difference in the response of aquatic vs. terrestrial species (Fig. S2).
Fig. 3.
(A) Comparison of the % change in mass per °C in aquatic and terrestrial animals. Aquatic species (mean, −3.65% °C−1) show a significantly stronger temperature-size response than terrestrial species (mean, −1.43% °C−1). Within the aquatic group, there is no significant difference between marine and freshwater species. Note how species within the order Diptera are found in both terrestrial and freshwater environments, with differences between these two reflecting the broader patterns across environment types. (B) Mean ±95% CI for the % change in mass per °C in aquatic and terrestrial species. Size changes are significantly different between these two environments (P < 0.0001).
Discussion
These findings support the hypothesis that oxygen availability is a major correlate and cause of the TSR in aquatic environments (12–14). Specifically, the size response not only is more strongly negative in aquatic species, as predicted by our first hypothesis, but also becomes stronger with increased species size, as predicted by our second hypothesis—but crucially only in aquatic species. We critically assessed alternate explanations based on temperature- and size-dependent differences between aquatic and terrestrial environments, including oxygen solubility and diffusivity, and viscosity and density of the medium (Alternative Hypotheses for Temperature-Size Changes), but found that none of these explains the observed patterns.
In unicells and small metazoans, in both aquatic and terrestrial environments, diffusion meets oxygen demands without the need for special adaptations such as ventilation of specialized respiratory organs. It has been demonstrated that if the complication of a boundary layer of stagnant water enveloping an aquatic organism is ignored, a body radius of up to ∼1 mm can meet metabolic oxygen requirements by diffusion through the organism’s body surface (15, 16). This maximum size will be reduced by the presence of a boundary layer. The thickness of the boundary layer relative to organism volume increases for smaller species (17); thus, we expect the variability in microplankton size response to temperature to be influenced more by flow conditions than by species size. Our data (Fig. 2) show no detectable effect of species size on the strength of the temperature-size response below about 0.01 mg DM. This predicted high importance of water movement is consistent with the observation of the converse TSR in the diatom Phaeodactylum tricornutum, which was confined to experiments in which the rate of air bubbling was experimentally increased along with increased temperature (6).
We now argue that oxygen affects the strength of the TSR increasingly in larger aquatic species. From a geometric perspective, larger organisms generally have reduced ratios of surface area to body mass. In addition, metabolic rate is more sensitive to warming than is oxygen diffusion (12, 15). However, oxygen supply is much lower and more costly to increase (by active ventilation of respiratory surfaces) in water than in air (13, 14). Thus, the challenge of meeting oxygen requirements increases with both body size and temperature, especially for aquatic species (12, 15). Above a threshold size of ∼0.01 mg DM, suggested by our findings, larger aquatic organisms therefore are expected to increasingly adopt mechanisms to either increase oxygen supply or reduce demand in the warmth, as the reduced ratio of surface area for respiratory uptake to body mass combines with greater thermal sensitivity of demand (metabolism) relative to oxygen supply (12, 13). Some mechanisms to maintain aerobic scope by increasing oxygen supply, such as increasing permeability or disproportionately increasing respiratory surface areas, cannot be sustained indefinitely. Moreover, ventilating these surfaces to increase oxygen diffusion rates from water into tissues requires large amounts of energy. For example, tench (Tinca tinca) expend approximately a third of their resting metabolic energy on ventilation (13). Therefore, we hypothesize that as species size increases, in the size range included in this meta-analysis, maintaining aerobic scope increasingly relies on reducing oxygen demand by maturing at a smaller size at increased temperatures; this entails a strengthening of the temperature-size response in larger aquatic species, as is observed.
By contrast, oxygen supply is many orders of magnitude greater for terrestrial organisms, and the ventilation costs are much cheaper (13), which should improve the potential to meet the additional oxygen demand at warmer temperatures by adjusting permeability, surface area, or ventilation of respiratory exchange surfaces, without resorting to strong reductions in size at maturity (18). Nor does the potential to meet the additional demand seem to be diminished in larger terrestrial species, as the size response does not become more negative in larger species. Therefore, we suggest that other factors override the effects of oxygen on the size response of terrestrial ectotherms.
Explanations for variation in terrestrial size responses need to account for why these become more variable as species size increases (Fig. 2) above a minimum size of 0.01 mg DM. Specifically, size responses of larger terrestrial species range from a TSR of the same magnitude as that of their smaller counterparts to a converse TSR. Multiple mechanisms may drive these differences, and a quantitative understanding of the forces that favor larger size in warmer conditions should improve predictions of size responses in terrestrial environments. One such mechanism consists of the size-dependent benefits from the ability to raise and retain body temperatures above ambient among sun-basking ectotherms (19). Grasshoppers (Orthoptera, Fig. 3), along with several butterfly species (Lepidoptera), are known exceptions to the TSR (1, 5, 20); they show an increase in size with increasing temperature in Fig. 1, and typically are heliothermic. Our analysis, however, suggests that the converse TSR in these taxa is part of a size-dependent continuum rather than being a taxonomic outlier. The heliothermic strategy may define the upper edge of the distribution of temperature-size responses in terrestrial species (Fig. 2), and further investigation might test whether the importance of heliothermy declines in terrestrial species with more negative size responses.
Further mechanistic understanding comes from relating the observed stronger TSR in larger aquatic metazoans and the oxygen supply hypothesis to the observation at the heart of the TSR that developmental rate usually is more sensitive than growth rate to temperature (1, 5, 11, 21–23). Our main prediction is that growth rate will be relatively less sensitive than developmental rate to warming in aquatic than in terrestrial metazoans above 0.1 mg DM, and that this difference will increase with increased species size. A secondary prediction is that this difference in thermal sensitivity between growth and development may not be evident during early phases of ontogeny in either terrestrial or aquatic species, as oxygen limitation likely is unimportant (24). However, differences between terrestrial and aquatic species may be expected as growth proceeds: to mitigate any progressive reduction of aerobic scope by oxygen limitation in aquatic species in the warm, organisms would be expected to progressively reduce their investment in size increases during ontogeny; hence growth would demonstrate a lower sensitivity to warming than development, resulting in a reduced size at maturity and stronger TSR, especially in larger species. Indeed, aquatic crustaceans do demonstrate such diminishing thermal sensitivity of growth but not development rates during ontogeny (21, 22), which varies among species (21), although interspecific differences have not yet been linked to species size. Although similarly suitable data are not available for terrestrial species, the optimal temperature for both growth and development rates was found to decline during development of a lepidopteran larva (25), suggesting a mechanism different from that observed in aquatic crustaceans.
Several challenges to understanding temperature-size responses remain. Overall, we suggest that beyond our description of the major effect of oxygen on the body size response in aquatic metazoans, other drivers must be identified and quantitatively understood, especially those affecting terrestrial and very small aquatic species. Also, to extend the scope of our findings, studies on larger species are needed in all environments, especially marine, as these are represented here only by small (<10 mg DM) species.
Our finding that the plastic body size responses to temperature are environment dependent parallels at least three geographical body size trends, both intraspecific and interspecific. First, intraspecific shifts in adult size with increasing latitude (correlated with lower temperatures) among 45 terrestrial arthropod species changed from positive in small species to increasingly negative in larger species (26), which parallels our observed progression toward reversal of the TSR with increased size of terrestrial species. As plastic body size responses to temperature generally are adaptive (4, 5), an adaptive response to temperature (large size being increasingly favored in warmer terrestrial environments) may at least contribute to the pattern of size clines that had been otherwise, and contentiously, explained as effects of seasonality or season-length constraints (26, 27). Second, the largest known member in each of 24 diverse terrestrial ectotherm taxa is tropical, whereas the size of the largest member at different latitudes generally decreases with increased latitude (28, 29). Third, and in contrast to the terrestrial latitudinal cline, the phenomenon of polar gigantism in ectotherms is confined to aquatic species (30). Thus, the selective factors that produce the major difference in growth response that we report between environments may be the same as those dominating the differences between aquatic and terrestrial environments in the evolution of body size and the sorting of different-sized species along latitudinal temperature gradients.
Size responses to climate change, including increased frequencies and intensities of warmer weather, are expected to affect ecosystems substantially by modifying the overall size structure, as well as size-dependent biogeochemical rates and food web dynamics (10, 31). Any attempt to predict such consequences must consider the stark differences between environments we have demonstrated in the temperature-size responses of species.
Methods
Data for multicellular organisms were compiled using an expanded dataset from J. Forster (23). Newly published data were added using the Institute for Scientific Information (ISI) Web of Knowledge using the search terms “(adult OR pupa* OR larva*) AND temperature AND (weight OR *mass OR size).” Entomological journals were individually searched for extra datasets. Data were included for both sexes when available, and for multiple studies of a single species. Included were only the laboratory studies in which sizes were measured at a range of constant temperatures but food concentrations had been maintained at or above saturation (therefore removing the confounding impact of food limitation). We were careful to include only studies in which food supply was considered nonlimiting.
The minimum period of acclimation for the inclusion of adult mass data was set so that only individuals who had been raised from egg or first larval stages were included. We included only nonharmful temperatures within the analysis, limiting the data to temperatures at which the animals survived to adulthood and in which there was no evidence of growth rate declining with increasing temperature. Adult data were collected as lengths, volumes, and dry, wet, or carbon mass. Measurements subsequently were converted to dry mass (milligrams) using appropriate conversions (Dataset S1). Data for unicellular organisms were compiled using the dataset from D. Atkinson (6), combined with published data for bacteria (Dataset S1). Data were searched for with the ISI Web of Knowledge using the search terms “(protist OR protozoa* OR unicell*) AND temperature AND (*volume OR *mass OR size).” Furthermore, individual relevant journals (e.g., Journal of Aquatic Microbial Ecology, Journal of Plankton Research) were searched. Data for Blepharisma americanum were from our own previously unpublished results. All data are presented in Dataset S1; an overview of the data collected is presented in Table 1.
Table 1.
Summary of data compiled in this meta-analysis and used in determining species-specific temperature-size responses
| Organism | Environment | Species, n | Studies, n | Data points, n |
| Multicellular | Terrestrial | 54 | 124 | 880 |
| Multicellular | Freshwater | 32 | 60 | 247 |
| Multicellular | Marine | 22 | 27 | 149 |
| Unicellular | Freshwater | 25 | 57 | 295 |
| Unicellular | Marine | 36 | 44 | 319 |
| Total | 169 | 312 | 1890 |
We used an information theoretic approach (Akaike Information Criteria), with nesting for different studies and sexes within each species (30) to test different equation forms for the response of body mass to temperature; we found the metazoan data to be best modeled by an exponential equation form (vs. power, linear, and Arrhenius models; SI Modeling the Data; see Table S1). The species-specific slopes of [the natural log] ln (dry mass) vs. temperature were subsequently transformed to percentage change in dry mass per degree Celsius for ease of interpretation, using the formula (exp(slope) − 1)*100 = % change in mass per °C. Comparing across species, the effect of environment type (freshwater, marine, and terrestrial), body size, class, and temperature on the species-specific temperature-size responses (i.e., percentage change in mass per degree Celsius) were incorporated within a series of GLMs with the basic structure:
where PCM is the species-specific change in mass (% °C−1), determined by the linear mixed-effects model; T is the species-specific midtemperature (°C, the midpoint between the highest and lowest rearing temperature used); E is the environment type (freshwater, marine, terrestrial); DM is the species average dry mass (milligrams); and C is the class or taxon used to define different groups (following the same divisions shown in Fig. 3). These divisions were made by order, rather than class, for arthropods because of the diversity and range of data for this phylum. Beyond this simple structure, we allowed the interaction of these four parameters and determined the GLM that best described the data by testing which parameters (both alone and with interactions) significantly improved the fit of the model (P < 0.05). Application of the GLM revealed no significant difference between marine and freshwater environments; thus, these were grouped together as “aquatic.” We provide the output for the best-fit GLM in Fig. S1. To test whether the differences in adult responses between environments mght be attributed to differences in progeny mass responses to temperature, we conducted similar analyses on the latter. However, no differences in the % mass changes °C−1 by environment, midexperimental temperature, taxon, or dry mass were found for the progeny (Fig. S2). We tested whether any of the patterns were a consequence of systematic shifts in the goodness of fit in the temperature-size responses of adults by screening all the data to include only species-specific regressions in which R2 ≥ 0.8. The subsequent patterns were unaltered from those presented here (SI Modeling the Data and Figs. S3 and S4).
Supplementary Material
Acknowledgments
J.F. was supported by Natural Environment Research Council Studentship NE/G523655/1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data set reported in this paper are listed in Dataset S1.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210460109/-/DCSupplemental.
References
- 1.Atkinson D. Temperature and organism size—a biological law for ectotherms. Adv Ecol Res. 1994;25:1–58. [Google Scholar]
- 2.Atkinson D, Sibly RM. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol Evol. 1997;12(6):235–239. doi: 10.1016/s0169-5347(97)01058-6. [DOI] [PubMed] [Google Scholar]
- 3.Kingsolver JG. The well-temperatured biologist. (American Society of Naturalists Presidential Address) Am Nat. 2009;174(6):755–768. doi: 10.1086/648310. [DOI] [PubMed] [Google Scholar]
- 4.Angilletta MJ, Jr, Steury TD, Sears MW. Temperature, growth rate, and body size in ectotherms: Fitting pieces of a life-history puzzle. Integr Comp Biol. 2004;44(6):498–509. doi: 10.1093/icb/44.6.498. [DOI] [PubMed] [Google Scholar]
- 5.Kingsolver JG, Huey RB. Size, temperature, and fitness: Three rules. Evol Ecol Res. 2008;10(2):251–268. [Google Scholar]
- 6.Atkinson D, Ciotti BJ, Montagnes DJS. Protists decrease in size linearly with temperature: ca. 2.5% degrees C(-1) Proc Biol Sci. 2003;270(1533):2605–2611. doi: 10.1098/rspb.2003.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson D, Porter JR. Temperature, plant development and crop yields. Trends Plant Sci. 1996;1(4):119–124. [Google Scholar]
- 8.Daufresne M, Lengfellner K, Sommer U. Global warming benefits the small in aquatic ecosystems. Proc Natl Acad Sci USA. 2009;106(31):12788–12793. doi: 10.1073/pnas.0902080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheridan JA, Bickford D. Shrinking body size as an ecological response to climate change. Nature Clim Change. 2011;1(8):401–406. [Google Scholar]
- 10.Hildrew AG, Raffaelli DG, Edmonds-Brown R. Body Size: The Structure and Function of Aquatic Ecosystems. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 11.Zuo W, Moses ME, West GB, Hou C, Brown JH. A general model for effects of temperature on ectotherm ontogenetic growth and development. Proc Biol Sci. 2012;279(1734):1840–1846. doi: 10.1098/rspb.2011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson D, Morley SA, Hughes RN. From cells to colonies: At what levels of body organization does the ‘temperature-size rule’ apply? Evol Dev. 2006;8(2):202–214. doi: 10.1111/j.1525-142X.2006.00090.x. [DOI] [PubMed] [Google Scholar]
- 13.Pauly D. Gasping Fish and Panting Squids: Oxygen, Temperature and the Growth of Water Breathing Animals. Oldendorf/Luhe, Germany: International Ecology Institute; 2010. [Google Scholar]
- 14.Verberk WCEP, Bilton DT, Calosi P, Spicer JI. Oxygen supply in aquatic ectotherms: Partial pressure and solubility together explain biodiversity and size patterns. Ecology. 2011;92(8):1565–1572. doi: 10.1890/10-2369.1. [DOI] [PubMed] [Google Scholar]
- 15.Woods HA. Egg-mass size and cell size: Effects of temperature on oxygen distribution. Am Zool. 1999;39(2):244–252. [Google Scholar]
- 16.Schmidt-Nielsen K. Animal Physiology: Adaptation and Environment. 2nd Ed. Cambridge, UK: Cambridge Univ Press; 1979. [Google Scholar]
- 17.Vogel S. Comparative Biomechanics: Life’s Physical World. Princeton, NJ: Princeton Univ Press; 2003. [Google Scholar]
- 18.Verberk WCEP, Bilton DT. Can oxygen set thermal limits in an insect and drive gigantism? PLoS ONE. 2011;6(7):e22610. doi: 10.1371/journal.pone.0022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson RD. Body size and limits to the daily range of body temperature in terrestrial ectotherms. Am Nat. 1985;125(1):102–117. [Google Scholar]
- 20.Willott SJ, Hassall M. Life-history responses of British grasshoppers (Orthoptera: Acrididae) to temperature change. Funct Ecol. 1998;12(2):232–241. [Google Scholar]
- 21.Forster J, Hirst AG, Woodward G. Growth and development rates have different thermal responses. Am Nat. 2011;178(5):668–678. doi: 10.1086/662174. [DOI] [PubMed] [Google Scholar]
- 22.Forster J, Hirst AG. The temperature-size rule emerges from ontogenetic differences between growth and development rates. Funct Ecol. 2012;26(2):483–492. [Google Scholar]
- 23.Forster J, Hirst AG, Atkinson D. How do organisms change size with changing temperature? The importance of reproductive method and ontogenetic timing. Funct Ecol. 2011;25(5):1024–1031. [Google Scholar]
- 24.Angilletta MJ, Jr, Dunham AE. The temperature-size rule in ectotherms: Simple evolutionary explanations may not be general. Am Nat. 2003;162(3):332–342. doi: 10.1086/377187. [DOI] [PubMed] [Google Scholar]
- 25.Petersen C, Woods HA, Kingsolver JG. Stage-specific effects of temperature and dietary protein on growth and survival of Manduca sexta caterpillars. Physiol Zool. 2000;25(1):35–40. [Google Scholar]
- 26.Blanckenhorn WU, Demont M. Bergmann and converse Bergmann latitudinal clines in arthropods: Two ends of a continuum? Integr Comp Biol. 2004;44(6):413–424. doi: 10.1093/icb/44.6.413. [DOI] [PubMed] [Google Scholar]
- 27.Chown SL, Gaston KJ. Body size variation in insects: A macroecological perspective. Biol Rev Camb Philos Soc. 2010;85(1):139–169. doi: 10.1111/j.1469-185X.2009.00097.x. [DOI] [PubMed] [Google Scholar]
- 28.Makarieva AM, Gorshkov VG, Li B-L. Gigantism, temperature and metabolic rate in terrestrial poikilotherms. Proc Biol Sci. 2005;272(1578):2325–2328. doi: 10.1098/rspb.2005.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makarieva AM, Gorshkov VG, Li B-L. Temperature-associated upper limits to body size in terrestrial poikilotherms. Oikos. 2005;111(3):425–436. [Google Scholar]
- 30.Moran AL, Woods HA. Why might they be giants? Towards an understanding of polar gigantism. J Exp Biol. 2012;215(Pt 12):1995–2002. doi: 10.1242/jeb.067066. [DOI] [PubMed] [Google Scholar]
- 31.Hansen B, Bjornsen PK, Hansen PJ. The size ratio between planktonic predators and their prey. Limnol Oceanogr. 1994;39(2):395–403. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.