Abstract
The BAG6 complex was first identified as an upstream loading factor for tail-anchored membrane proteins entering the TRC40-dependent pathway for posttranslational delivery to the endoplasmic reticulum. Subsequently, BAG6 was shown to enhance the proteasomal degradation of mislocalized proteins by selectively promoting their ubiquitination. We now show that the BAG6-dependent ubiquitination of mislocalized proteins is completely reversible and identify a pivotal role for the small glutamine-rich tetratricopeptide repeat-containing protein α (SGTA) in specifically antagonizing this process. SGTA does not simply mask the exposed hydrophobic transmembrane domain of a mislocalized protein, thereby preventing BAG6 recruitment. Rather, SGTA actively promotes the deubiquitination of mislocalized proteins that are already covalently modified, thus reversing the actions of BAG6 and inhibiting its capacity to promote substrate-specific degradation. This SGTA-mediated effect is independent of its tetratricopeptide motifs, suggesting it does not require the actions of Hsp70 and Hsp90 chaperones. These data reveal that, in a cellular context, mislocalized protein ubiquitination is the result of a dynamic equilibrium reflecting competition between pathways that promote either protein maturation or degradation. The targeted perturbation of this equilibrium, achieved by increasing steady-state SGTA levels, results in a specific stabilization of a model mislocalized protein derived from the amyloid precursor protein, an effect that is completely negated by ensuring efficient precursor delivery to the endoplasmic reticulum. We speculate that a BAG6/SGTA cycle operates during protein maturation and quality control in the cytosol and that together these components dictate the fate of a specific subset of newly synthesized proteins.
Keywords: membrane protein targeting, protein homeostasis
Protein targeting to the endoplasmic reticulum can be either co- or posttranslational and involves the binding of a hydrophobic signal sequence by delivery factors such as signal recognition particle or components of the TRC/GET pathway, respectively (1). Inefficient recognition and/or delivery of precursor proteins destined for the endoplasmic reticulum can lead to their cytosolic accumulation, resulting in toxicity such as that observed in neurodegenerative disorders such as prionopathies (2, 3). The disposal of such mislocalized proteins in mammalian cells has recently been shown to depend on the BAG6 complex comprised of the BAG6 protein together with TRC35 and UBL4A. The BAG6 complex recognizes mislocalized proteins, recruits the E2 conjugating enzyme, UbcH5, and an unidentified E3 ligase(s), and thereby selectively promotes the rapid ubiquitination and proteasomal degradation of these substrates (4). This role for the BAG6 complex in cytosolic quality control extends other studies showing that the BAG6 complex plays a comparable role during the ubiquitination of substrates destined for endoplasmic reticulum-associated degradation (5, 6) and the efficient removal of polypeptides resulting from defective ribosomal protein synthesis (7).
Before the identification of a role in the quality control of selected polypeptide substrates located in the cytosol (8), BAG6 was also shown to facilitate the posttranslational integration of so-called tail-anchored membrane proteins into the endoplasmic reticulum (9, 10). In this case, the BAG6 complex functions as an upstream loading factor that binds the hydrophobic transmembrane spanning regions of newly synthesized tail-anchored proteins shortly after their synthesis (11). Tail-anchored protein substrates are subsequently handed on to a second component, TRC40, which mediates their ATP-dependent delivery to the endoplasmic reticulum membrane (1, 11, 12). A similar pathway for tail-anchored protein biogenesis is found in Saccharomyces cerevisiae, where the TRC40 homolog Get3 mediates delivery to the endoplasmic reticulum (1, 12). An upstream loading complex is also present, although in this case it is composed of Get4 (equivalent to TRC35), Get5 (equivalent to UBL4A), and small glutamine-rich tetratricopeptide repeat-containing protein 2 (Sgt2), but with no obvious equivalent of the BAG6 protein involved (1, 12, 13). Strikingly, a mammalian ortholog of Sgt2, SGTA (small glutamine-rich tetratricopeptide repeat-containing protein α), is a known interacting partner of BAG6 (14) that has been shown to associate with both tail-anchored proteins and a range of other hydrophobic polypeptides (9, 15–17).
Despite clear evidence that SGTA has the capacity to both associate with BAG6 and bind a potentially overlapping range of substrates, its potential role during the cytosolic quality control of mislocalized proteins has not been investigated. To address this issue, we have exploited an in vitro system that recapitulates BAG6-dependent ubiquitination of mislocalized proteins to show that SGTA specifically antagonizes the actions of the BAG6 complex, thereby reducing mislocalized protein ubiquitination. SGTA does not function by simply masking the hydrophobic BAG6 recognition motif, but rather actively facilitates the deubiquitination of substrates that have already been previously modified. Our data show that mislocalized proteins could undergo multiple cycles of ubiquitination and deubiquitination before their ultimate fate is decided, and we propose that a BAG6/SGTA cycle operates to enforce protein quality control in the cytosol.
Results and Discussion
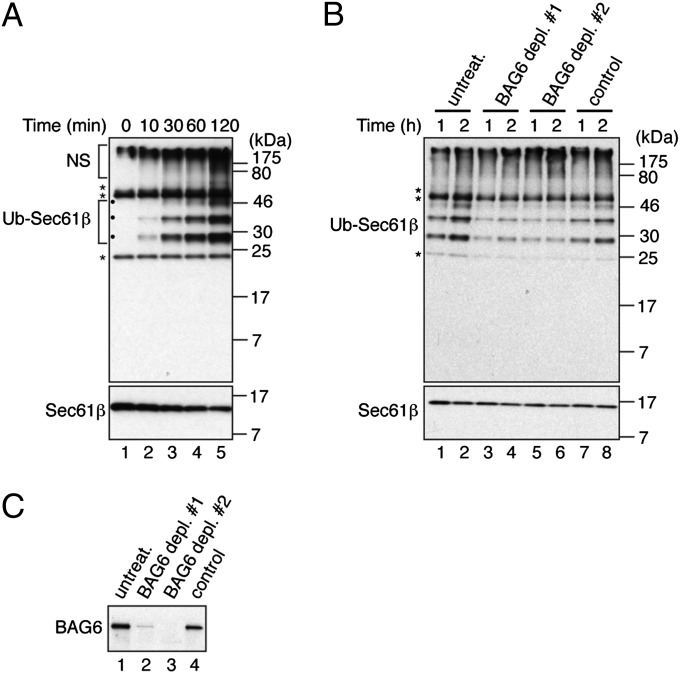
Using the purified recombinant tail-anchored protein Sec61β, we recapitulated the previously reported ubiquitination of mislocalized proteins (4), by adding rabbit reticulocyte lysate supplemented with FLAG-tagged ubiquitin. A time-course analysis showed a steady increase in Sec61β-specific ubiquitination (Fig. 1A, Ub-Sec61β species) quite distinct from any endogenous polyubiquitinated species that arise (Fig. S1). Furthermore, the appearance of these products correlates well with a decrease in the level of unmodified precursor (Fig. 1A, Sec61β). Notably, steady-state levels of Ub-Sec61β were significantly reduced when reticulocyte lysate was depleted of BAG6 (Fig. 1 B and C). We conclude that the lysate-dependent in vitro modification of recombinant Sec61β is greatly facilitated by the BAG6 complex (ref. 4).
Fig. 1.
Cytosolic ubiquitination of Sec61β is BAG6-dependent. (A) Purified recombinant Sec61β (2 μM) was mixed with rabbit reticulocyte lysate supplemented with 40 μM FLAG-tagged ubiquitin and incubated at 30 °C. At indicated times samples were treated with 5 mM N-ethylmaleimide (NEM) followed by immunoprecipitation of Sec61β via an opsin-derived epitope tag. Ubiquitinated and unmodified species were visualized by Western blotting with anti-FLAG and anti-Sec61β antibodies, respectively. (B) Similar reactions were performed using untreated, control-treated, and BAG6-depleted lysates, and the efficiency of BAG6 depletion was confirmed by Western blotting (C). ●, Specific ubiquitinated Sec61β species (Fig. S1). Anti-FLAG–reactive, high-molecular-weight species bound nonspecifically to Protein A Sepharose (NS) and cross-reacting species corresponding to heavy (**) and light (*) chains of antibodies used for immunoisolation are identified (Fig. S1).
SGTA Antagonizes BAG6-Mediated Ubiquitination of Mislocalized Proteins.
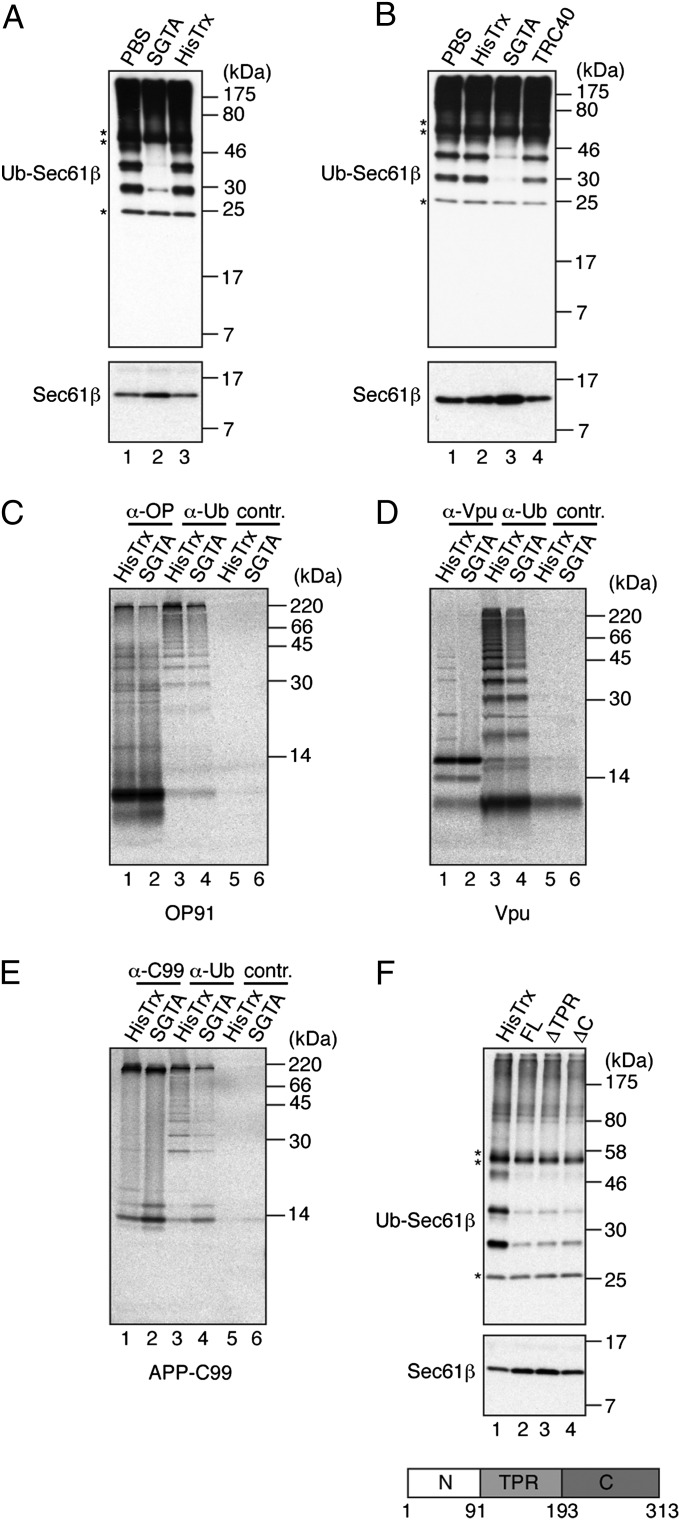
Strikingly, in the presence of recombinant HisTrx-tagged SGTA (hereafter called SGTA), but not buffer or HisTrx controls, the BAG6-dependent ubiquitination of Sec61β was almost completely abolished and there was a corresponding increase in the amount of unmodified substrate recovered (Fig. 2A). In contrast, the addition of purified TRC40, the main endoplasmic reticulum delivery factor for Sec61β (9, 18, 19), had a minimal effect on Sec61β ubiquitination and the level of unmodified substrate (Fig. 2B). Hence, substrate binding alone does not appear to prevent Sec61β ubiquitination, suggesting a more specific role for SGTA. A substantial SGTA-mediated reduction in substrate ubiquitination was also observed with recombinant forms of two other tail-anchored proteins, RAMP4 and Synaptobrevin 2 (Fig. S2 A and B).
Fig. 2.
SGTA inhibits the ubiquitination of a range of hydrophobic precursors. (A) Sec61β ubiquitination reaction as for Fig. 1 (2 h) but in the presence of 4 μM HisTrx-SGTA (SGTA), HisTrx, or an equal volume of PBS. Samples were analyzed by Western blotting as for Fig. 1. (B) Specificity of the SGTA-dependent effect was confirmed by comparing Sec61β ubiquitination in the presence of TRC40. (C–E) Substrates indicated were translated in rabbit reticulocyte lysate in the presence of 2 μM HisTrx-SGTA (SGTA) or 2 μM HisTrx control, treated with NEM, and then immunoprecipitated with antibodies against the substrate (lanes 1 and 2), ubiquitin (lanes 3 and 4), or a control (lanes 5 and 6). Products were resolved by SDS/PAGE and visualized by phosphorimaging. APP-C99, C-terminal 99 amino acids of amyloid precursor protein (APP) fused with its N-terminal signal sequence; OP91, N-terminal 91 residues of bovine rhodopsin; Vpu, viral protein U of HIV (Fig. S2). (F) Sec61β ubiquitination was as for A but in the presence of 4 μM HisTrx, full-length His-S-SGTA (FL), or SGTA deletion mutants lacking the tetratricopeptide repeats (ΔTPR) or the C-terminal glutamine-rich region (ΔC). SGTA organization with domain boundaries is shown.
A role for BAG6 in promoting the ubiquitination of mislocalized proteins was first investigated by synthesizing hydrophobic polypeptides in a cell-free system lacking endoplasmic reticulum-derived membranes (4). When we used the same system to generate three distinct membrane protein precursors, evidence of their ubiquitination was apparent in every case (Fig. 2 C–E, lane 3). Strikingly, the level of ubiquitinated product resulting from each precursor was substantially reduced by the presence of SGTA during synthesis (Fig. 2 C–E, lane 4, quantification indicates an approximate twofold reduction in each case; Fig. S2E). Hence, an SGTA-mediated reduction in ubiquitination is a wide-ranging phenomenon that is not restricted to tail-anchored proteins. Notably, all of the substrates analyzed contain at least one predicted transmembrane domain: an N-terminal fragment of bovine rhodopsin (OP91), the HIV protein Vpu, and the C-terminal 99 residues of amyloid precursor protein (APP) fused to its signal sequence (APP-C99). Furthermore, the ubiquitination of mislocalized rhodopsin and Vpu has been shown to be facilitated by BAG6 (4). However, in contrast to the behavior of these precursors, the efficient in vitro ubiquitination of two alternative model substrates, Ub-R-GFP and UbG76V-GFP, recognized on the basis of the N-end rule or the ubiquitin fusion degradation pathway, respectively, was unaffected, suggesting that SGTA does not perturb protein ubiquitination per se (Fig. S2 C and D). The tetratricopeptide repeats of SGTA are known to mediate interactions with molecular chaperones of the Hsp70 and Hsp90 families (ref. 20 and references therein), whereas the C-terminal glutamine-rich region is implicated in binding hydrophobic precursor proteins (13, 16). To better understand the actions of SGTA, we investigated the effect of removing each of these domains on the ubiquitination of mislocalized proteins. Recombinant SGTA lacking either the tetratricopeptide repeats or the glutamine-rich C terminus is as effective as the full-length protein at preventing the ubiquitination of Sec61β (Fig. 2F, lanes 2–4). Taken together, these data indicate that SGTA specifically antagonizes the modification of mislocalized proteins bearing a hydrophobic domain and suggest this process does not require the recruitment of molecular chaperones via its tetratricopeptide repeats.
SGTA Promotes the Deubiquitination of Mislocalized Proteins.
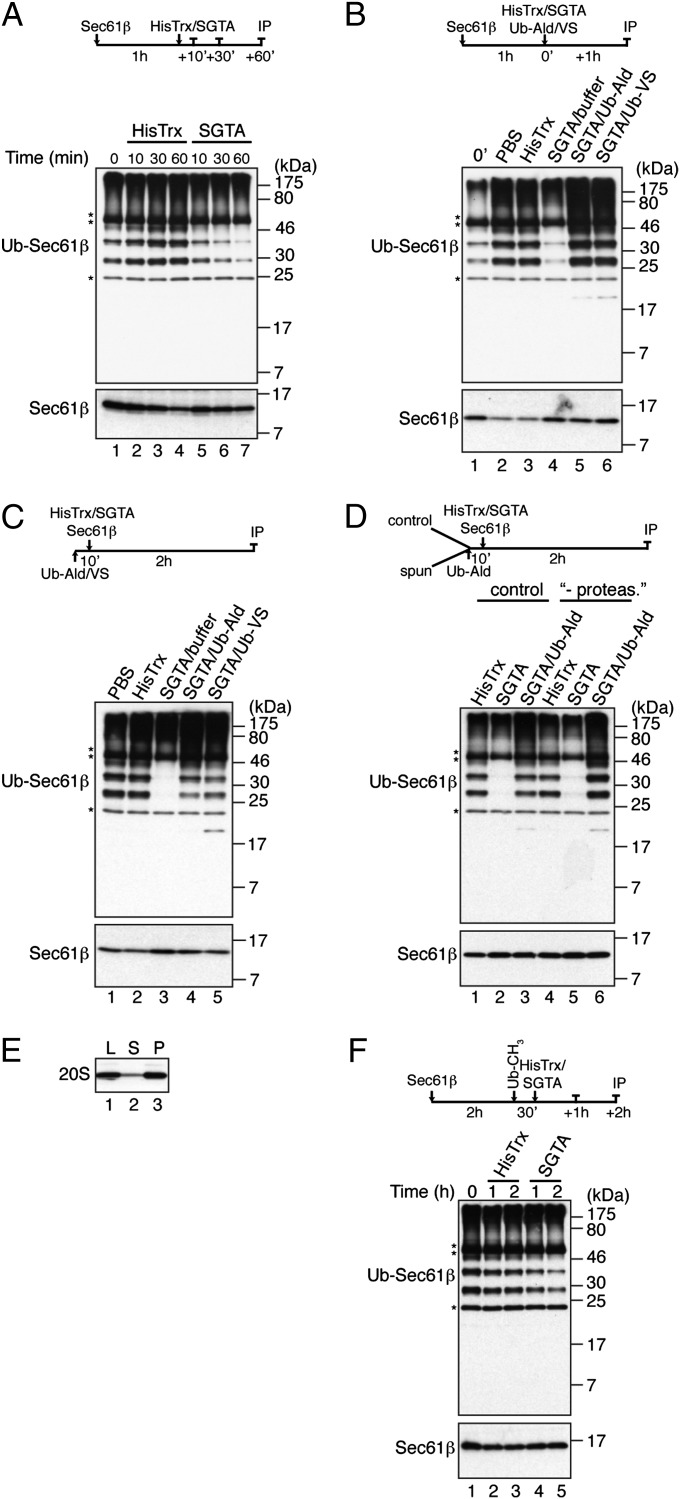
To further delineate the role of SGTA we determined its effect when added to a reaction containing preubiquitinated Sec61β. Remarkably, whereas Sec61β ubiquitination continued under control conditions the addition of SGTA actually resulted in a time-dependent decrease in ubiquitinated products (Fig. 3A, Ub-Sec61β species). One explanation for this observation is that SGTA acts on preformed ubiquitinated species and facilitates their deubiquitination. In support of this hypothesis, inhibitors of deubiquitinating enzymes, ubiquitin-aldehyde (Ub-Ald) and ubiquitin vinyl-sulfone (Ub-VS) (21, 22), completely abolished the otherwise substantial SGTA-dependent reduction in “pre-ubiquitinated” Sec61β species (Fig. 3B). Likewise, including these inhibitors together with both the substrate and SGTA at the start of the reaction resulted in increased levels of ubiquitinated Sec61β (Fig. 3 C and D), also suggesting that rapid deubiquitination is central to the SGTA-mediated reduction in ubiquitinated mislocalized proteins. Any possibility that proteasomal degradation contributes to the SGTA-mediated loss of ubiquitinated Sec61β (Fig. 3A) can be excluded, because this process occurs equally well with lysate that has been substantially depleted of proteasomes before the in vitro ubiquitination assay (Fig. 3 D and E). A potential alternative to SGTA selectively promoting the deubiquitination of mislocalized proteins could be its ability to inhibit a normally rapid reubiquitination pathway that operates following a constitutive, and nonselective, deubiquitination process. However, when ubiquitinated Sec61β was accumulated in cytosol depleted of proteasomes, and then further ubiquitination was inhibited using an appropriate concentration of methylated ubiquitin (Fig. S3), the addition of SGTA continued to promote a substantial reduction in the level of ubiquitinated Sec61β (Fig. 3F). Taken together, these data all support a model in which SGTA actively promotes the deubiquitination of mislocalized proteins that have already been modified.
Fig. 3.
SGTA mediates deubiquitination of mislocalized proteins. (A) Sec61β was ubiquitinated for 1 h at 30 °C as for Fig. 1, 4 μM HisTrx-SGTA (SGTA) or 4 μM HisTrx was added, reactions continued for the times indicated, and samples were analyzed as before. (B) Reactions were as for A except 5 μM ubiquitin aldehyde (Ub-Ald) or 2 μM HA-tagged ubiquitin vinyl-sulfone (Ub-VS) was added with SGTA where indicated. (C) As for A except where indicated Ub-Ald or Ub-VS and SGTA were present from the start of a 2-h ubiquitination reaction. (D) Lysates before and after proteasome depletion were used in a ubiquitination reaction as described for C. (E) Rabbit reticulocyte lysate was depleted of proteasomes by ultracentrifugation and analyzed by Western blotting with anti-20S proteasome serum. L, load, P, pellet; S, supernatant. (F) Sec61β was ubiquitinated in proteasome-depleted rabbit reticulocyte lysate for 2 h at 30 °C, further polyubiquitination was inhibited with 120 μM methylated ubiquitin for 30 min (Fig. S3), then SGTA or HisTrx was added to 4 μM final concentration and reactions continued for 1 or 2 h.
SGTA Coexpression Significantly Enhances Cellular Levels of a Mislocalized Protein.
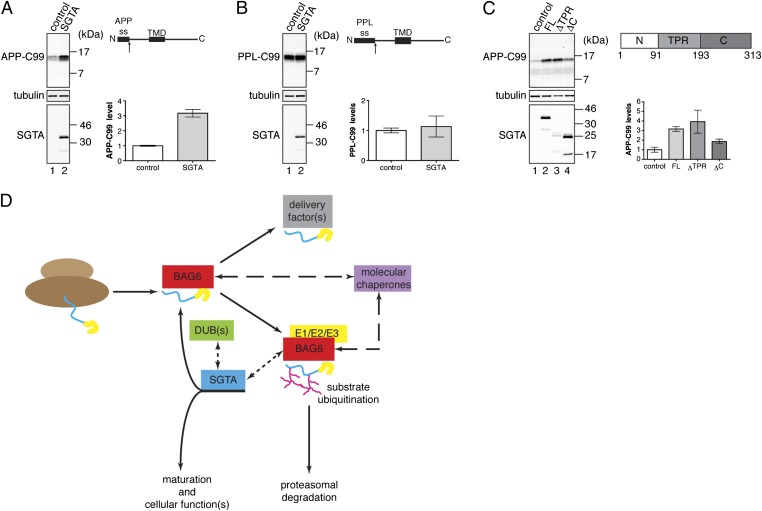
We identified APP-C99 as a candidate mislocalized protein for analysis in mammalian cells, on the basis that its naturally occurring signal sequence for targeting to the endoplasmic reticulum is comparatively inefficient (23), and therefore prone to generating mistargeted species. This hypothesis was further supported by a substantial increase in steady-state levels of APP-C99 obtained in cultured cells upon the inclusion of a proteasome inhibitor (Fig. S4A, ref. 4). Compellingly, the SGTA-mediated protection of APP-C99 from ubiquitination observed in vitro (Fig. 2E) directly mirrors the outcome of SGTA with this substrate in mammalian cells. Hence, cotransfection of APP-C99 with SGTA resulted in ∼3.5-fold increase in steady-state levels of the substrate (Fig. 4A) that is otherwise efficiently removed by proteasomal degradation (Fig. S4A). Furthermore, the stabilizing effect of SGTA upon APP-C99 correlates well with the amount of exogenous SGTA that is coexpressed (Fig. S4B). Taken together, these data indicate that SGTA counteracts BAG6-mediated ubiquitination in vivo (Figs. 2 and 3) and thereby inhibits subsequent proteasomal degradation of mislocalized APP-C99. To further explore the potential link between SGTA-mediated stabilization and mislocalization, we reengineered APP-C99 to minimize mislocalized protein production by replacing its endogenous signal sequence with a highly efficient one from preprolactin (24). When this alternative version of the precursor was analyzed, steady-state levels of the resulting PPL-C99 appeared much higher in control cells and, crucially, were unaffected by SGTA coexpression (Fig. 4B). Thus, increased levels of SGTA preferentially stabilize mislocalized proteins in mammalian cells, a role that can be effectively bypassed by ensuring efficient delivery of the precursor protein to the endoplasmic reticulum. Building on the in vitro analysis of SGTA deletion mutants (Fig. 2F), we compared the effect of coexpressing the same SGTA variants on steady-state levels of APP-C99. Once again, the tetratricopeptide repeats proved dispensable and the effect of the SGTAΔTPR mutant was comparable to that of full-length SGTA (Fig. 4C, APP-C99). Interestingly, this effect was clearly apparent despite comparatively low-level expression of SGTAΔTPR (Fig. 4C, SGTA). We speculate that the inability of the SGTAΔTPR mutant to interact with Hsp70s and Hsp90s may prevent it from being sequestered into alternative pathways, thereby enhancing its availability for modulating mislocalized protein quality control. Although efficiently expressed (Fig. 4C, SGTA), the SGTAΔC mutant had a more modest effect on APP-C99 levels (Fig. 4C, APP-C99). Hence, although the C terminus of SGTA is dispensable for promoting deubiquitination in vitro (Fig. 2F), it may make some additional, as yet undefined, contribution in a cellular context. Taken together, our data support a model in which SGTA reverses BAG6-mediated substrate-specific ubiquitination by promoting the deubiquitination of selected substrates, including mislocalized proteins.
Fig. 4.
SGTA coexpression increases steady-state levels of mislocalized proteins. HeLa cells were cotransfected with plasmids encoding APP-C99 (A) or PPL-C99 (C-terminal 99 residues of APP fused to the preprolactin signal sequence) (B), plus pcDNA5-SGTA-V5 (SGTA) or empty vector (control). Substrate levels were examined 22 h posttransfection by quantitative Western blotting of total cell lysate and signals normalized to tubulin and plotted relative to the amount of substrate in control cells, SEs for n ≥ 3. Overexpressed exogenous SGTA was visualized by immunoblotting with anti-V5 antibody (Fig. S4B). (C) HeLa cells were cotransfected with APP-C99 as for A except that SGTA deletion mutants lacking the tetratricopeptide repeats (ΔTPR) or the C-terminal glutamine-rich region (ΔC) were used in addition to full-length SGTA (FL). (D) A model for a BAG6/SGTA cycle. SGTA antagonizes the BAG6-dependent ubiquitination of selected substrates by facilitating their deubiquitination. At present we cannot discriminate between models where deubiquitination occurs on BAG6 or following substrate release. In either case, this process would stabilize the substrate and thereby promote its potential maturation and subsequent cellular function(s). For mislocalized proteins deubiquitination may enable productive interactions with specific delivery factors and/or molecular chaperones that facilitate the acquisition of a native structure and thereby counteract premature degradation. Alternatively, if deubiquitinated precursors are unable to initiate productive interactions, we propose they might be reubiquitinated via BAG6 to facilitate their degradation. Dashed lines are used to indicate connections between components that may be direct or indirect.
Ubiquitination of Mislocalized Proteins Is Dynamic and Reversible.
In this study we identify a unique role for SGTA in the cytosolic quality control of mislocalized proteins, showing that SGTA antagonizes BAG6-mediated substrate ubiquitination and proteasomal degradation (4) via a mechanism that requires active deubiquitination (Fig. 4D). This SGTA-dependent reduction of mislocalized protein ubiquitination is observed with a range of substrates and correlates with the substantial stabilization of a model mislocalized protein upon SGTA coexpression. Overexpression of full-length SGTA at a level greater than fivefold that of the endogenous protein is sufficient to shift the normal equilibrium between ubiquitination and deubiquitination in favor of deubiquitination (Fig. 4D), resulting in the stabilization of substrates normally removed by proteasomal degradation (Fig. S4A).
In the case of tail-anchored proteins that undergo posttranslational integration at the endoplasmic reticulum, SGTA may correct their premature ubiquitination, thereby extending the time frame for their successful delivery to, and insertion at, the endoplasmic reticulum. Notably, Sgt2 acts as an upstream component of the GET pathway for tail-anchored protein biogenesis in yeast (13), consistent with our current finding that its mammalian ortholog also promotes a biosynthetic fate for newly made precursors. Likewise, the posttranslational delivery of short secretory proteins to the endoplasmic reticulum (25, 26) suggests that the SGTA-dependent correction of premature substrate ubiquitination may be a common event. In practice, even cotranslational protein synthesis at the endoplasmic reticulum (1) can result in mislocalized proteins, for example as a result of endoplasmic reticulum stress (3). However, although BAG6 promotes the degradation of mislocalized prion protein (4), prolonged exposure of mice to these substrates and the resulting constitutive activity of a cytosolic quality control pathway(s) leads to neurodegeneration (3). Thus, the cell must carefully regulate substrate access to the BAG6-dependent pathway for accelerated protein degradation, and we propose that SGTA can act as a molecular governor for BAG6 activity by reversing its actions. Because the role of BAG6 in promoting substrate-specific degradation is known to extend beyond mislocalized proteins to include the metabolic enzyme phosphoenolpyruvate carboxykinase (27), it is possible that SGTA modulates BAG6 activity across a range of ubiquitin-dependent cellular processes.
BAG6/SGTA Cycle for Protein Quality Control in the Cytosol?
Our data show that the selective ubiquitination of mislocalized proteins does not represent an automatic and irreversible commitment to proteasomal degradation. Rather, the process is highly dynamic and its substrates are subjected to the competing attentions of distinct components favoring either protein synthesis and maturation or protein degradation (8). Both SGTA and BAG6 have the potential to recruit and modulate molecular chaperones via their tetratricopeptide repeats and BAG domain, respectively (ref. 9 and references therein). However, the removal of the tetratricopeptide repeats from SGTA does not affect its ability to promote deubiquitination or stabilize mislocalized proteins. On this basis we conclude that Hsp70s and Hsp90s are unlikely to contribute to the role of SGTA in protein quality control. The contribution of the BAG domain of BAG6 to the fate of mislocalized proteins remains to be addressed. We speculate that a subset of newly synthesized proteins, selected on the basis of inappropriately exposed hydrophobic residues, enter a BAG6/SGTA cycle (Fig. 4D). These precursors would undergo a reiterative maturation process leading to either the acquisition of a native structure or, failing that, their proteasome-mediated degradation. In this scenario, the BAG6/SGTA cycle would mirror aspects of the calnexin cycle that operates in the lumen of the endoplasmic reticulum to promote glycoprotein folding while ensuring efficient degradation of terminally misfolded or aberrant precursors.
Materials and Methods
In Vitro Ubiquitination Assays.
Rabbit reticulocyte lysate was supplemented with 40 μM FLAG-tagged ubiquitin and 4 μM recombinant cytosolic proteins where stated; purified tail-anchored protein was added to 2 μM final concentration and reactions incubated at 30 °C for indicated times. Upon completion 5 mM N-ethylmaleimide (NEM) was added and samples were incubated at 30 °C for 10 min, quenched with 10 mM DTT for 5 min, and diluted in 200 μL of immunoprecipitation buffer [10 mM Tris⋅Cl (pH 7.5), 140 mM NaCl, 1 mM EDTA, 1% Triton-X100] supplemented with 1 mM PMSF, protease inhibitor mixture (Sigma), and anti-opsin antibody. Following overnight incubation at 4 °C, antibody-bound material was recovered on Protein A Sepharose (GenScript) and washed extensively with immunoprecipitation buffer and proteins were eluted with SDS sample buffer. Products were resolved by SDS/PAGE and analyzed by Western blotting followed by enhanced chemiluminescence detection using anti-FLAG to monitor substrate ubiquitination and anti-Sec61β to visualize the unmodified substrate. Radiolabeled substrates were generated by in vitro translation (28) in the absence of endoplasmic reticulum-derived membranes, and samples were processed as for recombinant proteins. Anti-substrate, anti-ubiquitin, or a control serum were used for immunoprecipitation and products visualized using a Fuji BAS-3000 phosphorimager and quantified using AIDA software.
Cell Culture.
HeLa cells were grown in a 12-well dish to 80–90% confluency then cotransfected with 0.5 μg of substrate-coding plasmid (SI Materials and Methods) and 0.5 μg of pcDNA5-SGTA-V5 or empty pcDNA3.1 vector control using Lipofectamine 2000 (Invitrogen). Cells were grown for another 22 h and lysed with nonreducing SDS sample buffer supplemented with 10 mM NEM and protease inhibitor mixture (Sigma). Following 10 min at room temperature, samples were harvested, incubated for 10 min at 37 °C, supplemented with 20 mM DTT, and incubated for a further 20 min. Alternatively, cells were transfected with plasmids encoding the substrate and empty vector and treated overnight with 10 nM bortezomib, 100 µM leupeptin plus 1 µg/mL pepstatin, or DMSO control. Samples were resolved by SDS/PAGE and analyzed by quantitative Western blotting (Licor Biosciences) using anti-C99 antibody to monitor substrate level, anti-tubulin to correct for differences in loading, and anti-V5 antibody to examine SGTA overexpression. Each transfection was independently repeated at least three times; the amount of substrate was quantified with Odyssey 2.1 software and plotted relative to the matched control using GraphPad Prism 4.0 software. To titrate the effect of SGTA expression upon APP-C99 levels, 0.5 µg of APP-C99 encoding plasmid was cotransfected with 0.5-µg mixtures of empty vector and SGTA encoding plasmids in different ratios. Samples were processed as before, except SGTA was detected using an anti-SGTA antibody to compare the levels of exogenous and endogenous proteins. The two forms were distinguished on the basis of their different mobility.
Depletion of Cytosolic Factors.
BAG6 was immunodepleted from rabbit reticulocyte lysate as before (9) using antibodies raised against the N terminus or residues 112–130 of human BAG6. Proteasomes were depleted by centrifuging rabbit reticulocyte lysate for 2 h at 300,000 × g and the resulting 20S-depleted supernatant was used for subsequent experiments.
Additional information is given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Padraig D’Arcy, Ruth Geiss-Friedlander, Stig Linder, Janni Petersen, Martin Pool, Peri Roboti, Blanche Schwappach, Lisa Swanton, Fabio Vilardi, and Phil Woodman for their valuable contributions at various stages of the project. This work was supported by project grant funding from the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209997109/-/DCSupplemental.
References
- 1.Shao S, Hegde RS. Membrane protein insertion at the endoplasmic reticulum. Annu Rev Cell Dev Biol. 2011;27:25–56. doi: 10.1146/annurev-cellbio-092910-154125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belin D, Bost S, Vassalli J-D, Strub K. A two-step recognition of signal sequences determines the translocation efficiency of proteins. EMBO J. 1996;15(3):468–478. [PMC free article] [PubMed] [Google Scholar]
- 3.Rane NS, Kang SW, Chakrabarti O, Feigenbaum L, Hegde RS. Reduced translocation of nascent prion protein during ER stress contributes to neurodegeneration. Dev Cell. 2008;15(3):359–370. doi: 10.1016/j.devcel.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hessa T, et al. Protein targeting and degradation are coupled for elimination of mislocalized proteins. Nature. 2011;475(7356):394–397. doi: 10.1038/nature10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q, et al. A ubiquitin ligase-associated chaperone holdase maintains polypeptides in soluble states for proteasome degradation. Mol Cell. 2011;42(6):758–770. doi: 10.1016/j.molcel.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claessen JH, Ploegh HL. BAT3 guides misfolded glycoproteins out of the endoplasmic reticulum. PLoS ONE. 2011;6(12):e28542. doi: 10.1371/journal.pone.0028542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minami R, et al. BAG-6 is essential for selective elimination of defective proteasomal substrates. J Cell Biol. 2010;190(4):637–650. doi: 10.1083/jcb.200908092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ast T, Schuldiner M. Protein degradation: BAGging up the trash. Curr Biol. 2011;21(18):R692–R695. doi: 10.1016/j.cub.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Leznicki P, Clancy A, Schwappach B, High S. Bat3 promotes the membrane integration of tail-anchored proteins. J Cell Sci. 2010;123(Pt 13):2170–2178. doi: 10.1242/jcs.066738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariappan M, et al. A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature. 2010;466(7310):1120–1124. doi: 10.1038/nature09296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodsky JL. The special delivery of a tail-anchored protein: Why it pays to use a dedicated courier. Mol Cell. 2010;40(1):5–7. doi: 10.1016/j.molcel.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabu C, Schmid V, Schwappach B, High S. Biogenesis of tail-anchored proteins: the beginning for the end? J Cell Sci. 2009;122(Pt 20):3605–3612. doi: 10.1242/jcs.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Brown EC, Mak G, Zhuang J, Denic V. A chaperone cascade sorts proteins for posttranslational membrane insertion into the endoplasmic reticulum. Mol Cell. 2010;40(1):159–171. doi: 10.1016/j.molcel.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winnefeld M, et al. Human SGT interacts with Bag-6/Bat-3/Scythe and cells with reduced levels of either protein display persistence of few misaligned chromosomes and mitotic arrest. Exp Cell Res. 2006;312(13):2500–2514. doi: 10.1016/j.yexcr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Leznicki P, Warwicker J, High S. A biochemical analysis of the constraints of tail-anchored protein biogenesis. Biochem J. 2011;436(3):719–727. doi: 10.1042/BJ20101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liou ST, Wang C. Small glutamine-rich tetratricopeptide repeat-containing protein is composed of three structural units with distinct functions. Arch Biochem Biophys. 2005;435(2):253–263. doi: 10.1016/j.abb.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Rual JF, et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437(7062):1173–1178. doi: 10.1038/nature04209. [DOI] [PubMed] [Google Scholar]
- 18.Favaloro V, Spasic M, Schwappach B, Dobberstein B. Distinct targeting pathways for the membrane insertion of tail-anchored (TA) proteins. J Cell Sci. 2008;121(Pt 11):1832–1840. doi: 10.1242/jcs.020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefanovic S, Hegde RS. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell. 2007;128(6):1147–1159. doi: 10.1016/j.cell.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 20.Buchanan G, et al. Control of androgen receptor signaling in prostate cancer by the cochaperone small glutamine rich tetratricopeptide repeat containing protein alpha. Cancer Res. 2007;67(20):10087–10096. doi: 10.1158/0008-5472.CAN-07-1646. [DOI] [PubMed] [Google Scholar]
- 21.Borodovsky A, et al. Chemistry-based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem Biol. 2002;9(10):1149–1159. doi: 10.1016/s1074-5521(02)00248-x. [DOI] [PubMed] [Google Scholar]
- 22.Hershko A, Rose IA. Ubiquitin-aldehyde: A general inhibitor of ubiquitin-recycling processes. Proc Natl Acad Sci USA. 1987;84(7):1829–1833. doi: 10.1073/pnas.84.7.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161(1):41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine CG, Mitra D, Sharma A, Smith CL, Hegde RS. The efficiency of protein compartmentalization into the secretory pathway. Mol Biol Cell. 2005;16(1):279–291. doi: 10.1091/mbc.E04-06-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao S, Hegde RS. A calmodulin-dependent translocation pathway for small secretory proteins. Cell. 2011;147(7):1576–1588. doi: 10.1016/j.cell.2011.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson N, et al. TRC40 can deliver short secretory proteins to the Sec61 translocon. J Cell Sci. 2012;125(Pt 15):3612–3620. doi: 10.1242/jcs.102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang W, et al. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol Cell. 2011;43(1):33–44. doi: 10.1016/j.molcel.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson CM, et al. Ribophorin I associates with a subset of membrane proteins after their integration at the sec61 translocon. J Biol Chem. 2005;280(6):4195–4206. doi: 10.1074/jbc.M410329200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.