Abstract
Blind mole rats Spalax (BMR) are small subterranean rodents common in the Middle East. BMR is distinguished by its adaptations to life underground, remarkable longevity (with a maximum documented lifespan of 21 y), and resistance to cancer. Spontaneous tumors have never been observed in spalacids. To understand the mechanisms responsible for this resistance, we examined the growth of BMR fibroblasts in vitro of the species Spalax judaei and Spalax golani. BMR cells proliferated actively for 7–20 population doublings, after which the cells began secreting IFN-β, and the cultures underwent massive necrotic cell death within 3 d. The necrotic cell death phenomenon was independent of culture conditions or telomere shortening. Interestingly, this cell behavior was distinct from that observed in another long-lived and cancer-resistant African mole rat, Heterocephalus glaber, the naked mole rat in which cells display hypersensitivity to contact inhibition. Sequestration of p53 and Rb proteins using SV40 large T antigen completely rescued necrotic cell death. Our results suggest that cancer resistance of BMR is conferred by massive necrotic response to overproliferation mediated by p53 and Rb pathways, and triggered by the release of IFN-β. Thus, we have identified a unique mechanism that contributes to cancer resistance of this subterranean mammal extremely adapted to life underground.
Keywords: aging, necrosis
Blind mole rats, Spalax (BMR), belong to a family of subterranean rodents of the Muroidea superfamily, prevalent in the Middle East, ranging in the Eastern Mediterranean and North Africa. BMRs are solitary mammals that spend their lives in underground burrows. Living under extreme hypoxic conditions, BMRs evolved strong hypoxic tolerance (1, 2).
BMRs are very long-lived for their size. The maximum lifespan documented for the animals kept in our animal facility is 21 y (3). In comparison, mice and rats belonging to the same superfamily have a maximum lifespan of 4 y (4, 5). Furthermore, BMRs show a striking resistance to cancer. Our observation of thousands of captive animals did not show a single case of spontaneous tumor development over a 40-y period. Cancer accounts for ∼23% of human mortality (6). In mice and rats, cancer mortality is very high, reaching 90% in some strains (7, 8).
Animals have evolved multiple mechanisms to protect themselves from cancer. These mechanisms include cell-cycle checkpoints, DNA repair, programmed cell death, and replicative senescence controlled by a network of tumor-suppressor genes, such as p53 and Rb. Anticancer adaptations differ between species, which may explain the differences in cancer susceptibility (9–11). Mice have been used extensively as models for cancer research. However, mice are more prone to cancer and are likely to possess fewer anticancer defenses, thus limiting a potential for discovery of novel anticancer pathways. Hence, there is great value in studying anticancer mechanisms in cancer-resistant species. Subterranean mammals are good candidates for these studies.
We showed previously that the BMR p53 gene contains an arginine to lysine substitution at a site corresponding to human p53 position 174, where mutations are often found in human tumors (12). The R174K substitution affects the DNA-binding domain of p53; the resulting protein is capable of inducing cell-cycle arrest but is defective in initiating apoptosis. We hypothesized that R174K substitution evolved in BMR as an adaptation to the hypoxic environment in underground tunnels to prevent hypoxia-induced apoptosis (12). Mice with the corresponding p53 mutation are defective in p53-dependent apoptosis (13). These mice show longer tumor latency than p53−/− mice, but are more tumor-prone than the wild-type mice (13). Thus, the extreme tumor resistance of BMR is intriguing.
Our previous studies of another long-lived subterranean rodent, the naked mole rat (Heterocaphalus glaber), identified a novel anticancer mechanism termed early contact inhibition (ECI) (14). Naked mole rat cells proliferate slowly in culture and display hypersensitivity to contact inhibition (14). Abrogation of ECI makes naked mole rat cells more prone to malignant transformation. Naked mole rats inhabit East Africa and belong to the Hystricognath group of rodents, which also includes guinea pigs. Naked mole rats are evolutionarily distant from BMR, which is closer to Old World mice and rats. Both mole rats, however, lead subterranean lifestyles protected from predators and the aboveground extreme climatic fluctuations, which allowed these species to evolve extreme longevity and cancer resistance.
Here we set out to investigate cancer resistance in the BMR. The analysis of BMR-cultured cells reveals that, unlike the naked mole rat, BMR fibroblasts do not display early contact inhibition, but instead proliferate rapidly for 7–20 population doublings (PDs) after which the cells begin to secrete IFN-β and the culture undergoes concerted cell death (CCD), which is characterized by a large fraction of necrotic cells. Inactivation of Rb and p53 tumor suppressors rescued the cell-death phenotype. Our results suggest that the BMR has evolved a unique anticancer mechanism mediated by strong induction of the necrotic cell-death response to hyperproliferation. Furthermore, we conclude that the two long-lived cancer-resistant rodents have achieved their cancer resistance by two distinct mechanisms.
Results
BMR Fibroblasts Do Not Show ECI.
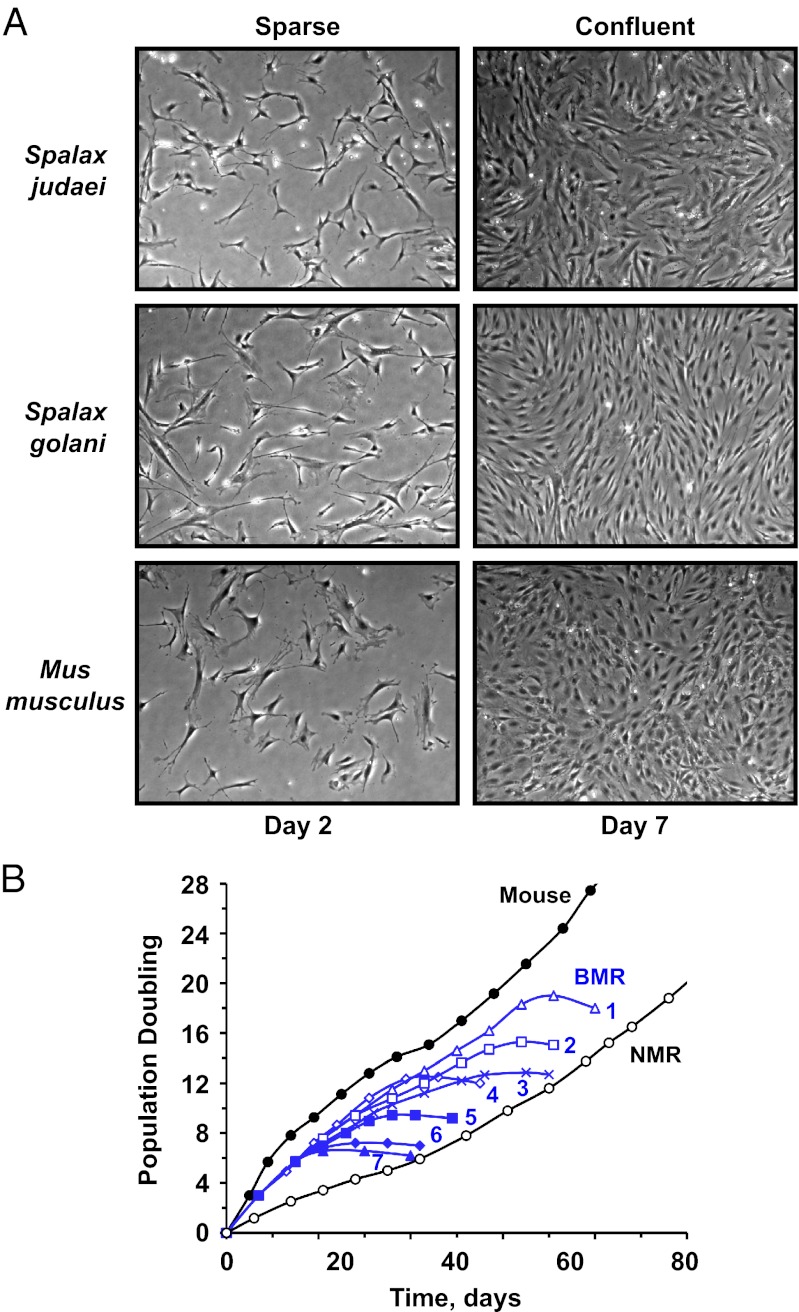
To understand the molecular mechanism responsible for the cancer resistance of BMR, we isolated primary fibroblasts from BMR lung and skin and examined their proliferation in culture. The experiments were performed on fibroblasts isolated from two animals of each species: Spalax judaei and Spalax golani (2). All fibroblast lines showed similar growth characteristics; therefore, we will refer to them as BMR fibroblasts. BMR fibroblasts proliferated rapidly and reached high cell density on a plate (Fig. 1A). Cell density was similar to the density attained by mouse fibroblasts. We concluded that BMR fibroblasts, unlike naked mole rat fibroblasts, do not display the ECI phenotype. Therefore, BMR is likely to have evolved a distinct anticancer mechanism.
Fig. 1.
BMR fibroblasts display a unique concerted cell death phenotype. All cells were cultured at 3% oxygen concentration. (A) Fibroblasts of both S. judaei and S. golani species become confluent at high cell density and do not undergo early contact inhibition typical of naked mole rat cells. Cells were seeded at 5 × 105 cells per 10-cm dish and photographed at indicated time points. (Magnification: 100×.) (B) BMR fibroblast cultures undergo CCD irrespective of growth conditions. Mouse, denotes mouse fibroblasts, and NMR denotes naked mole rat fibroblasts cultured under our standard conditions of 15% FBS and EMEM media from ATCC. Blue growth curves correspond to BMR fibroblasts cultured in different concentrations of FBS and either EMEM media from ATCC or low-serum fibroblast growth media (FGM) from Lonza. FGM media was used with different concentrations of fibroblast growth factor indicated by (no. in blue) -F or 1/2F (1), 15% FBS, FGM (2), 15% FBS, FGM-F (3), 2% FBS, FGM (4), 15% FBS, EMEM (5), 2% FBS, FGM 1/2F (6), 5% FBS, EMEM (7), 0% FBS, FGM. BMR cells grew rapidly for several PDs, then invariably stopped proliferation and underwent CCD.
BMR Cells Undergo Concerted Cell Death.
BMR fibroblasts proliferated rapidly for 7–20 PDs, and then the cells arrested proliferation for ∼3 d, after which all cells on the plate detached and died, with no attached cells remaining within 4 d of the onset of cell death. Our laboratory routinely cultures primary fibroblasts from 20 rodent species, and we have never observed such a synchronous death of cell cultures. We termed this phenomenon “concerted cell death” (CCD). Our standard growth media for all primary rodent fibroblasts is EMEM supplemented with 15% (vol/vol) FBS. In an attempt to find growth conditions where the cells would not die, we have also cultured BMR cells in low-serum and serum-free fibroblast growth media. However, variations of cell culture media changed the lifespan by a few PDs, after which all cultures invariably underwent CCD (Fig. 1B).
To identify the type of cell death in BMR cultures, we collected the dying cells, stained them with Annexin-V and propidium iodide (PI), and then analyzed by flow cytometry. The cell death was preferentially necrotic, with 46% of the cells dying by necrosis and 28% by apoptosis (Fig. 2B, control samples).
Fig. 2.
CCD requires Rb and p53 pathways. (A) At day 27 BMR cells were stably transfected with plasmids encoding SV40 LT antigen, Large T K1 (a mutant version of LT antigen that inactivates p53 but not Rb), and a control plasmid. Cells transfected with the wild-type LT did not undergo CCD. (B) CCD occurs by necrosis and apoptosis. Cell death was analyzed by Annexin-V/PI staining and flow cytometry in actively growing (day 47) and dying (day 67) BMR cultures shown in A.
CCD Is Abrogated by SV40 Large T Antigen.
We next tested whether CCD is controlled by the major tumor suppressor pathways, Rb and p53. We transfected BMR cells with SV40 large T antigen (LT) or its mutant derivatives and selected chromosomal integrants. Wild-type LT is a viral oncoprotein that binds and inactivates both p53 and pRb. The mutant derivative LTK1 inactivates only p53, and LTΔ434–444 inactivates only pRb and its family members (p107 and p130) (15). BMR cells expressing LT proliferated continuously for over 50 PDs and did not undergo CCD (Fig. 2). LTK1 did not rescue CCD, but rather accelerated its onset (Fig. 2), but LTΔ434–444 was toxic to the cells and did not yield any chromosomally integrated clones despite multiple attempts. This result indicates that both Rb and p53 pathways must be inactivated to abrogate CCD.
CCD Is Not Caused by Telomere Shortening.
We then tested whether CCD is induced by rapid telomere shortening. Importantly, BMR cultures expressed high levels of telomerase activity (Fig. 3A), as expected for a small-sized rodent (10). In addition, time-resolved fluorescence (TRF) assay showed that BMR has telomeres with a mean length of 50 kb, which is much longer than telomeres in the species with replicative senescence, such as humans (Fig. 3B). Furthermore, telomere length did not change in cultured cells undergoing CCD. These results indicate that CCD is not triggered by telomere shortening.
Fig. 3.
CCD of BMR cells is not caused by telomere shortening. (A) BMR fibroblasts have endogenous telomerase activity. Telomeric repeat amplification protocol assay was performed on extracts from growing (G) or dying (D) BMR cells, and HeLa cells as a positive control. (B) Telomeres do not shorten in dying BMR cells. Telomere length in growing (G) and dying (D) BMR cells was measured using TRF assay. Young (Y) and senescent (S) human fibroblasts were used as a reference.
Concerted Cell Death of BMR Cells Is Mediated by Release of IFN-β.
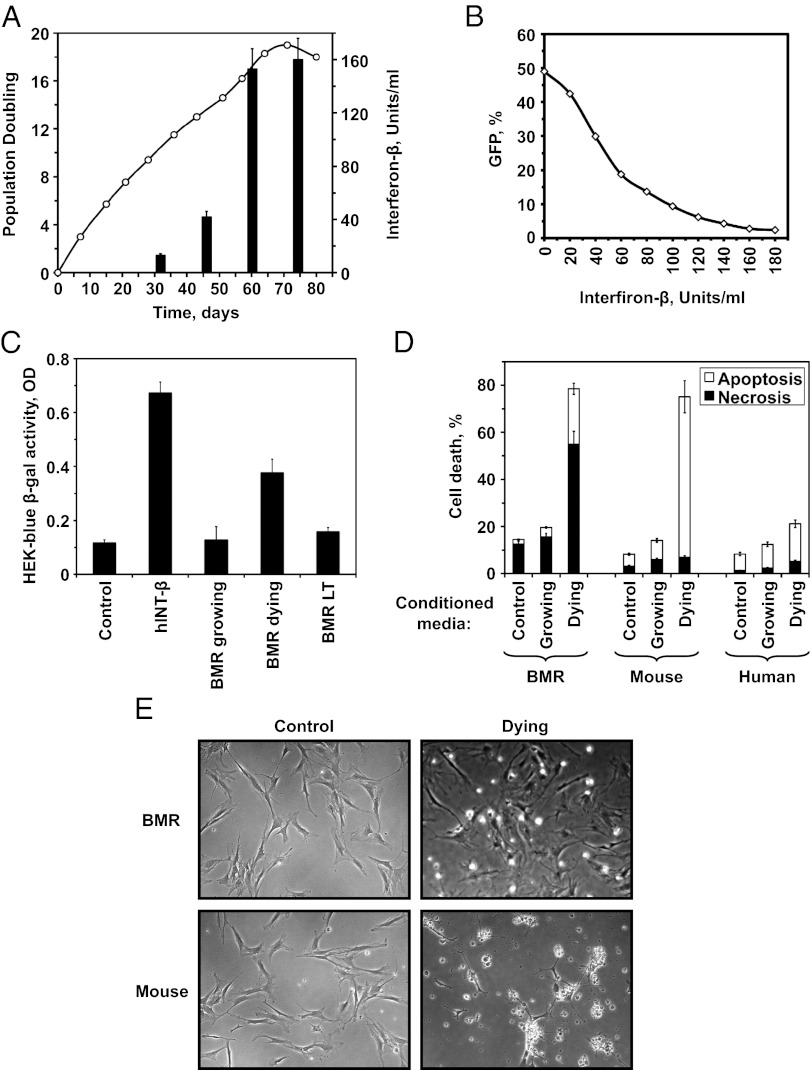
We then set out to identify the mechanism of CCD. We hypothesized that the pattern of cells dying synchronously is consistent with a response to IFN. We tested whether BMR cell cultures secrete IFN-β using two assays. In the first assay, HT1080 cells were infected with vesicular stomatitis virus encoding a GFP gene (VSV-GFP). The number of cells containing green fluorescent virus particles was quantified by flow cytometry. HT1080 cells were incubated overnight with the media conditioned by BMR cells at different stages of growth. HT1080 cells were then infected with VSV-GFP, and the presence of IFN was quantified by the ability of the conditioned media to halt VSV-GFP infection and reduce the number of GFP+ cells (Fig. 4A). The number of units of IFN-β was determined via a calibration curve built by adding human IFN to VSV-GFP–infected HT1080 cells (Fig. 4B). Young, growing BMR cultures did not release any detectable IFN, but just before the onset of CCD BMR cells secreted high levels of IFN-β (Fig. 4A). In the second assay, BMR-conditioned media was added to HEK-blue cells containing a β-galactosidase gene under an IFN-β–responsive promoter. Young, growing BMR cultures did not induce β-galactosidase above the control level, but conditioned media from the dying cultures showed a threefold elevated expression of the reporter gene. Notably, transfection with SV40 LT antigen that rescues CCD also abrogated IFN-β release (Fig. 4C).
Fig. 4.
CCD is triggered by release of IFN-β by BMR cells. (A) IFN release coincides with the onset of CCD. The growth curve for BMR cells is overlaid on the bar graph showing IFN levels in the media. IFN levels were measured by VSV-GFP assay. In this assay HT1080 cells are incubated with fresh media, or conditioned media containing INT-β; the cells are then infected with a GFP-encoding VSV, and the level of IFN in the test media corresponds to the reduction in the number of GFP positive HT1080 cells. (B) Calibration curve used to quantify the amount of BMR IFN in A. The curve was built by performing a VSV assay with known amounts of human INT-β. (C) IFN release by dying BMR cells determined by HEK-blue assay, which measured the induction of β-gal reporter in HEK cells under IFN-inducible promoter. Control indicates untreated HEK-blue cells. hINT-β are HEK-blue cells to which 300 U/mL of human IFN-β was added. Other bars show HEK-blue cells treated with BMR-conditioned media. (D) Conditioned media from dying BMR cells causes death of mouse cells. Media conditioned by BMR cells was added to growing BMR, mouse, or human fibroblasts. Cell death was quantified by Annexin-V/PI staining and flow cytometry. (E) Microscopic examination of CCD. Photographs of early passage mouse and BMR cells before and after addition of conditioned media from dying BMR cells. (Magnification: 100×.) Upon addition of the BMR conditioned media mouse cells display typical apoptosis with membrane blebbing, but BMR cells show predominantly necrotic cell death with cells detouching from the plate and breaking up without membrane bebbing.
Finally, we tested whether BMR-conditioned media from dying cells would induce CCD in young BMR cells or in cells for other species. Fresh media, media conditioned by young, growing BMR cells, and media conditioned by dying BMR cells were added to young BMR, mouse, or human cells. Media conditioned by dying BMR cells caused massive necrotic cell death of young BMR cells, and massive apoptotic death of mouse cells (Fig. 4D). Media conditioned by dying BMR cells did not cause massive death of human cells, which is likely explained by the divergence between rodent and human interferons. In summary, these results indicate that CCD is triggered by IFN-β release.
Discussion
In this study we show that cells of a cancer-resistant rodent, BMR, display an unusual cell death mechanism that rapidly wipes out entire cultures of cells, leaving no survivors. CCD occurs by necrosis and is triggered by the release of INF-β. This response required functional p53 and Rb pathways. When cells are cultured in vitro they are subjected to growth factors from FBS and are forced to proliferate because of frequent subculture at low cell density. These strong progrowth signals may be recognized by BMR cells as a potentially oncogenic signaling imbalance and trigger a cell death response. This mechanism resembles oncogene-induced senescence of human cells, where cells enter irreversible arrest in response to hyperproliferative signals, such as expression of activated oncogenes (16, 17). Another similarity with oncogene-induced senescence is that CCD of BMR cells is independent of telomere shortening and occurs in cells with long telomeres. We hypothesize that in vivo CCD efficiently clears away premalignant cells contributing to the cancer resistance of the BMR. Cell culture experiments revealed that a similar necrotic cell death takes place in primary BMR cell cultures upon extensive in vitro proliferation.
Strikingly, BMR cells use necrosis rather than apoptosis to oncogenic insults. This finding can be explained by the unusual sequence of BMR p53, which is deficient in activating an apoptosis cascade, and evolved as an adaptation to subterranean life under hypoxic conditions (12). Nevertheless, necrotic death of BMR cells requires a functional p53 pathway. Despite necrosis being commonly viewed as less precise or as an inefficient way of eliminating unwanted cells, BMRs have evolved a highly efficient antitumor mechanism based on necrotic response. An advantage of necrosis could be in eliminating all cells surrounding the premalignant lesion, which may provide an added antitumor effect by eliminating reactive tumor stroma, including tumor-activated fibroblasts (18).
In the future, it would be interesting to move these studies into in vivo system by testing whether CCD protects BMRs from chemically induced carcinogenesis. It would also be interesting to mix BMR cells with malignantly transformed mouse cells and test whether BMR cells suppress carcinogenesis in a xenograft mouse model.
Remarkably, cells of another cancer-resistant subterranean rodent, the naked mole rat, do not display CCD, and achieve high PDs in culture (11). Furthermore, naked mole rat cells easily undergo apoptosis and do not favor necrosis in response to stress (14). This finding is consistent with the naked mole rat p53 gene, which has arginine in position 174 (19), and is thus similar to the mouse or human p53 in its propensity to induce apoptosis. Naked mole rat cells display early contact inhibition that acts as an additional tumor suppressor mechanism in this species. In contrast BMR cells proliferate to high cell density. Thus, BMRs and naked mole rats have evolved two distinct anticancer mechanisms that provide remarkable cancer resistance to these small rodents.
Our earlier comparative studies of 15 rodent species suggested that small, long-lived rodents evolve novel anticancer mechanisms (10, 11). The current in-depth analysis of two small, long-lived species of subterranean rodents belonging to different superfamilies have shown that these mechanisms are distinct for each species, as they evolved phylogenetically independently in each species, possibly associated with hypoxic conditions underground and the species longevity. We speculate that many unique anticancer adaptations will be found in long-lived rodent species. This knowledge could then be used for treatment or prevention of cancer in humans.
Experimental Procedures
Cell Isolation and Culture.
Primary blind mole rat (BMR) and mouse fibroblasts were isolated from lung and under arm skin, as described previously (20). The experiments were performed on fibroblasts isolated from two animals of each BMR species: Spalax judaei and Spalax golani (2) and three mice Mus musculus. All BMR fibroblast isolates showed similar growth characteristics.
Normal human fibroblasts were HCA2 neonatal foreskin fibroblasts. Human Fibrosarcoma (HT-1080) cells were purchased from American Type Culture Collection (ATCC CCL-121).
Under standard conditions, all primary fibroblasts were cultured at 37 °C, 5% CO2, 3% O2, on treated polystyrene culture dishes (Corning) in EMEM media (ATCC) supplemented with 15% FBS (Gibco), nonessential amino acids, sodium pyruvate, 100 units/mL penicillin, and 100 μg/mL streptomycin (Gibco) or FGM-2 BulletKit (Lonza) supplemented with 15% FBS (Gibco).
For experiments with different types of media (Fig. 1B), BMR cells were cultured in different concentrations of FBS (0%, 2%, or 15%) and either EMEM media from ATCC or low-serum fibroblast growth media (FGM) from Lonza. FGM media was used with different concentrations of fibroblast growth factor. In addition, we unsuccessfully tried to grow BMR cells in low serum growth medium 106 (Gibco) supplemented with different concentrations of FBS (0%, 2%, or 15%) and with different concentrations of low serum growth supplements (Gibco).
We did not observe significant differences in the growth of BMR cells on different surfaces, including treated polystyrene culture dishes (Corning), BD PureCoat Amine (positively charged surface), BD PureCoat Carboxyl (negatively charged surface), collagen, fibronectin, gelatin, and glass.
Analysis of Cell Growth.
Cells were seeded at 5 × 105 cells per 100-mm dish. When cells reached 80% confluence, they were harvested, counted, and the number of population doublings was calculated.
Analysis of Telomeres.
Telomeric repeat amplification protocol and time-resolved fluorescence assays were performed as previously described (11).
Analysis of Cell Death.
Cell death was analyzed using Annexin-V-FLUOS assay kit (Roche) according to the manufacturer’s instructions. Briefly, floating and adherent cells were harvested, stained with Annexin-V and propidium iodide, and analyzed on a BD Biosciences FACS Canto flow cytometer.
Transfections.
Fibroblasts were seeded at 5 × 105 cells/100-mm plate 2 d before transfection. For transfection, cells were harvested, counted, and 106 cells were transfected with 5 μg of plasmid DNA using Amaxa Nucleofector II on program U-020 and solution NHDF (Amaxa). Transfections included a mock with no DNA, pSG5 Large T (Addgene 9053), pSG5 Large T K1 (Addgene 9055), pSG5 Large T∆434–444 (Addgene 9054), and no electroporation control.
IFN-β Assays.
Vesicular stomatitis virus encoding a GFP gene assay.
Vesicular stomatitis virus encoding a GFP gene (VSV-GFP) was previously described (21). It consists of VSV with GFP linked to the cytoplasmic domain of the VSV-G protein. The addition of GFP to VSV-G does not cause a reduction in titer, is stable through multiple passages, and is incorporated into virions with almost the same efficiency as wild-type VSV-G. The virus was expanded as follows: 200 µL of the virus was added onto 1.5 × 106 HeLa cells plated 2 d earlier; 48 d after infection, media with the virus was collected, filtered, and stored at −80 °C. The titer of the virus was determined for individual cell lines using standard plaque assay. Aliquots of VSV stock were stored at −80 °C until use.
For the experiments, the viruses were thawed slowly on ice and diluted in EMEM medium to obtain a working stock of 100 pfu/µL. VSV was used according to bio-safety procedures in a P2-level safety facility/room. The use of VSV-GFP in this project was reviewed and approved by the Institutional Biosafety Committee at the University of Rochester.
For the assays, media conditioned with BMRs at different stages of growth, mouse, or human HCA2 fibroblasts were collected after 3–5 d of growing cells, spinned down to remove debris, and stored at +4 °C. For the VSV-GFP infection we used HT1080 cells, that were seeded at a density of 60,000 cells per well in six-well plates in EMEM with 15% FBS and incubated for 24 h. Then, the media was discarded and the conditioned media was added to each well. In addition, we used human IFN-β human (100 U per well) as a positive control and human IFN-γ (100 U per well) as a negative control. After 3–4 h of incubation in conditioned media, cells were infected with VSV-GFP at a multiplicity of infection 0.1 or 1 for a 12–16 h period. GFP signal appears in infected cells after 12–16 h, and the cells die about 6 h later. FACS analysis was performed 16 h after infection to quantify GFP+ cells.
HEK-Blue cells assay.
HEK-Blue IFN-α/β cells (Invivogen) allow the detection of bioactive human type I IFNs by monitoring the activation of the ISGF3 pathway. The assay was performed according to the manufactured protocol (Invivogen). Briefly, HEK-Blue cells were plated in 96-well plates and incubated overnight. Then, 20 μL of test medium was added to each well. Human IFN-β was used as a positive control and human IFN-γ was used as a negative control. Cells were incubated overnight. The next day, 30 μL of the supernatant from Hek-Blue cells was added to 170 μL of Quanti-Blue Reagent (Invivogen) for 3 h at 37 °C. The colorimetric reaction was measured at 650 nm on a plate reader.
Acknowledgments
This work was supported by grants from the National Institutes of Health and Ellison Medical Foundation (to V.G.); an Ellison Medical Foundation grant (to A.S.); and the Ancell-Teicher Research Foundation of Genetics and Molecular Evolution (E.N.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Nevo E. Mosaic Evolution of Subterranean Mammals: Regression, Progression, and Global Convergence. London: Oxford Univ Press; 1999. [Google Scholar]
- 2.Nevo E, Ivanitskaya I, Beiles A. Adaptive Radiation of Blind Subterranian Mole Rats. Leiden, The Netherlands: Backhuys; 2001. [Google Scholar]
- 3.Edrey YH, et al. Sustained high levels of neuregulin-1 in the longest-lived rodents; A key determinant of rodent longevity. Aging Cell. 2012;11(2):213–222. doi: 10.1111/j.1474-9726.2011.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turturro A, et al. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 5.de Magalhães JP, Costa J, Toussaint O. HAGR: The Human Ageing Genomic Resources. Nucleic Acids Res. 2005;33(Database issue):D537–D543. doi: 10.1093/nar/gki017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heron M. Deaths: Leading Causes for 2008, National Vital Statistics Reports. 2012;60(6):9–11. [PubMed] [Google Scholar]
- 7.Lipman R, Galecki A, Burke DT, Miller RA. Genetic loci that influence cause of death in a heterogeneous mouse stock. J Gerontol A Biol Sci Med Sci. 2004;59(10):977–983. doi: 10.1093/gerona/59.10.B977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burek JD, Hollander CF. Incidence patterns of spontaneous tumors in BN/Bi rats. J Natl Cancer Inst. 1977;58(1):99–105. doi: 10.1093/jnci/58.1.99. [DOI] [PubMed] [Google Scholar]
- 9.Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention: Fundamental differences in human and mouse telomere biology. Nat Med. 2000;6(8):849–851. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]
- 10.Seluanov A, et al. Telomerase activity coevolves with body mass not lifespan. Aging Cell. 2007;6(1):45–52. doi: 10.1111/j.1474-9726.2006.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seluanov A, et al. Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan. Aging Cell. 2008;7(6):813–823. doi: 10.1111/j.1474-9726.2008.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashur-Fabian O, et al. Evolution of p53 in hypoxia-stressed Spalax mimics human tumor mutation. Proc Natl Acad Sci USA. 2004;101(33):12236–12241. doi: 10.1073/pnas.0404998101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu G, et al. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet. 2004;36(1):63–68. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- 14.Seluanov A, et al. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc Natl Acad Sci USA. 2009;106(46):19207–19208. doi: 10.1073/pnas.0905252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn WC, et al. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol Cell Biol. 2002;22(7):2111–2123. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Micco R, Fumagalli M, d’Adda di Fagagna F. Breaking news: High-speed race ends in arrest—How oncogenes induce senescence. Trends Cell Biol. 2007;17(11):529–536. doi: 10.1016/j.tcb.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 18.Mueller MM, Fusenig NE. Friends or foes—Bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4(11):839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 19.Kim EB, et al. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature. 2011;479(7372):223–227. doi: 10.1038/nature10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seluanov A, Vaidya A, Gorbunova V. Establishing primary adult fibroblast cultures from rodents. J Vis Exp. 2010 doi: 10.3791/2033. (44)pii:2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz RM. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67(6):2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]