Abstract
The paired male accessory glands of Drosophila melanogaster enhance sperm function, stimulate egg production, and reduce female receptivity to other males by releasing a complex mixture of glycoproteins from a secretory epithelium into seminal fluid. A small subpopulation of about 40 specialized secretory cells, called secondary cells, resides at the distal tip of each gland. We show that these cells grow via mechanisms promoted by mating. If aging males mate repeatedly, a subset of these cells delaminates from and migrates along the apical surface of the glandular epithelium toward the proximal end of the gland. Remarkably, these secretory cells can transfer to females with sperm during mating. The frequency of this event increases with age, so that more than 50% of triple-mated, 18-d-old males transfer secondary cells to females. Bone morphogenetic protein signaling specifically in secondary cells is needed to drive all of these processes and is required for the accessory gland to produce its normal effects on female postmating behavior in multiply mated males. We conclude that secondary cells are secretory cells with unusual migratory properties that can allow them to be transferred to females, and that these properties are a consequence of signaling that is required for secondary cells to maintain their normal reproductive functions as males age and mate.
Keywords: reproduction, insect, BMP, prostate
Secretory glands of the male reproductive system play a unique role in higher organisms, modulating germ-cell activities and secreting components of the seminal fluid that ultimately function in females. In the fruit fly, Drosophila melanogaster, the paired accessory glands promote normal sperm storage and utilization, and influence female postmating behaviors, increasing egg production and reducing subsequent receptivity to males (1). The glands secrete proteases, antiproteases, other glycoproteins, and cellular material into seminal fluid via mechanisms that have not been fully characterized (2–4).
Previous studies have demonstrated that sex peptide, a secretory product of the accessory gland, promotes egg laying, reduces the receptivity of females to remating, and is required for efficient release of sperm from female storage organs (5–7). However, other accessory gland products are needed for the long-term maintenance of sex peptide-mediated functions (8), and additional sex peptide-independent factors are also involved (9, 10).
Each accessory gland consists of a single-layer epithelium of binucleate cells surrounded by a muscular sheath. Although most of the epithelium consists of so-called main cells, which secrete key accessory gland proteins like sex peptide (6), it also includes about 40 distally located, distinctively rounded secondary cells that contain large secretory vacuoles (2, 11).
Here we show that adult secondary cells grow preferentially compared with main cells, a process accelerated by mating. Unexpectedly, in multiply mated males, some of these secretory cells delaminate apically from the epithelium, migrate within the gland, and can be transferred to females upon mating, processes that are enhanced with age. We demonstrate that all these events are driven by bone morphogenetic protein (BMP) signaling in secondary cells, and that this signaling is essential in multiply mated males for the accessory gland to properly suppress female receptivity to remating. Our data suggest that the overall strategy by which the accessory glands perform their reproductive functions is genetically programmed to change in response to aging and mating history, potentially to optimize reproductive success.
Results
Secondary Cells Grow and Delaminate in an Age- and Mating-Dependent Fashion.
To establish specific tools to test secondary cell function, we screened multiple GAL4 transcriptional drivers (12). Driver lines were crossed to animals carrying a transgene encoding a nuclear-localized GFP (GFPnls) under the transcriptional control of upstream activation sequences (UAS), the binding sites for GAL4. An esg-GAL4 line (13, 14) was strongly expressed by secondary cells in accessory glands of newly eclosed adults (Fig. S1). A small proportion of main cells expressed esg-GAL4, although only during the first day of adulthood. Expression in secondary cells also diminished with time, so few—if any—cells expressed GFP by 6 d (Fig. S1). Jiang et al. (15) have developed a system, the esgts Flp-Out (F/O) system, to study adult epithelia where the esg-GAL4 driver is combined with a ubiquitously expressed, temperature-sensitive form of the GAL4 inhibitor GAL80 (tub-GAL80ts) (16) and an interrupted copy of an actin-GAL4 construct, which is activated by UAS-FLP–induced recombination. When males were shifted from 18 °C to 28.5 °C within 1 d of eclosion, esg-GAL4–regulated, FLP-induced events occurred in all secondary cells, but not main cells, activating the actin-GAL4 construct, and secondary cell-specific actin-GAL4–driven expression was then maintained throughout adulthood (Fig. 1A and Fig. S2A).
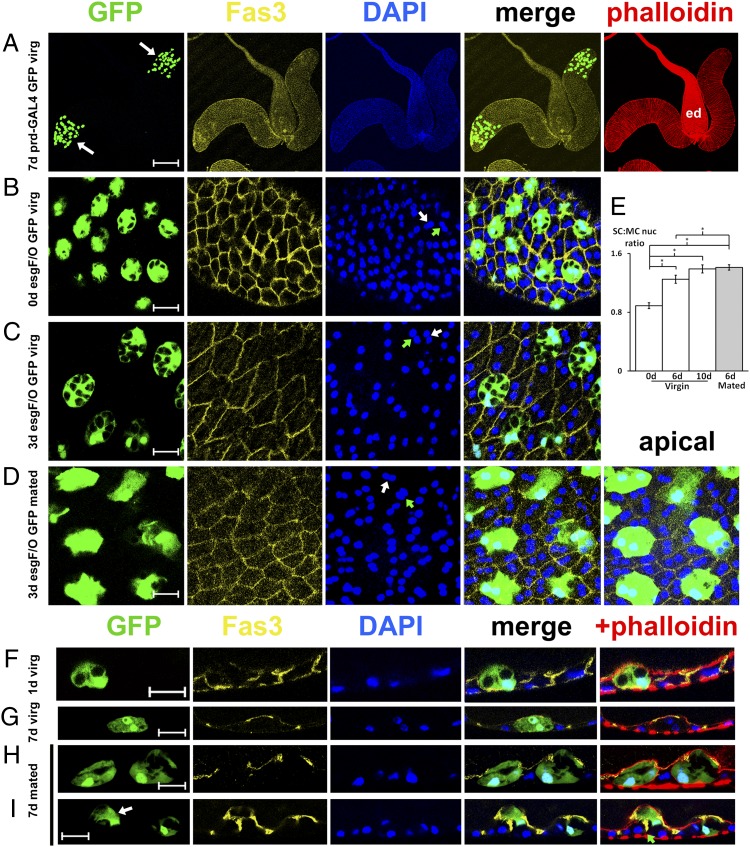
Fig. 1.
Growth and delamination of adult secondary cells is stimulated by mating. (A) Two accessory glands and ejaculatory duct (ed) from a 7-d-old virgin male expressing nuclear GFP (green; arrows) in secondary cells at the distal tips of the glands under the control of prd-mf5.4-GAL4. Preparations were stained with TRITC-phalloidin (F-actin, in red), DAPI (binucleate staining in blue), and an antibody to the septate junction protein Fas3 (yellow). (B–E) Epithelial layer from the distal tip of an accessory gland of esgts F/O UAS-GFPnls virgin males at eclosion (B) and after 3 d of adulthood (C), and of 3-d-old males of the same genotype after mating to multiple females (D). The size of both nuclei in each secondary cell (green arrows) increases with age and mating relative to surrounding main cell nuclei (white arrows; data in E, ± 2× SEM). (F–I) Transverse confocal sections of the distal tips of accessory glands from virgin (G) and mated (H and I) 7-d-old males, and a 1-d-old virgin (F) expressing nuclear GFP (green) under esgts F/O control and stained as in A. Mating induces selective growth and in some cases delamination (white arrow in I; this cell does not touch the underlying basal lamina, marked in right panel by a green arrow) of secondary cells from the apical surface of the epithelium. For E, *P < 0.001, n = 5 glands, one-way ANOVA. [Scale bars: 200 μm (A), 20 μm (B–D), and 15 μm (F–I).] Images in these and all panels in Figs. 3 and 4 are representative of glands from at least 10 males of the same age and genotype.
Using this system to distinguish secondary cells and marking apico-lateral cell outlines with the septate junction protein, Fasciclin 3 (Fas3) (17), we observed that the size of secondary cells and their nuclei relative to main cells increased over time in virgin males (Fig. 1 B and C, and Fig. S2A). Unlike main cells, which became more squamous with age, secondary cells retained their apical-basal height and bulged into the glandular lumen, while still contacting the basement membrane (compare Fig. 1G with Fig. 1F). Much of the apical surface of each cell was shrouded by a thin main-cell covering (Fig. S2G). Because the nuclei of secondary cells and surrounding main cells remain roughly spherical in all genotypes studied, we compared nuclear area for these two cell types. There was a significant increase in both the absolute size (Fig. S3A) and the relative size of secondary cell nuclei with age (Fig. 1E) (P < 0.01, n = 5 glands, one-way ANOVA), reflecting the change in cell size. Main cell nuclear size remained unaltered (Fig. S3B). Flies fed with the nucleotide analog bromodeoxyuridine did not incorporate this molecule in secondary cell nuclei (Fig. S4), indicating that the nuclear size increase was not associated with global DNA replication. Some other cell types in flies also exhibit nuclear growth in the absence of DNA replication (18).
As previously reported (19), when flies were mated with multiple females, the epithelial monolayer became more cuboidal and densely packed (Fig. 1D and Fig. S2). Surprisingly, the growth of the secondary cells but not main cells, as measured by nuclear size, was accelerated (Fig. 1 E and H, and Fig. S3). Furthermore, some secondary cells, but not main cells, reduced contact with the basement membrane and started to delaminate apically (Fig. 1I and Fig. S2E). Specific proteins involved in apico-lateral cell–cell adhesion, like Fas3 (Fig. 1G), continued to be localized at cell–cell junctions in delaminating secondary cells (Fig. 1 H and I), suggesting that the epithelium was not compromised during delamination. Actin-GAL4–induced secondary cell-specific GFP expression was also consistently elevated by mating (Fig. 1D and Fig. S2C), indicating that transcription of selected cytoskeletal proteins is also up-regulated by mating. GFP, which was expressed in the nucleus and cytosol of secondary cells, but not the secretory vacuoles, was occasionally observed in the glandular lumen (5 of 25 multiply mated 6-d-old males), sometimes in discrete structures (Fig. S2F). This finding is consistent with previous observations that the accessory glands of Drosophila and other insects secrete intracellular contents, potentially via holocrine or apocrine mechanisms (3, 20).
Some Secondary Cells from Multiply Mated Males Migrate Proximally and Can Be Transferred to Females During Mating.
Remarkably, we observed proximally located secondary cells in a small proportion of flies aged between 3 and 10 d that had mated to multiple females (8 of 200 males) (Fig. 2A), but not in virgin males. These cells were not free-floating, but contacted the apical epithelial surface (Fig. 2 B–E). Like other migratory cells (21), they asymmetrically accumulated F-actin at their surface in lamellipodia-like structures (Fig. 2 B, D, and E).
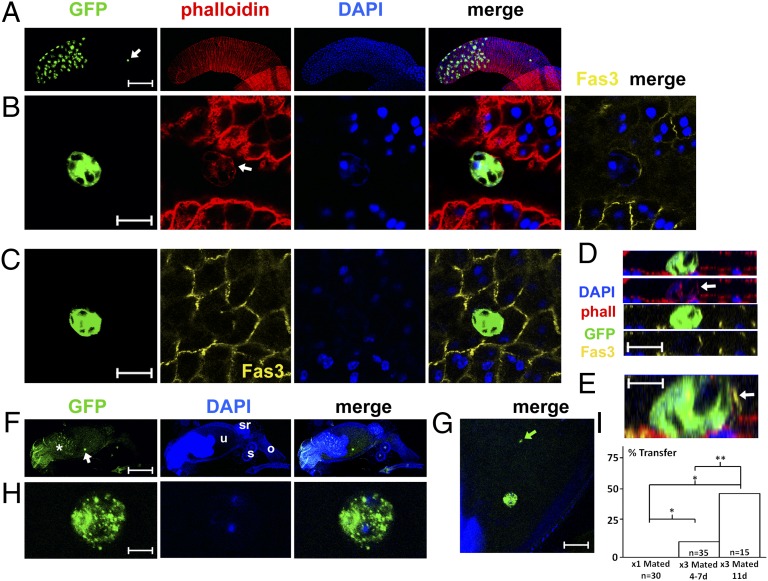
Fig. 2.
Secondary cells occasionally migrate in mated flies and can transfer to females. (A–E) Confocal images show an example of a secondary cell (arrow in A) that has delaminated apically and migrated proximally along the glandular epithelium. Gland is from a multiply mated 3-d-old male expressing a Rab5-GFP fusion protein (green) under the control of esgts F/O and stained with TRITC-phalloidin (red), DAPI (blue), and an antibody to the septate junction protein Fas3 (yellow). (B) High-magnification view showing the apical surface of this migrating cell with F-actin at one of its edges (arrow). (C) The basal part of the cell is embedded in the epithelium. (D and E) Confocal z-sections reveal one edge of the cell contains higher concentrations of actin and Fas3 (arrows; epithelial surface at bottom of panels). (F–H) Dissected reproductive tract from a wild-type female, shortly after mating with an 18-d-old male, previously mated at 11 and 14 d, expressing GFP in secondary cells. At this stage, delaminated secondary cells frequently express GFP in punctate structures within the cytoplasm (see also Fig. S7C). A transferred binucleate secondary cell is marked with a white arrow in F and shown at high magnification in H. Some GFP+ cell debris is also highlighted (green arrow) in G. (I) Percentage of females containing transferred secondary cells after mating with virgin 4- to 7-d-old males (1×) or previously doubly mated males (3×), first mated at 4–7 d or 11 d (latter equivalent to F–H). *P < 0.001, **P = 0.026, two-tailed Fisher’s exact test. In F, *, autofluorescent mating plug; o, oviduct; s, pair of spermathecae; sr, seminal receptacle; and u, uterus. [Scale bars: 200 μm (A and F), 15 μm (B–D), 5 μm (E and H), and 20 μm (G).]
To test whether these cells are transferred to females upon mating, we used males of different ages to set up repeated single pair matings, separated by 3 or 4 d to allow secondary cells to delaminate between matings. We dissected the reproductive organs of females within 1 h of the completion of the final mating. We never observed transfer of secondary cells from males after a single mating (n > 10 for each age, matings at 1, 4, 7, and 11 d). However, 4- and 7-d-old males, which were mated three separate times over a period of 1 wk, transferred one or more GFP+ secondary cells to females during the final mating in a significant proportion of cases (4 of 35 females) (Fig. 2 F–H and Fig. 2I). Even more females from a third mating with males mated at 11, 14, and 18 d contained transferred secondary cells (7 of 15 females) (Fig. 2I). During the process of mating, other secondary cells may break into fragments, because small GFP+ structures were frequently observed in females mated with older males (15 of 15 females from the third mating of 18-d-old males) (Fig. 2G).
These data suggest that secondary cells have delaminated in many previously mated 18-d-old males. Indeed, we found that 10 of 10 glands from males of this age contained one or more proximally located secondary cells in their accessory glands, when they were dissected rather than mated for a third time. Presumably, the low frequency of migration that we observed in 6-d-old multiply mated males (8 of 200) is explained by the fact that these cells are transferred upon each mating and it then takes several days for new secondary cells to delaminate and migrate proximally.
We did not observe secondary cells or their remnants within the two female sperm storage structures, the spermathecae and the seminal receptacle, which begin to accumulate sperm within 5 min of the start of mating (22). Transferred secondary cells are therefore likely to be expelled by the female when she ejects the seminal fluid upon egg laying about 90 min after mating. However, if these cells play an active role in accessory gland function, this role could be complete by this time, because the long-term effects of mating, which require sex peptide, have been fully initiated before egg laying (23). Overall, we conclude that all secondary cells grow as adult males age, a process accelerated by mating, and that a subset of these cells delaminates, migrates proximally along the glandular epithelium and can be transferred to females after multiple matings, particularly in aging males.
BMP Signaling Drives Growth, Delamination, and Migration of Secondary Cells.
To determine what signals might regulate the unusual cellular behaviors of secondary cells, we overexpressed activating and inactivating components of several different signaling cascades within these cells in virgin males from eclosion onwards using the esgts F/O system. Hyperactivation of the Ras/ERK MAPK and PI3K/Akt pathways, which are frequently involved in growth regulation (24, 25), produced no obvious changes in cell or nuclear size (Fig. S5 A and B). However, expression of an activated form of the BMP type I receptor Thick veins (Tkv) (26) strongly stimulated cellular and nuclear growth (compare Fig. 3 A and B; Fig. 3E and Fig. S5 D and E), increased actin-GAL4 UAS-GFP expression, led to GFP accumulation in the lumen of some glands (Fig. S6C), and induced delamination of a subset of secondary cells in virgin males (Fig. 3D and Fig. S6B versus Fig. 3C and Fig. S6A), phenocopying changes normally seen only in mated flies. By shifting temperature after 3 d of adulthood, it was possible to up-regulate BMP signaling in a random subset of secondary cells, which grew faster than normal secondary cells (Fig. S7A), showing that this effect is cell-autonomous. Growth stimulation appears cell type-specific, because up-regulation of BMP signaling in adult main cells did not affect their cell or nuclear size (Fig. S5C).
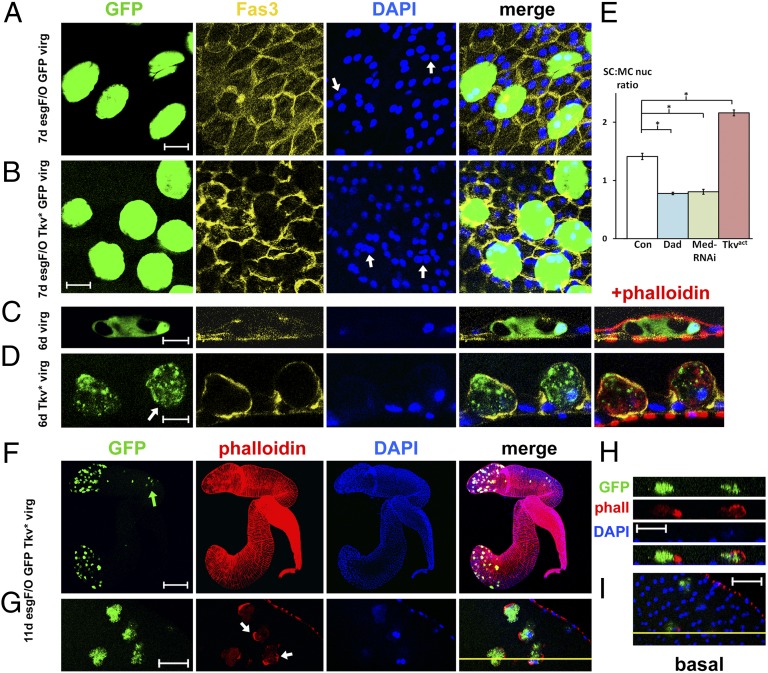
Fig. 3.
BMP signaling promotes growth, delamination and migration of secondary cells. (A–D) Images of accessory glands from 6- to 7-d-old virgin males expressing nuclear GFP with (B and D) or without (A and C) an activated form of the BMP type I receptor Tkv (Tkv*) under the control of esgts F/O after a temperature shift at eclosion. Preparations were stained with TRITC-phalloidin (red), DAPI (blue), and an antibody to the septate junction protein Fas3 (yellow). Secondary cells with increased BMP signaling are enlarged and have much larger nuclei than their neighbors (compare nuclei marked by arrows in B vs. A); some cells detach from the basement membrane (cell marked in D is almost completely detached). (E) Relative size (± 2× SEM) of secondary cell nuclei versus surrounding main cell nuclei was determined for 6-d-old normal males and males with altered BMP signaling in secondary cells, expressing activated Tkv, Dad, or an RNAi against Med. *P < 0.001, n = 5 glands, one-way ANOVA. (F–I) A subset of adult secondary cells overexpressing activated Tkv under esgts F/O control in an 11-d-old virgin male delaminates and migrates proximally (green arrow). (G–I) High-magnification view of the gland marked in F, showing asymmetric actin accumulation at the cell surface in apical section (arrows in G) and basal contact with the glandular epithelium (I) [see also confocal z-section (H) along the line drawn in G and I; the epithelium is at the bottom of each panel in H). [Scale bars: 20 μm (A and B), 10 μm (C and D), 200 μm (F), and 25 μm (G–I).]
If males initiating expression of activated Tkv in secondary cells immediately after eclosion were aged for 11–13 d, 9.2 ± 2.6 of these cells per animal (n = 6) fully delaminated and moved more proximally in the gland (Fig. 3F and Fig. S6C), a phenotype never seen in normal virgin flies (Fig. S6D). These cells maintained contact with the epithelium, exhibited one or more lamellipodia-like structures (Fig. 3 G–I), and most of them migrated to the proximal third of the gland (Fig. 3F and Fig. S6C), suggesting that the movement is directional.
We tested whether BMP signaling is normally involved in secondary cell function by overexpressing the BMP-regulated transcriptional repressor Dad (27) in newly eclosed males under esgts F/O control. Dad-expressing secondary cells in 6-d-old virgin males were smaller than main cells, and their nuclei showed a significant 40% reduction in size compared with non-Dad–expressing controls (P < 0.001, n = 5, one-way ANOVA) (Figs. 3E, 4B, and Fig. S5D). Indeed, there was no increase in nuclear size compared with newly eclosed males, suggesting that all or most post-eclosion growth requires BMP signaling. A similar phenotype was observed when transcripts from the Medea (Med) gene, encoding the co-Smad transcription factor required for BMP signaling (28), were knocked down with a UAS-RNAi construct (Fig. 3E and Fig. S5D). Dad-expressing secondary cells in mated males not only failed to grow normally (Fig. S7 D vs. B), but also did not delaminate (Fig. 4D and Fig. S7E) or transfer to females, even in 18-d-old triply mated males (0 of 15 males), demonstrating that BMP signaling is required for all these specialized processes.
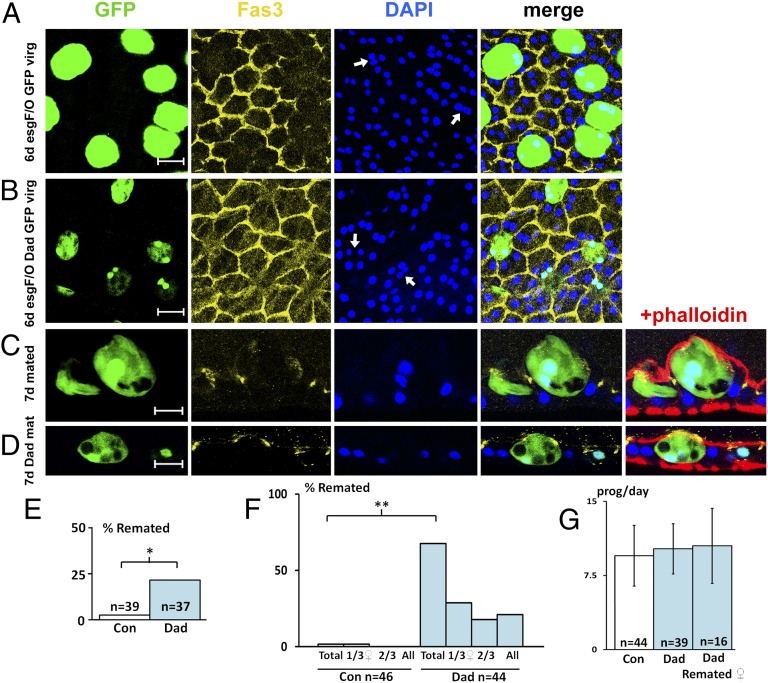
Fig. 4.
BMP signaling is required for normal secondary cell growth and mating-induced delamination. Images of accessory glands from 6- to 7-d-old virgin (A and B) or multiply mated (C and D) males expressing nuclear GFP with (B and D) or without (A and C) the BMP transcriptional repressor Dad under the control of esgts F/O after a temperature shift at eclosion. Preparations were stained with TRITC-phalloidin (red), DAPI (blue), and an antibody to the septate junction protein Fas3 (yellow). (A and B) Secondary cells with reduced BMP signaling are smaller and have smaller nuclei than their neighbors (compare nuclei marked by arrows in B vs. A) (Fig. 3E). (C and D) Transverse sections of multiply-mated males. (E) Percentage of females remating 4 d after mating with a 1- to 4-d-old control virgin male or a male expressing Dad in its secondary cells. (F) Percentage of Dad-expressing and control multiply-mated males that fail to protect three females from remating 3 d later (marked Total): chart also shows how many of these males failed to protect one of three, two of three, or all of these females. (G) Rate of progeny production by females treated as in F during the 2 d after the first mating to control or Dad-expressing multiply mated males. Column on right presents data for females that remated. *P < 0.01, **P < 0.001, two-tailed Fisher’s exact test. Mann–Whitney analysis showed no significant difference in G. [Scale bars: 20 μm (A and B) and 15 μm (C and D).]
To determine whether the activity of the BMP signaling cascade is altered by mating, we used a Dad-GFP transcriptional reporter as a signaling read-out (29). Dad transcription is normally controlled by BMP signaling. BMP signaling appeared elevated in secondary cells (Fig. S7 F and G), but there was no obvious increase in signaling after mating, suggesting either that mating-dependent changes are too transient to be detected with this reporter, or that other events in addition to BMP signaling, such as contraction of the surrounding muscular sheath, normally contribute to the altered behavior of secondary cells upon mating.
BMP Signaling in Secondary Cells Is Required to Fully Modulate Female Postmating Behavior in Multiply Mated Males.
We hypothesized that reduced BMP signaling might affect the normal function of secondary cells. To test this theory, adult males either expressing GFP alone or GFP and Dad in these cells under esgts F/O control were initially mated up to three times in single-pair matings. We focused on young males (up to 4-d-old at first mating), because at least 10% of older control males (7- to 11-d-old) failed to block female receptivity to males after mating. We did not observe any significant change in the level of egg laying or fecundity in females mated with males expressing Dad in their secondary cells versus esgts F/O controls (Fig. S8 A and B). However, significantly more Dad-expressing males compared with control males (8 of 37 vs. 1 of 39; P < 0.01; two-tailed Fisher’s exact test) (Fig. 4E) were unable to fully prevent females from remating 4 d later during a 3-h period.
We reasoned that a primary role of BMP signaling in adult secondary cells might be to maintain accessory gland function when males mate repeatedly. Indeed, whereas almost all multiply mated, 4-d-old control males were able in subsequent matings to block the receptivity of a further three females to remating (98%; 45 of 46 males), significantly fewer males expressing Dad in adult secondary cells (39%; 16 of 44 males; P < 0.001) were able to do this (Fig. 4F). During the second round of matings, there was no significant difference either in the length of time that males mated (Fig. S8D) or in the number of males that remated within 40 min (Fig. S8 E and F) for Dad-expressing vs. control males, suggesting that the effects on female postmating behavior were not a result of altered male-mating behavior. Furthermore, the fecundity of males in this second round of matings was unaffected by expression of Dad in secondary cells (Fig. 4G and Fig. S8C), even for those matings in which females subsequently remated (right column in Fig. 4G), demonstrating that there was not a general defect in fertility. Our data therefore indicate that secondary cells play a selective role in modulating postmating female behavior, and that persistent BMP signaling is required in these cells to maintain this function, particularly in males that have mated multiple times over a short period.
Discussion
We have shown that the secondary cells of the fly accessory gland selectively grow during aging in adults, a process enhanced by repeated mating. These cells exhibit a range of behaviors, induced by mating, that are atypical of secretory cells in glands, including active delamination and migration. Although migrating cells were initially observed in less than 5% of repeatedly mated males, introducing a delay between two previous matings and dissecting the resulting 18-d-old males revealed migrating cells in all animals, suggesting that this process is common in aged, mated animals.
The growth, delamination and migratory activities of secondary cells all require cell-autonomous BMP signaling. One or more of these BMP-regulated processes modulates long-term, postmating behavior in females, particularly when males are repeatedly mated over short periods of time, requiring rapid replenishment of luminal content in the accessory gland. Although the numbers of vacuoles in secondary cells with high levels of BMP signaling seem more variable than controls, vacuole number in Dad-expressing secondary cells appears relatively normal, suggesting that reduced BMP signaling does not simply block the general secretory machinery. However, reduced signaling presumably affects the synthesis or function of one or more secondary cell products, leading either to direct effects in mated females or to indirect effects through modulation of main cell function or products in males.
Unexpectedly, some secondary cells are transferred to females after multiple matings, particularly in aged flies, raising the possibility that these delaminating cells continue to function together with sperm even outside the male. Transfer is not essential for these cells to mediate their BMP-regulated effects in females, because not all mated females receive these cells. However, it is possible that transfer could contribute to changes in accessory gland function as the glandular epithelium undergoes BMP-dependent structural alterations during aging and mating. A recent study from Minami et al. (30) indicates that secondary cells are required for normal male fecundity and effects on female postmating behaviors. Our work now clearly demonstrates that BMP-mediated events in secondary cells are involved in maintaining these latter functions specifically during adulthood.
Our data highlight some surprising parallels between the accessory gland and the prostate, in addition to those previously reported (2). Like the prostate, the structure of the accessory gland epithelium changes significantly with age. Furthermore, BMP signaling is implicated in normal prostate development (31) and in the progression of prostate cancer (32–34). Importantly, prostate cells have been identified in human semen and the phenotype of these cells may be altered in prostate cancer (35, 36). Although many of these cells are likely to have sloughed off from the epithelium, our study raises the possibility that some actively delaminate into seminal fluid.
We have found that the secondary cells of the accessory gland require BMP signaling to regulate the synthesis or function of one or more important components of the seminal fluid as flies age and mate. However, this signaling simultaneously drives cell loss and changes in the morphology and function of the epithelium, which appears to lack regenerative capacity in flies. The prostate gland of most human males over 50 y of age is hyperplastic (37), and it is tempting to speculate that this reflects a regenerative response to similar events in this organ. A more detailed analysis of secondary cell biology should help to further elucidate the processes that underlie functional changes in the accessory gland epithelium and test whether these are shared by male reproductive glands in other organisms.
Materials and Methods
Fly Strains and Culture.
The following fly strains were used: esgts F/O = w; esg-GAL4, UAS-GFPnls; act > CD2 > GAL4, UAS-FLP and w; esg-GAL4, UAS-GFPnls (15), prd-mf5.4-GAL4 (38), Acp26Aa-GAL4 (6), UAS-TkvQD (26), UAS-Dad (27), Dad-GFP (29), and UAS-Rab5-GFP (39). UAS-Med-IR (JF02218) and other fly stocks were obtained from the Bloomington Drosophila Stock Centre. Flies were fed on standard cornmeal agar medium. No dried yeast was added to the vials.
Fly Genetics.
When specific expression of UAS-transgenes in adult secondary cells under esgts F/O control was required, fly crosses were initially cultured at the nonpermissive temperature, 18 °C, using a cross between esgts F/O and w1118 flies as a control (all test and control males have wild-type eye color). Each morning, newly eclosed males of the appropriate genotype were separated from females and for standard experiments, transferred to 28.5 °C immediately. GFP marks all secondary cells that activate act-GAL4, but relative expression levels do not reflect levels of expression of other transgenes (Fig. S5F). Size phenotypes were typically consistent in all GFP-expressing secondary cells. Flies carrying other GAL4 drivers were maintained at 28.5 °C for comparability.
For single-pair matings, individual males were added to a normal 1- to 5-d-old virgin w1118 female (for a specific group of control and test flies, females were of identical age), and mating was observed. The flies were separated after mating and females cultured at 25 °C for 4 d, transferring them to new vials each day, so eggs and progeny could be counted. Each female was then introduced to a dominantly marked white-eyed male and left for 3 h to test if it remated. Any progeny from this second mating could be easily distinguished, because all or most of the progeny from the first cross were red-eyed.
Multiply mated males for staining experiments were generated by placing a newly eclosed male with at least six virgin females and leaving the cross for 3 or 6 d at 28.5 °C. For multiple-mating experiments to test effects on female receptivity, individual 3-d-old virgin males either expressing GFP alone or GFP and Dad under esgts F/O control were mated to a group of five virgin females overnight. These males were then separated and introduced to a further three virgin females for 24 h, observing mating during the first 3 h. Females from the first cross were individually separated into new vials to check for mating. To assay receptivity to remating, the three females from the second round of mating were separated into individual vials for 3 d (to check they had mated and count progeny), before transferring to a new vial and adding two dominantly marked white-eyed males to test if the females remated over a 3-h period.
For BrdU labeling, adult male flies were placed in vials containing yeast paste supplemented with 5 mg/mL BrdU (40).
Immunohistochemistry and Imaging.
Flies were anesthetized using CO2 and dissected with forceps in 4% paraformaldehyde dissolved in PBS. Dissected accessory glands were transferred to Eppendorf tubes, fixed for a total of 20–30 min at 22 °C, and then washed 6 × 10 min in 1 mL PBST [PBS, 0.03% Triton X-100 (Sigma-Aldrich)]. Standard procedures (detailed in SI Materials and Methods) were used for staining. Anti-Fas3 (41) (Developmental Studies Hybridoma Bank) and anti-BrdU (Developmental Studies Hybridoma Bank) monoclonal supernatants were diluted 1:10 in PBSTG; 50–100 μL of diluted antibody were incubated with each preparation overnight.
Glands were imaged using a Zeiss Axioplan 2 confocal microscope with a LSM 510 laser module and z-sections processed using LSM software. Nuclear areas were measured using Axiovision software (Zeiss). For each genotype, one accessory gland from five different males was analyzed. For each gland, the areas of both nuclei from three different secondary cells, which were strongly expressing GFP, and from three main cells adjacent to different secondary cells, were measured, averaged for each cell, and the absolute size mean for each gland calculated. To assess relative size, each secondary cell value was divided by the average area of four neighboring main cell nuclei, and the mean relative size for each gland calculated.
Statistical Analysis.
Frequencies of secondary cell transfer and remating were compared using a two-tailed Fisher’s exact test. All nuclear size data were shown to be normally distributed by the Shapiro–Wilk test, and therefore analyzed using one-way ANOVA and subsequently applying Bonferroni’s correction. For egg laying, mating duration and progeny count data, which were nonparametric, a Mann–Whitney analysis was applied.
Supplementary Material
Acknowledgments
We thank Bruce Edgar, Marcus Noll, Tom Neufeld, Stephen Goodwin, Marianna Wolfner, Daimark Bennett, Enrique Martin-Blanco, and the Bloomington Drosophila Stock Centre for providing Drosophila stocks, and the Developmental Studies Hybridoma Bank at Iowa for monoclonal antibodies. We also thank Stuart Wigby for helpful comments. This study was funded in part by Cancer Research-UK and a Urology Foundation Scholarship (to A.L.); the Wellcome Trust (L.C.); and the John Fell Fund, Oxford (C.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214517109/-/DCSupplemental.
References
- 1.Wolfner MF. Tokens of love: Functions and regulation of Drosophila male accessory gland products. Insect Biochem Mol Biol. 1997;27(3):179–192. doi: 10.1016/s0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]
- 2.Rylett CM, Walker MJ, Howell GJ, Shirras AD, Isaac RE. Male accessory glands of Drosophila melanogaster make a secreted angiotensin I-converting enzyme (ANCE), suggesting a role for the peptide-processing enzyme in seminal fluid. J Exp Biol. 2007;210(Pt 20):3601–3606. doi: 10.1242/jeb.009035. [DOI] [PubMed] [Google Scholar]
- 3.Walker MJ, et al. Proteomic identification of Drosophila melanogaster male accessory gland proteins, including a pro-cathepsin and a soluble gamma-glutamyl transpeptidase. Proteome Sci. 2006;4:9. doi: 10.1186/1477-5956-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kravets FG, et al. Prostasomes: Current concepts. Prostate. 2000;43(3):169–174. doi: 10.1002/(sici)1097-0045(20000515)43:3<169::aid-pros2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Chen PS, et al. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 1988;54(3):291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- 6.Chapman T, et al. The sex peptide of Drosophila melanogaster: Female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci USA. 2003;100(17):9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avila FW, Ravi Ram K, Bloch Qazi MC, Wolfner MF. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics. 2010;186(2):595–600. doi: 10.1534/genetics.110.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ram KR, Wolfner MF. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc Natl Acad Sci USA. 2009;106(36):15384–15389. doi: 10.1073/pnas.0902923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heifetz Y, Lung O, Frongillo EA, Jr, Wolfner MF. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr Biol. 2000;10(2):99–102. doi: 10.1016/s0960-9822(00)00288-8. [DOI] [PubMed] [Google Scholar]
- 10.Avila FW, Wolfner MF. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc Natl Acad Sci USA. 2009;106(37):15796–15800. doi: 10.1073/pnas.0904029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertram MJ, Akerkar GA, Ard RL, Gonzalez C, Wolfner MF. Cell type-specific gene expression in the Drosophila melanogaster male accessory gland. Mech Dev. 1992;38(1):33–40. doi: 10.1016/0925-4773(92)90036-j. [DOI] [PubMed] [Google Scholar]
- 12.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 13.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439(7075):475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136(3):483–493. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang H, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137(7):1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004(220):pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 17.Narasimha M, Uv A, Krejci A, Brown NH, Bray SJ. Grainy head promotes expression of septate junction proteins and influences epithelial morphogenesis. J Cell Sci. 2008;121(Pt 6):747–752. doi: 10.1242/jcs.019422. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15(11):1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taniguchi K, et al. Binucleation of Drosophila adult male accessory gland cells increases plasticity of organ size for effective reproduction. J Organ Biol. 2011;1:e101. [Google Scholar]
- 20.Sirot LK, et al. Towards a semen proteome of the dengue vector mosquito: Protein identification and potential functions. PLoS Negl Trop Dis. 2011;5(3):e989. doi: 10.1371/journal.pntd.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008;88(2):489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- 22.Bloch Qazi MC, Heifetz Y, Wolfner MF. The developments between gametogenesis and fertilization: Ovulation and female sperm storage in Drosophila melanogaster. Dev Biol. 2003;256(2):195–211. doi: 10.1016/s0012-1606(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100(17):9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17(11 Reviews):1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 25.Goberdhan DCI, Wilson C. The functions of insulin signaling: Size isn’t everything, even in Drosophila. Differentiation. 2003;71(7):375–397. doi: 10.1046/j.1432-0436.2003.7107001.x. [DOI] [PubMed] [Google Scholar]
- 26.Hoodless PA, et al. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85(4):489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- 27.Tsuneizumi K, et al. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature. 1997;389(6651):627–631. doi: 10.1038/39362. [DOI] [PubMed] [Google Scholar]
- 28.Pyrowolakis G, Hartmann B, Müller B, Basler K, Affolter M. A simple molecular complex mediates widespread BMP-induced repression during Drosophila development. Dev Cell. 2004;7(2):229–240. doi: 10.1016/j.devcel.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Ninov N, et al. Dpp signaling directs cell motility and invasiveness during epithelial morphogenesis. Curr Biol. 2010;20(6):513–520. doi: 10.1016/j.cub.2010.01.063. [DOI] [PubMed] [Google Scholar]
- 30.Minami R, et al. The homeodomain protein defective proventriculus is essential for male accessory gland development to enhance fecundity in Drosophila. PLoS ONE. 2012;7(3):e32302. doi: 10.1371/journal.pone.0032302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook C, et al. Noggin is required for normal lobe patterning and ductal budding in the mouse prostate. Dev Biol. 2007;312(1):217–230. doi: 10.1016/j.ydbio.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokobza SM, Ye L, Kynaston HG, Jiang WG. Growth and differentiation factor-9 promotes adhesive and motile capacity of prostate cancer cells by up-regulating FAK and Paxillin via Smad dependent pathway. Oncol Rep. 2010;24(6):1653–1659. doi: 10.3892/or_00001030. [DOI] [PubMed] [Google Scholar]
- 33.Ding Z, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470(7333):269–273. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YC, et al. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Res. 2011;71(15):5194–5203. doi: 10.1158/0008-5472.CAN-10-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barren RJ, 3rd, et al. Method for identifying prostate cells in semen using flow cytometry. Prostate. 1998;36(3):181–188. doi: 10.1002/(sici)1097-0045(19980801)36:3<181::aid-pros6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 36.Gardiner RA, et al. Abnormal prostatic cells in ejaculates from men with prostatic cancer—A preliminary report. Br J Urol. 1996;78(3):414–418. doi: 10.1046/j.1464-410x.1996.00089.x. [DOI] [PubMed] [Google Scholar]
- 37.Fitzpatrick JM. The natural history of benign prostatic hyperplasia. BJU Int. 2006;97(Suppl 2):3–6, discussion 21–22. doi: 10.1111/j.1464-410X.2006.06097.x. [DOI] [PubMed] [Google Scholar]
- 38.Xue L, Noll M. Dual role of the Pax gene paired in accessory gland development of Drosophila. Development. 2002;129(2):339–346. doi: 10.1242/dev.129.2.339. [DOI] [PubMed] [Google Scholar]
- 39.Wucherpfennig T, Wilsch-Bräuninger M, González-Gaitán M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;161(3):609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox DT, Spradling AC. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell. 2009;5(3):290–297. doi: 10.1016/j.stem.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel NH, Snow PM, Goodman CS. Characterization and cloning of fasciclin III: A glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell. 1987;48(6):975–988. doi: 10.1016/0092-8674(87)90706-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.