Abstract
The transsynaptic complex of neuroligin (NLGN) and neurexin forms a physical connection between pre- and postsynaptic neurons that occurs early in the course of new synapse assembly. Both neuroligin and neurexin have, indeed, been proposed to exhibit active, instructive roles in the formation of synapses. However, the process by which these instructive roles play out during synaptogenesis is not well understood. Here, we examine one aspect of postsynaptic neuroligin with regard to its synaptogenic properties: its basal state as a constitutive dimer. We show that dimerization is required for the synaptogenic properties of neuroligin and likely serves to induce presynaptic differentiation via a transsynaptic clustering of neurexin. Further, we introduce chemically inducible, exogenous dimerization domains to the neuroligin molecule, effectively bestowing chemical control of neuroligin dimerization. This allows us to identify the acute requirements of neuroligin dimerization by chemically manipulating the monomeric-to-dimeric conversion of neuroligin. Based on the results of the inducible dimerization experiments, we propose a model in which dimerized neuroligin induces the mechanical clustering of presynaptic molecules as part of a requisite step in the coordinated assembly of a chemical synapse.
Keywords: electrophysiology, hippocampus, development, synaptic adhesion molecule
The synapse is among the most complex of cellular structures, dense with highly organized protein interactions. Although our knowledge of this molecular matrix is expanding rapidly, the precise dynamics that govern the formation of a synapse, with its matched asymmetric sides, are still not fully understood. However, some particularly important interactions are beginning to emerge. For instance, the transsynaptic complex of presynaptic neurexin and postsynaptic neuroligin (NLGN) has been proposed to lie at the heart of an emerging synapse (1, 2).
Independent manipulations of either neuroligin or neurexin can result in modifications of both pre- and postsynaptic assembly, suggesting an instructive transsynaptic role for the neuroligin/neurexin complex in synaptic formation. Most strikingly, in experiments using a coculture system of neurons and nonneuronal cells, neuroligin expressed in nonneuronal cells is able to induce the formation of functional presynaptic terminals onto those cells from cocultured neurons (3), whereas neurexin expression in nonneuronal cells supports the formation of postsynaptic specializations at the junctions of those nonneuronal cells and cocultured neurons (4). Whether or not the neuroligin/neurexin complex is absolutely required for synapse formation is not clear, given that dissociated hippocampal and cortical cultures from triple-knockout mice lacking neuroligins 1, 2, and 3 display normal synapse density, although these mice do die at birth from respiratory failure as a consequence of reduced synaptic transmission in the brainstem (5). Whereas this finding certainly suggests that the neuroligin family is not essential for synaptic formation, it remains possible that compensation by another family of postsynaptic adhesion molecules such as leucine-rich repeat transmembrane (LRRTM) proteins (6–8) or the cerebellin/glutamate receptor delta complex (9) could be masking the effects of a germ-line knockout of the three major neuroligin subtypes.
Like the synapse, the neuroligin/neurexin complex is itself asymmetric. Neuroligin exists natively as a dimer, whereas neurexin, in an unbound state, is monomeric (10). The complex, then, is an asymmetric tetramer consisting of a neuroligin dimer and two neurexin molecules—the neurexin molecules brought in close proximity to each other via their interaction with the neuroligin dimer (10–12). As stated above, in vitro evidence suggests that neurexin clustering may be an early step in the differentiation of an axon segment into a presynaptic terminal (13). Such clustering could be achieved by the monomeric-to-dimeric conversion of neurexin upon neuroligin binding. Importantly, this clustering would be entirely dependent on the presence of neuroligin in a dimerized state.
We set out to define the functional requirement of neuroligin dimerization with an aim to specifically test the hypothesis that the clustering of neurexin via an interaction with dimerized neuroligin is required for presynaptic assembly. We find strong evidence to support this hypothesis and propose a model whereby postsynaptic neuroligin drives presynaptic differentiation via the clustering of neurexin, while also being required for some, but not all, aspects of postsynaptic assembly.
Results
Functional Neuroligin Requires an Intact Dimerization Domain.
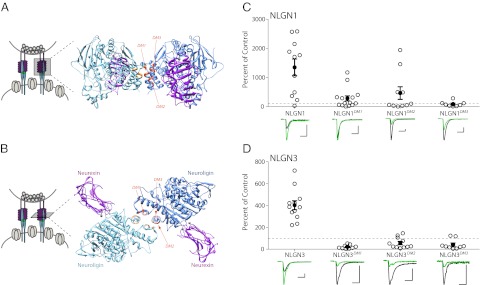
To directly test the physiological requirement for neuroligin dimerization, we constructed mutants of neuroligin that have been previously shown to eliminate dimerization (13, 14). We tested three separate mutants that each contain alanine substitutions at the interface of the neuroligin dimer (Fig. 1 A and B). These mutants have been shown to retain surface localization and neurexin binding and, indeed, have been shown in one case to result in increases in synapse density when expressed in neurons. However, the mutants also display deficits in function compared with wild-type neuroligin, including the lack of aggregation of neurexin-expressing cells in a nonneuronal cell-adhesion assay and the lack of an increase in synaptic cluster size when expressed in neurons (13, 14).
Fig. 1.
Mutations of neuroligin affecting dimerization abolish the synaptogenic effects of postsynaptic expression. (A) Structure of the neuroligin 1/neurexin 1β extracellular domains viewed side-on, looking through the synaptic cleft. (Left) Schematic (neuroligin in blue, neurexin in purple) indicates the viewing angle. (Right) Two neuroligin molecules, shown in dark and light blue, form a dimer with each neuroligin molecule bound to neurexin, shown in purple. Calcium ions at the neuroligin/neurexin interface are shown in gray. Locations of the dimerization-inhibiting mutations are indicated in orange. Structure from Araç et al. (10). (B) As in A, but viewed from the presynaptic side of the synapse, looking toward the postsynaptic side. (C) Postsynaptic expression of wild-type NLGN1 results in increased AMPAR-mediated EPSCs compared with control (P < 0.01, n = 11), whereas the expression of dimerization-null mutants do not (NLGN1DM1 P > 0.05, n = 15; NLGN1DM2 P > 0.05, n = 10; NLGN1DM3 P > 0.05, n = 8). Open circles represent individual pairs; closed circles indicate mean ± SEM. (D) Postsynaptic expression of wild-type NLGN3 also results in increased AMPAR-mediated EPSCs compared with control (P < 0.001, n = 12), whereas expression of dimerization-null mutants results in decreased AMPAR-mediated EPSCs (NLGN3DM1 P < 0.005, n = 9; NLGN3DM2 P < 0.05, n = 11; NLGN3DM3 P < 0.05, n = 9). As in C, open circles represent individual pairs; closed circles indicate mean ± SEM. Expression of wild-type NLGN1 and NLGN3, previously shown in Shipman et al. (25) are repeated here for clarity. Sample traces in C and D show individual paired recordings with control AMPAR-mediated currents in black and experimental in green (scale bar, 20 pA/20 ms.)
The postsynaptic expression of wild-type neuroligin results in a profound enhancement of excitatory synaptic currents (15, 16). Therefore, we evaluated the effect of expressing exogenous, dimerization-null mutants of neuroligin on this enhancement of postsynaptic currents. To do so we expressed wild-type or mutated neuroligin using sparse biolistic transfection of organotypic hippocampal slice cultures. This resulted in transfection of only one to a few hippocampal pyramidal cells per slice, allowing for the simultaneous recording of whole-cell currents from a transfected neuron and a neighboring control cell. Evoking action potentials in the Schaffer axon collaterals with an extracellular stimulating electrode resulted in simultaneous excitatory synaptic currents in both the experimental and control cell, the relative magnitude of which serves as a readout of the effect of the manipulation on synaptic strength (Fig. S1). We found that the introduction of dimerization-null mutations into NLGN1 eliminated the neuroligin-induced enhancement of synaptic currents seen with expression of wild-type NLGN1 (Fig. 1C). To test the generality of this requirement for dimerization in neuroligin function, we made homologous mutations in neuroligin 3 (NLGN3). These dimerization-null mutants of NLGN3 also completely lacked the synapse promoting effects of wild-type NLGN3, with their expression instead resulting in a depression of synaptic strength (Fig. 1D). We conclude that dimerization is essential for the normal physiological function of neuroligin.
Although the original characterizations of dimerization-null mutants of NLGN1 reported preserved surface expression (13, 14), a more recent study found evidence for endoplasmic reticulum retention of dimerization-null mutants (17). To assess whether a trafficking deficit could explain our findings, we examined surface expression of wild-type and dimerization-null mutants of both NLGN1 and NLGN3 in neurons by staining for the presence of an HA-tag in the extracellular domain of the proteins under nonpermeabilizing conditions. In all cases, we found evidence for surface expression of the protein (Fig. S2 A and B), indicating that the dimerization-null mutants are competent for trafficking.
Nonetheless, it is possible that there exists a trafficking deficit in the mutants that is simply overwhelmed by the relatively long duration or high degree of our overexpression but that still influences our findings. In the same study that found intracellular retention of the dimerization-null mutants, a single amino acid substitution in the transmembrane domain was shown to mitigate this retention (17). To directly test whether a subtle trafficking deficit may be contributing to the synaptic phenotype that we observe, we introduced this single amino acid substitution (N702L) into wild-type and dimerization-null NLGN3, which we expressed in individual neurons in slice culture to assess the effect on synaptic currents. We found that this N702L substitution had no effect on the synaptic phenotype of either wild-type or dimerization-null mutants of neuroligin—wild-type neuroligin still enhanced currents and two different dimerization-null mutants still depressed currents (Fig. S2C). Based on the combination of surface staining and the lack of any effect of this mutation, we conclude that the synaptic phenotype of the dimerization-null neuroligin is likely due to an action at the cell surface rather than a trafficking effect.
Dimerization of Neuroligin Is Required for Its Transsynaptic Effects.
Neuroligin has been implicated in the formation (18), validation (14), and maintenance (19) of synapses. The overexpression of neuroligin in particular has been shown to result in an increase in the number of synapses as evidenced by an increase in the frequency of miniature excitatory postsynaptic currents (20, 21), an increase in the density of spines (18, 22), and an increase in both pre- and postsynaptic markers by immunostaining (18, 21). We sought to determine which aspects of neuroligin-induced synaptogenesis are dependent on dimerization. For the remainder of the experiments we chose to use NLGN3, owing to the more severe effects of dimerization-null mutations and less variable effects of overexpression. We used just one dimerization-null mutant for consistency (NLGN3DM2 in Fig. 1, hereafter referred to as NLGN3D-N), although we saw no differences between different dimerization mutants on any test of function. Based on the evidence that dimerization-null mutants of neuroligin can still induce spinogenesis (14), but that clustering of neurexin has been shown to induce presynaptic differentiation (13), we hypothesized that there may be a differential requirement for neuroligin dimerization with regard to post- and transsynaptic effects. Indeed, we were able to replicate the previous finding that expression of a dimerization-null mutant of neuroligin can increase the density of spines (Fig. 2A), even though we showed that this same mutant does not retain the ability to enhance synaptic currents. To further investigate the synaptic deficit of the dimerization-null mutant, we moved to dissociated hippocampal cultures to allow for immunostaining of the pre- and postsynaptic components of a synapse.
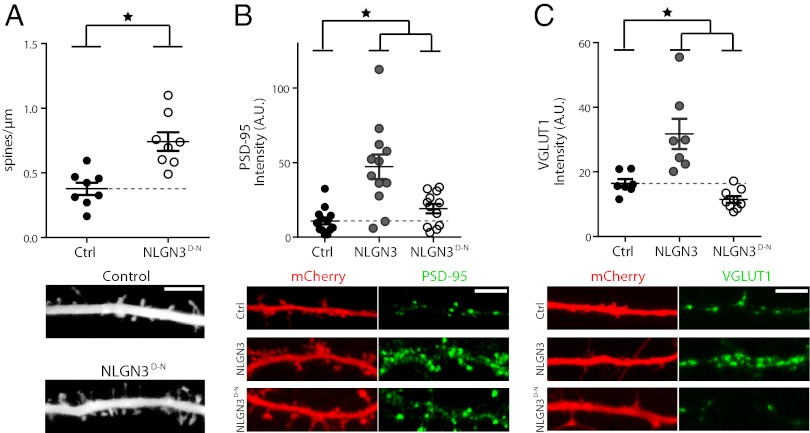
Fig. 2.
Dimerization-null neuroligin mutant retains the ability to enhance the postsynaptic site in the absence of presynaptic enhancements. (A) Postsynaptic expression of NLGN3D-N results in an increased spine density along the apical dendrite of CA1 pyramidal neurons in organotypic hippocampal cultures compared with control neurons [P < 0.0001, n = 8 control (ctrl), n = 8 experimental). Circles represent individual cells; horizontal bars indicate mean ± SEM. Sample images show individual sections of dendrite (Scale bar, 5 µm.) (B) Postsynaptic expression of either wild-type or dimerization-null NLGN3 increases PSD-95 immunofluorescence intensity in dissociated hippocampal neurons (NLGN3 P < 0.001, n = 12; NLGN3D-N P < 0.05, n = 12; ctrl n = 13). Circles represent individual cells; horizontal bars indicate mean ± SEM. In sample images, dendrites are shown using mCherry, red (Left), whereas PSD-95 staining is in green (Right). (Scale bar, 5 µm.) (C) Postsynaptic expression of wild-type NLGN3 increases VGLUT1 staining onto the postsynaptic cell (P < 0.005, n = 7; ctrl n = 7), whereas expression of NLGN3D-N decreases VGLUT1 staining onto the postsynaptic cell (P < 0.05, n = 9). Circles represent individual cells; horizontal bars indicate mean ± SEM. In sample images, dendrites are shown using mCherry, red (Left), whereas VGLUT1 staining is in green (Right). (Scale bar, 5 µm.)
Neuroligin has been shown to increase immunostaining for the postsynaptic scaffolding molecule PSD-95 (18), consistent with its enhancement of postsynaptic currents and spine density. We confirmed this enhancement of PSD-95 upon the expression of wild-type NLGN3 (Fig. 2B) and additionally found an increase in PSD-95 staining following expression of the dimerization mutant (Fig. 2B)—again, not surprisingly given our prior finding of an increase in spine density. However, the relative magnitude of PSD-95 enhancement by wild-type and dimerization-null NLGN3 was different, with wild-type inducing a significantly larger postsynaptic effect. This could indicate a postsynaptic impairment in the dimerization-null mutant, or that this postsynaptic effect is secondary to the transsynaptic effects presented below.
Typically, postsynaptic expression of neuroligin results in an increase in the presynaptic marker vesicular glutamate transporter 1 (VGLUT1) (18, 21) via a transsynaptic interaction with presynaptic neurexin. We could clearly show this presynaptic enhancement of VGLUT1 following the overexpression of wild-type NLGN3, but not with the dimerization-null mutant of NLGN3 (Fig. 2C). Rather, expression of the dimerization-null neuroligin actually reduced the amount of VGLUT1. Thus, the transsynaptic effects of neuroligin would seem to depend on intact dimerization. A closer examination of the PSD-95 immunostaining reveals an increase in PSD-95 puncta density following expression of dimerization-null NLGN3 with no significant change in puncta size or intensity (Fig. S3 A–D), whereas the VGLUT1 immunostaining shows a similar, but opposite effect. That is, a reduction in VGLUT1 puncta density with no change in puncta size or intensity (Fig. S3 E–H). Although not entirely conclusive, this finding is consistent with all-or-none effects on both pre- and postsynaptic assembly, rather than modifications of synaptic strength. It would appear, then, that the ability of neuroligin to induce the assembly of postsynaptic components of a synapse remains largely intact in the absence of dimerization, whereas its ability to induce the clustering of the functional presynaptic components of a synapse is compromised. Further, the dominant-negative effect of the NLGN3 dimerization mutants on synaptic currents must have a primarily presynaptic locus of origin.
Rigorous Test of Neuroligin Dimerization-Driven Synaptic Assembly.
Given the remarkable dependence of neuroligin on dimerization for its transsynaptic effects, we wondered how exactly the mutations that we introduced were exerting their effects on the protein. One possibility may be that there are protein interaction sites near the dimerization domain that mediate interactions other than dimerization, which could have been inadvertently affected by our dimerization mutations, or that there exist specific protein interaction sites that occur only in an uncompromised native neuroligin dimer. An alternative explanation would be that simple mechanical clustering of the interacting partners of neuroligin is the driving force behind neuroligin-induced synaptogenesis, and that such clustering is dependent on the presence of dimerized neuroligin.
We decided to test the mechanical clustering hypothesis by introducing chemically inducible dimerization domains into dimerization-null mutants of NLGN3. In this way, we can artificially cluster the monomeric NLGN3 mutants acutely. If this chemically induced dimerization is able to rescue the function of the dimerization-null mutants, we can conclude that there is a simple mechanical clustering requirement for neuroligin-induced synaptogenesis, whereas, conversely, a lack of rescue upon chemical-induced dimerization would suggest that the effect of these dimerization mutations is more complex. We used a chemically inducible dimerization domain initially based on FKBP12 (FK506-binding protein 12) and its small molecule ligand FK506 (23), modified to induce dimerization in the absence of endogenous interactions (24). We tested inclusion of the inducible dimerization domain in numerous positions along the length of neuroligin, including several near the original dimerization domain and several in more distant regions of the protein.
In a wild-type NLGN3, only inclusion of the chemically inducible dimerization domain at the extreme intracellular C terminus of neuroligin resulted in a functional protein, as evidenced by retention of the synaptogenic overexpression phenotype. Insertion of the dimerization domain in membrane-proximal regions of the intracellular tail or in any position in the extracellular domain resulted in elimination of endogenous function of the protein. We have previously shown that the extreme end of the cytoplasmic tail is not required for neuroligin function, explaining the tolerance to the inclusion of this artificial domain at that position (25). However, as the C terminus is quite distant from the endogenous dimerization domain and, indeed, is in the intracellular rather than extracellular compartment, we believe that this represents a rigorous test of the clustering hypothesis. Thus, the aim of this experiment was to express a monomeric NLGN3 by mutating the endogenous dimerization domain and then chemically induce dimerization in these expressed proteins through an artificial dimerization domain to test the synaptogenic effects of neuroligin clustering (Fig. 3A).
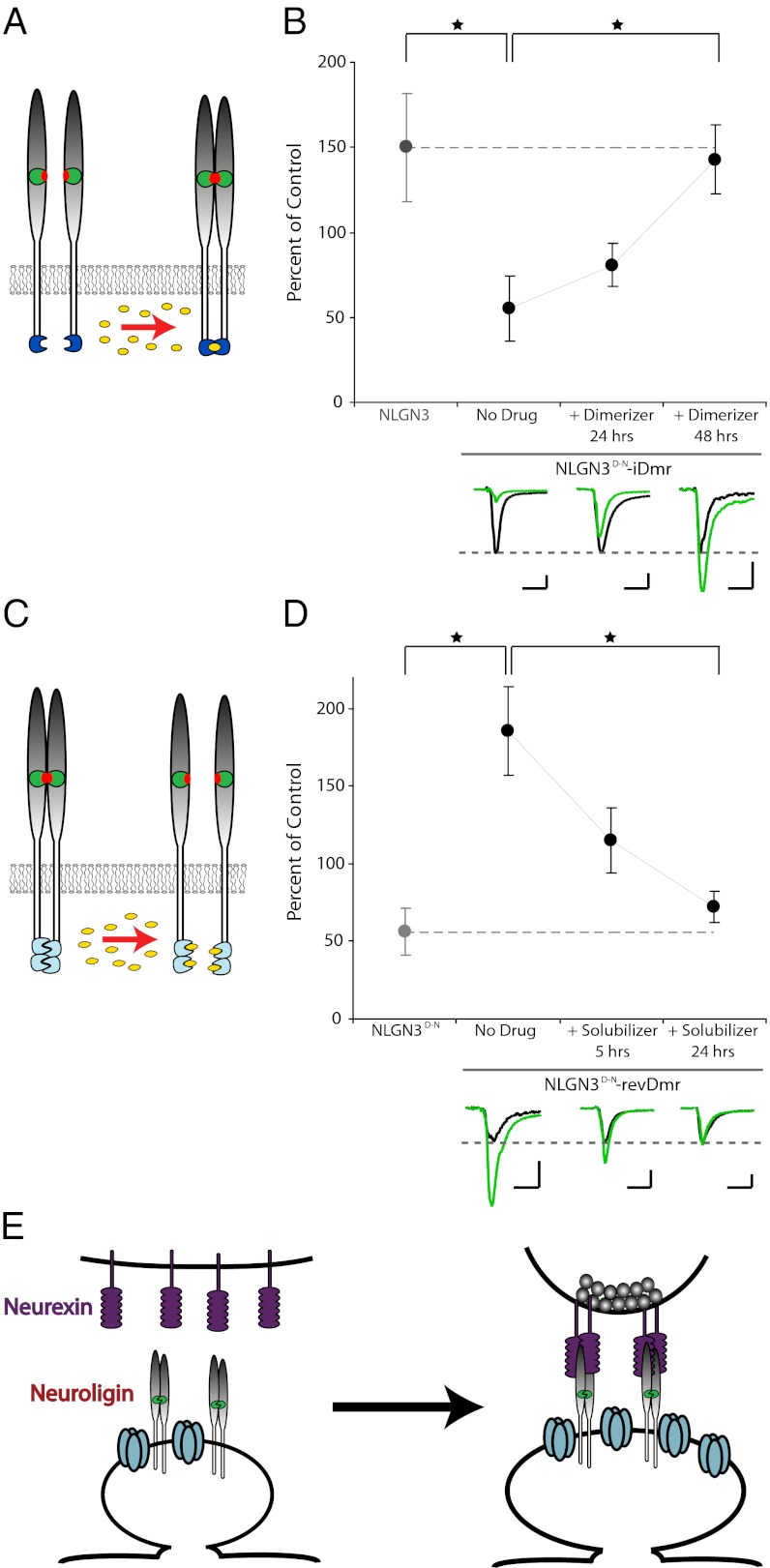
Fig. 3.
Chemically induced dimerization and monomerization can acutely alter the synaptic phenotype of neuroligin expression. (A) Schematic of induced-dimerization experiments. Neuroligin monomers are acutely dimerized via an exogenous, inducible dimerization domain (blue) in the presence of the small molecule B/B homodimerizer (yellow). (B) Acute dimerization rescues the synaptogenic phenotype of the dimerization-null neuroligin mutant. In the absence of the homodimerizing compound, expression of the NLGN3D-N-iDmr construct (n = 9) has an inhibitory effect on evoked AMPAR-mediated EPSCs compared with wild-type NLGN3 expression (n = 12, P < 0.005). 48 h of exposure to the B/B Homodimerizer (n = 10) results in an increase in the AMPAR-mediated current amplitudes compared with the no drug condition (P < 0.005), rescuing the phenotype to a wild-type level (48 h condition versus wild-type expression, P > 0.05; 24 h homodimerizer n = 10). Closed circles indicate mean AMPAR-mediated current amplitudes expressed as percent of control ± SEM. Example traces illustrate individual pairs, with control cells shown in black and experimental cells shown in green. (Scale bar, 20 pA/20 ms.) (C) Schematic of the induced-monomerization experiments. Dimerization-null mutant neuroligin is artificially dimerized via an exogenous domain (light blue), which, in the presence of the small molecule D/D solubilizer (yellow), is converted to a monomeric state. (D) Artificial dimerization via the exogenous dimerization domain rescues basal synaptogenic properties of neuroligin as evidenced by an increase in AMPAR-mediated synaptic currents upon expression of NLGN3D-N–revDmr (n = 12) compared with NLGN3D-N (n = 10) in the absence of drug (P < 0.005). NLGN3D-N expression, originally shown in Fig. 1D, is repeated here for clarity. Monomerization of NLGN3D-N–revDmr by the addition of the D/D solubilizer for 24 h (n = 10) results in elimination of the synaptogenic phenotype compared with the no-drug condition (P < 0.005), returning the synaptic phenotype to the dimerization-null condition (24-h condition versus NLGN3D-N expression, P > 0.05; 5 h solubilizer n = 8). Graphs and sample traces as in B. (Scale bar, 20 pA/20 ms.) (E) Model for neuroligin-induced synaptogenesis whereby postsynaptic, dimerizered neuroligin binds to and clusters presynaptic neurexin, leading to the differentiation of a presynaptic terminal.
We found that, as expected, mutation of the endogenous dimerization domain in a NLGN3 construct containing the artificial inducible dimerization domain (NLGN3D-N–iDmr) resulted in decreased excitatory synaptic currents rather than the increased currents that are evident upon expression of wild-type NLGN3 (Fig. 3B). We then exposed slices containing cells that expressed the NLGN3D-N–iDmr construct to the dimerizing compound. We found that, over the course of 48 h, the synaptogenic phenotype of neuroligin expression in the dimerization-null mutant was recovered by artificial dimerization. Specifically, in cells expressing the NLGN3D-N–iDmr construct, the amplitude of evoked excitatory synaptic currents compared with those currents in neighboring control cells was enhanced by the addition of the dimerizing compound, such that after 48 h the phenotype was indistinguishable from wild-type expression (Fig. 3B). Given that this inducible, artificial dimerization does not recapitulate the endogenous site, but rather induces dimerization via an independent domain, we conclude that dimerization of neuroligin is required for its synaptogenic phenotype due to a simple clustering mechanism. We did not see evidence for further enhancement with longer exposure to the dimerizing compound.
Given the ability of chemically induced dimerization to rescue function, we next tested whether chemically induced monomerization would acutely recapitulate the phenotype of expressing a monomeric neuroligin. This experiment was designed to distinguish between two alternative possibilities: first, that monomeric neuroligin is an ineffective synaptogenic molecule; or second, that the presence of monomeric neuroligin acutely inhibits synaptic function. To do this we used a strategy similar to that of the chemically induced dimerization. Using a dimerization-null NLGN3 as our starting point, we added a domain to the extreme C terminus, which natively forms a dimer but is converted into a monomer in the presence of a solubilizing compound, based on the same principles as the inducible dimerization domain (Fig. 3C). Similar to a wild-type neuroligin, expression of this artificially dimerized neuroligin (NLGN3D-N–revDmr, for reverse dimerization) resulted in enhanced excitatory synaptic currents, in stark contrast to the dimerization-null mutant (Fig. 3D)—once again confirming that the requirement for neuroligin dimerization is simple clustering rather than a special property of the endogenous dimerization domain. Furthermore, incubation of cells expressing the NLGN3D-N–revDmr construct in the solubilizing compound for 24 h resulted in an elimination of the enhancement of synaptic currents compared with the currents in neighboring control cells (Fig. 3D). Given the timeframe and magnitude of reduction in synaptic currents in the presence of the solubilizer, we believe that this result is consistent with the model that monomeric neuroligin actively inhibits the occurrence of fully functional synapses and suggests a lasting role for the neuroligin/neurexin complex at existing synapses.
Discussion
We set out to test the specific requirements of neuroligin dimerization for the formation and maintenance of synapses. Our results indicate that dimerization is required for the synaptogenic effects of neuroligin, but that this requirement is primarily due to a transsynaptic effect on the presynaptic site. Moreover, we find that the synaptogenic effects of the neuroligin dimer are achieved via a simple clustering mechanism. Thus, we put forward a model whereby postsynaptic neuroligin, in its native dimerized state, binds previously monomeric, axonal neurexin and induces differentiation into a functional presynaptic site (Fig. 3E). These conclusions largely confirm the hypothesis originally presented by Dean et al. (13) with respect to the clustering of neurexin.
We cannot state with absolute certainty whether the postsynaptic function of dimerization-null neuroligin is entirely unaffected. Although we saw evidence for increases in PSD-95 staining and spine density upon expression of the dimerization-null neuroligin, indicating retention of at least some postsynaptic function, the enhancement of PSD-95 staining by the dimerization-null mutant was of a smaller magnitude than was seen with expression of wild-type neuroligin. It is, therefore, possible that monomeric neuroligin is less effective at inducing the assembly of the postsynaptic components of a synapse. Alternatively, it could be that the postsynaptic sites induced by the dimerization-null neuroligin are less mature or are functionally stunted due to the lack of an opposing glutamate release site.
It should be noted that, although our interpretation of the results of this study has contrasted the function of monomeric versus dimeric neuroligin, we cannot rule out the formation of higher-order assemblies occurring downstream of neuroligin dimerization—through weaker dimer–dimer interactions or through intermediate proteins. Dean et al. (13) found recruitment of synaptic vesicles following the clustering of neurexin with multimerized, but not dimerized, antibody, suggesting that higher-order complexes of neuroligin might be necessary to exert a transsynaptic effect. Moreover, there is structural evidence in support of lateral neuroligin/neurexin sheets at the synapse (26). Acute dimerization of our expressed constructs via the chemically inducible dimerization could, therefore, be permissive for the assembly of higher-order complexes, which may be required for the synaptic effects that we observe.
It would seem, from the bulk of our results, that the presence of monomeric neuroligin actively excludes the presence of a functional presynaptic terminal through a transsynaptic action. That is, a diffuse arrangement of neurexin when bound to monomeric neuroligin—as opposed to the aggregated arrangement that normally occurs when bound to dimerized neuroligin—can inhibit the formation of presynaptic terminals. This may be a fundamental principle underlying neuroligin physiology with ramifications even outside the central nervous system; in fact, a recent study found that clustered neuroligin stimulates transcellular insulin release from pancreatic β cells (acting through an unknown partner other than neurexin), whereas diffuse neuroligin does not (27). Within the CNS, neuroligins are only one class of what is an emerging superfamily of postsynaptic neurexin ligands, including LRRTMs (6–8), cerebellins (9), and the G- protein–coupled receptor latrophilin-1 (28), which, together with neurexin, are situated within an even larger class of synaptic adhesion complexes, including those formed by cadherins (29), the synaptic cell adhesion molecule (SynCAM) family (30), netrin-G ligand-3 and receptor protein tyrosine phosphatases (31, 32), and teneurins (33). Given the apparent mechanistic requirement of neurexin clustering for neuroligin-induced synaptogenesis, it will be of considerable interest to explore the extent to which this is a shared function among neurexin ligands and synaptic adhesion complexes in general.
Materials and Methods
Details on experimental constructs can be found in SI Materials and Methods.
Slice Culture Preparation and Biolistic Transfection.
Hippocampal organotypic slice cultures were prepared from 6- to 8-d-old rats as previously described (34). All experiments were performed in accordance with University of California San Francisco Guidelines for Animal Use. Transfections were carried out 24 h after culturing using a Helios Gene Gun (Bio-Rad) with 1 µm DNA-coated gold particles. Slices were maintained at 34 °C at 5% CO2 with media changes every other day.
Dissociated Culture Preparation Transfection.
Dissociated cultures were prepared from E18–E19 rats. Hippocampi were surgically isolated, then dissociated by 0.25% trypsin followed by gentle mechanical trituration. Cells were plated on poly-d-lysine–treated glass coverslips at an approximate density of 20,000 cells per centimeter squared and maintained in neurobasal-based media (Invitrogen) supplemented with B27, penicillin–streptomycin, and l-glutamine at 37 °C at 5% CO2. Transfections were achieved using Lipofectamine 2000 (Invitrogen). Cells were transfected at 10 days in vitro (DIV) and analyzed 3–4 d later.
Electrophysiology.
Electrophysiological recordings were carried out on an upright Olympus BX51WI microscope using a Multiclamp 700B amplifier (Molecular Devices). Transfected and control neurons were recorded simultaneously on DIV 7–9 at 20–25 °C using glass patch electrodes filled with an internal solution containing 120 mM CsMeSO3, 20 mM CsCl, 10 mM Hepes, 4 mM NaCl, 0.5 mM EGTA, 0.3 mM CaCl2, 4 mM Mg-ATP, 0.3 mM Na-GTP, and 5 mM QX-314. During recording, slices were maintained in an artificial cerebral spinal fluid (ACSF) consisting of 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 11 mM glucose, 4 mM MgSO4, and 4 mM CaCl2 saturated with 95% O2 at 5% CO2. CA1 pyramidal neurons were identified visually, whereas transfected neurons were identified using fluorescence. Excitatory AMPA receptor (AMPAR)-mediated synaptic currents were isolated at a holding potential of −70 mV by the addition of 100 µM picrotoxin and 10 µM gabazine to block inhibition. A total of 50 nM NBQX [2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo(f)quinoxaline-7-sulfonamide] was added to reduce the occurrence of epileptiform activity. For the chemically induced dimerization/monomerization experiments, the small molecules B/B homodimerizer (AP20187) or D/D solubilizer (Clontech) were added to slice media at 100 nM for the specified duriation before recording. All recordings were analyzed using customized software (Igor). Statistical significance of paired whole-cell recordings was determined by a Wilcoxon signed-rank test, whereas comparisons between sets of paired data were performed using a Mann–Whitney u test of the ratios of experimental-to-control amplitudes.
Immunocytochemistry.
Cells were fixed with 4% (wt/vol) paraformaldehyde in PBS additionally containing 4% (wt/vol) sucrose. Cells were permeabilized and nonspecific binding sites were blocked using 10% donkey serum or 3% (vol/vol) BSA with 0.1% Triton X-100 in PBS. For surface staining, Triton X-100 was omitted from all solutions. Primary and secondary antibody incubations were carried out for 1 h (room temperature) or 18 h (4 °C) in blocking solution. Primary antibodies used in this study were guinea pig anti-VGLUT1 (AG208; Millipore) at a dilution of 1:5,000, mouse anti–PSD-95 (K28/43; NeuroMab) at a dilution of 1:650, and mouse anti-HA (16B12; Life Technologies) at a dilution of 1:100. Secondary antibodies used were donkey antiguinea pig conjugated to DyLight 488 (Jackson ImmunoResearch) and goat antimouse conjugated to Alexa Fluor 488 (Invitrogen).
Image Acquisition and Quantification.
Spine images of biolisitically transfected neurons in live hippocampal organotypic cultures were acquired on DIV 7–9 using an upright confocal microscope (Zeiss; LSM5 Pascal) from cells expressing either GFP alone (control condition) or GFP plus NLGND-N. Slices were submerged in Hepes-buffered ACSF containing 140 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM Hepes, and 10 mM glucose. Neurons were imaged along their primary apical dendrite for a length of 100 µm, starting 100 µm from the cell body. Statistical comparisons were performed using a Mann–Whitney u test. Images of immunostained synaptic proteins in dissociated culture were acquired on an inverted confocal microscope (Zeiss; LSM510 Meta). All images in a series were collected with identical settings for laser power, pinhole diameter, detector gain, amplifier gain, and amplifier offset. Images were analyzed using ImageJ using maximum intensity z projections. Three 50-µm sections of dendrite were analyzed for each cell. For the puncta analysis, an identical threshold was applied to all images in a series and a mask was created from which all puncta greater than 0.15 µm2 were selected and analyzed for punta density and size. This mask was applied to the original image to collect puncta intensity. Statistical comparisons were performed using a Mann–Whitney u test.
Supplementary Material
Acknowledgments
We are grateful to K. Bjorgan for technical assistance. This work was supported by grants from the National Institute of Mental Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217633109/-/DCSupplemental.
References
- 1.Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455(7215):903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17(1):43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu Z, Washbourne P, Ortinski P, Vicini S. Functional excitatory synapses in HEK293 cells expressing neuroligin and glutamate receptors. J Neurophysiol. 2003;90(6):3950–3957. doi: 10.1152/jn.00647.2003. [DOI] [PubMed] [Google Scholar]
- 4.Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119(7):1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varoqueaux F, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51(6):741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Ko J, Fuccillo MV, Malenka RC, Südhof TC. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 2009;64(6):791–798. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linhoff MW, et al. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61(5):734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Wit J, et al. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64(6):799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uemura T, et al. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141(6):1068–1079. doi: 10.1016/j.cell.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 10.Araç D, et al. Structures of neuroligin-1 and the neuroligin-1/neurexin-1 beta complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron. 2007;56(6):992–1003. doi: 10.1016/j.neuron.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Fabrichny IP, et al. Structural analysis of the synaptic protein neuroligin and its beta-neurexin complex: Determinants for folding and cell adhesion. Neuron. 2007;56(6):979–991. doi: 10.1016/j.neuron.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comoletti D, et al. Synaptic arrangement of the neuroligin/beta-neurexin complex revealed by X-ray and neutron scattering. Structure. 2007;15(6):693–705. doi: 10.1016/j.str.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean C, et al. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6(7):708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko J, et al. Neuroligin-1 performs neurexin-dependent and neurexin-independent functions in synapse validation. EMBO J. 2009;28(20):3244–3255. doi: 10.1038/emboj.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chubykin AA, et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54(6):919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Futai K, et al. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10(2):186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poulopoulos A, et al. Homodimerization and isoform-specific heterodimerization of neuroligins. Biochem J. 2012;446(2):321–330. doi: 10.1042/BJ20120808. [DOI] [PubMed] [Google Scholar]
- 18.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307(5713):1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, et al. Neuroligin-1 is required for normal expression of LTP and associative fear memory in the amygdala of adult animals. Proc Natl Acad Sci USA. 2008;105(26):9087–9092. doi: 10.1073/pnas.0803448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci USA. 2004;101(38):13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levinson JN, et al. Neuroligins mediate excitatory and inhibitory synapse formation: Involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem. 2005;280(17):17312–17319. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- 22.Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48(2):229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Spencer DM, Wandless TJ, Schreiber SL, Crabtree GR. Controlling signal transduction with synthetic ligands. Science. 1993;262(5136):1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- 24.Clackson T, et al. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc Natl Acad Sci USA. 1998;95(18):10437–10442. doi: 10.1073/pnas.95.18.10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shipman SL, et al. Functional dependence of neuroligin on a new non-PDZ intracellular domain. Nat Neurosci. 2011;14(6):718–726. doi: 10.1038/nn.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka H, et al. Higher-order architecture of cell adhesion mediated by polymorphic synaptic adhesion molecules neurexin and neuroligin. Cell Rep. 2012;2(1):101–110. doi: 10.1016/j.celrep.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Suckow AT, et al. Transcellular neuroligin-2 interactions enhance insulin secretion and are integral to pancreatic β cell function. J Biol Chem. 2012;287(24):19816–19826. doi: 10.1074/jbc.M111.280537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boucard AA, Ko J, Südhof TC. High affinity neurexin binding to cell adhesion G-protein-coupled receptor CIRL1/latrophilin-1 produces an intercellular adhesion complex. J Biol Chem. 2012;287(12):9399–9413. doi: 10.1074/jbc.M111.318659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arikkath J, Reichardt LF. Cadherins and catenins at synapses: Roles in synaptogenesis and synaptic plasticity. Trends Neurosci. 2008;31(9):487–494. doi: 10.1016/j.tins.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biederer T, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297(5586):1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 31.Kwon SK, Woo J, Kim SY, Kim H, Kim E. Trans-synaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatases LAR, protein-tyrosine phosphatase delta (PTPdelta), and PTPsigma via specific domains regulate excitatory synapse formation. J Biol Chem. 2010;285(18):13966–13978. doi: 10.1074/jbc.M109.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo J, et al. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci. 2009;12(4):428–437. doi: 10.1038/nn.2279. [DOI] [PubMed] [Google Scholar]
- 33.Mosca TJ, Hong W, Dani VS, Favaloro V, Luo L. Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature. 2012;484(7393):237–241. doi: 10.1038/nature10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37(2):173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.