Abstract
AMPA receptors (AMPARs) mediate the majority of fast excitatory neurotransmission, and their density at postsynaptic sites determines synaptic strength. Ubiquitination is a posttranslational modification that dynamically regulates the synaptic expression of many proteins. However, very few of the ubiquitinating enzymes implicated in the process have been identified. In a screen to identify transmembrane RING domain-containing E3 ubiquitin ligases that regulate surface expression of AMPARs, we identified RNF167. Predominantly lysosomal, a subpopulation of RNF167 is located on the surface of cultured neurons. Using a RING mutant RNF167 or a specific shRNA to eliminate endogenous RNF167, we demonstrate that AMPAR surface expression increases in hippocampal neurons with disrupted RNF167 activity and that RNF167 is involved in activity-dependent ubiquitination of AMPARs. In addition, RNF167 regulates synaptic AMPAR currents, whereas synaptic NMDAR currents are unaffected. Therefore, our study identifies RNF167 as a selective regulator of AMPAR-mediated neurotransmission and expands our understanding of how ubiquitination dynamically regulates excitatory synapses.
Keywords: hippocampus, glutamate receptor, GluA2
AMPA receptors (AMPARs) mediate most fast excitatory synaptic transmission in the mammalian brain, and alterations in excitatory synaptic strength are believed to be the cellular basis for learning and memory. AMPARs are tetrameric glutamate-gated ion channels composed of homologous subunits (GluA1-4). The subunit composition of AMPARs varies in different brain regions, and in hippocampal pyramidal neurons, they predominantly form GluA1A2 or GluA2A3 complexes (1, 2). AMPAR localization at the postsynaptic density (PSD) is highly regulated by trafficking events such as lateral diffusion, endocytosis, and exocytosis. The dynamic regulation of synaptic AMPARs is implicated in paradigms of learning and memory and in pathologic conditions.
Posttranslational modifications such as phosphorylation and ubiquitination dynamically regulate protein trafficking and synaptic function. However, our limited knowledge regarding the molecular players regulating synaptic protein ubiquitination is particularly striking and contrasts sharply with the extensive literature identifying kinases and scaffolding proteins that regulate synaptic function. Ubiquitination, first shown more than 30 y ago (3), is a multistep ATP-dependent enzymatic process, which covalently attaches the 76-aa protein ubiquitin to target protein. It requires the coordinated and sequential action of the E1 activating enzyme, an E2 conjugating enzyme, and finally an E3 ubiquitin ligase that confers substrate specificity. E3 ubiquitin ligases fall into two classes: HECT- or RING domain containing proteins and are commonly found in the cytosol, nucleus, or are peripherally associated with membrane. There are more than 600 RING domain E3 ligases, which are largely uncharacterized (4). The known functions of ubiquitin moieties are diverse, and their biological roles have now moved beyond the simple notion of being a marker for destruction. Ubiquitination is increasingly recognized as a key mechanism underlying various cellular processes, including protein quality control, membrane protein trafficking, receptor internalization, and down-regulation (5–11). By regulating many basic cell biological processes, ubiquitination has a major potential impact on health and disease.
Recently, the ubiquitination of the AMPAR subunits GluA1 and GluA2 was reported (11–13); however, little is known about the specific E3 ligases, which might be involved in the regulation of AMPARs. Therefore, a better understanding of the molecular determinants and the dynamics of ubiquitin signaling is of particular importance for a better understanding of synaptic plasticity and function. In the current study, we have screened a subset of transmembrane RING E3 ligases for effects on AMPARs. We now report that an endosomal/lysosomal E3 ligase, RNF167, regulates AMPAR surface expression. We show that a fraction of RNF167 is expressed on the cell surface of cultured neurons. In addition, inactivation of RNF167, by expressing a dominant negative or a specific shRNA, increases surface AMPARs in neurons and is involved in activity-dependent ubiquitination. We also find that RNF167 regulates synaptic AMPAR currents, whereas NMDAR currents are unaffected. Thus, our study identifies RNF167 as an endosomal ubiquitin ligase that regulates AMPAR-mediated synaptic transmission.
Results
RING domain E3 ubiquitin ligases are characterized by their highly conserved zinc-coordinating region, which serves as the docking site for E2 ubiquitin-conjugating enzymes and recruits them to a specific substrate. To identify E3 ligases that might regulate targeting of integral membrane proteins into particular subcellular compartments, we screened the human genome for transmembrane RING domain E3 ligases (14). Of the 49 nonredundant transmembrane RING-domain containing proteins identified, a subset of 5 was localized at the plasma membrane or endosomes (Fig. 1A). Because AMPARs are highly mobile and traffic through many endosomal compartments (7, 15, 16), we tested whether any of these ligases might modulate the trafficking or surface expression of AMPARs.
Fig. 1.
RNF167 is an E3 ligase that ubiquitinates GluA2 and regulates its surface expression. (A) Schematic representation of the subset of RING-domain E3 ubiquitin ligases that localize to endosomes. Signal peptide, transmembrane domains, and RING domains are indicated. (B) Surface expression of GluA2 was evaluated by using FACS analysis of HEK293T cells coexpressing Flag-GluA2 and WT or RING domain mutant E3 ligases. Data are presented as the mean of GluA2 surface expression relative to WT (% ± SEM, n = 3 independent experiments). (C) WT, but not mutant, RNF167 ubiquitinates GluA2. HEK293T cells expressing Flag-GluA2 and RNF167 were lysed, and Flag-GluA2 was immunoprecipitated and immunoblotted for the presence of ubiquitin. The asterisk (*) represents a nonspecific band found in total cell lysates. (D) Immunoprecipitated HA-tagged WT RNF167 expressed in HEK293T cells was deglycosylated by using endoglycosydase H (endo H) or peptide-N-glycosydase F (PNGaseF) and immunoblotted by using RNF167 antibody. (E) Mobility profiles of RNF167 glycosylation mutants. Thirty micrograms of total proteins from HEK293T cells expressing RNF167 WT or mutants were analyzed by immunoblotting for RNF167. See also Fig. S1 A–C.
Specifically, we screened AMPAR surface expression by using live cell FACS analysis, which allowed us to evaluate surface receptors within a heterogenous population of transfected HEK293T cells coexpressing YFP-tagged E3 ligases while excluding cells without an intact plasma membrane (DAPI positive). We compared WT E3 ligases with loss-of-function RING mutants, in which at least one conserved histidine residue within the RING domain was substituted for tryptophan, preventing zinc coordination and, thereby, generating a protein with possible dominant-negative effects (17). We evaluated GluA2 surface expression, because it is a constituent of most endogenous AMPARs. We found that GluA2 surface expression was significantly increased in cells expressing mutant RNF112 (134 ± 4% of WT; *P = 0.016), mutant RNF144 (118 ± 3% of WT; *P = 0.020), and mutant RNF167 (173 ± 14% of WT; *P = 0.034) (Fig. 1A). The mitochondrial E3 ligase MARCH5 (18) was used as a negative control in this assay and showed no effect on GluA2 surface expression.
Among the E3 ligases screened by FACS analysis, the expression of mutant RNF167 showed the most dramatic increase in surface GluA2, suggesting a critical role in regulating AMPARs, leading us to focus on RNF167. To evaluate whether GluA2 is ubiquitinated by RNF167, we coexpressed GluA2 with WT or mutant RNF167 in HEK293T cells and immunoprecipitated GluA2. By immunoblotting for ubiquitin, we found that GluA2 is ubiquitinated by WT but not mutant RNF167 (Fig. 1C). This result establishes that RNF167 is an E3 ligase and GluA2 is a substrate. In addition, the mutations generated in the RNF167 RING domain blocked the ability of RNF167 to ubiquitinate GluA2, validating that mutant RNF167 acts as a loss-of-function construct.
Bioinformatic analysis predicts that RNF167 is a type I transmembrane protein containing a signal peptide, a central membrane-spanning region, a C-terminal RING domain, and two putative N-terminal glycosylation sites. If RNF167 is glycosylated, this posttranslational modification might explain why RNF167 migrates diffusely at a larger molecular mass than its predicted 38 kDa (Fig. 1C). Therefore, we examined whether RNF167 is glycosylated by immunoprecipitating RNF167 from transfected HEK293T cells and treating with endoglycosydase H (endo H) or peptide-N-glycosydase F (PNGaseF). The smear was eliminated, resulting in a sharper lower molecular mass band (Fig. 1D), demonstrating that the diffuse pattern resulted from glycosylation of RNF167. We next generated mutations into the consensus glycosylation sites of RNF167, creating single (N33Q or N79Q) and double mutants (N33Q, N79Q). Specific immunoblotting of HEK293T cells lysates showed these mutations change RNF167 mobility on SDS/PAGE (Fig. 1E). Each single mutation created resulted in a mobility shift compared with WT RNF167, but only the double mutant (N33Q, N79Q) results in a pattern comparable to the PNGase F treated, deglycosylated RNF167 (Fig. 1D).
To examine the expression of endogenous RNF167, we first performed a PCR-based gene expression analysis by using a human multiple tissue cDNA panel. We found ubiquitous expression of RNF167 (Fig. 2A). Although the actin expression is similar between tissues, RNF167 expression is high in the liver, pancreas, and testis and lower in the brain. We next analyzed the RNF167 expression profile in the brain by using a specific RNF167 antibody. As expected from the PCR analysis (Fig. 2A), we found that RNF167 protein is expressed in the adult rat brain and at similar levels in the cortex and hippocampus (Fig. 2B).
Fig. 2.
RNF167 is broadly expressed in vivo and localizes to lysosomes. (A) PCR analysis of human RNF167 mRNA tissue expression. Shown are the expression profile of RNF167 (Upper) and β-actin (Lower). (B) Crude synaptosomes from adult female rat brain tissues were immunoblotted with specific antibodies as indicated. A typical result is shown. (C–G) Subcellular distribution of RNF167 was analyzed by using confocal microscopy. HeLa cells expressing HA-tagged RNF167 were stained for RNF167 (red) and the early endosome marker GFP-Rab5 (C, green), late endosome markers GFP-Rab7 (D, green), or GFP-Rab9 (E, green). (Scale bar: 10 µm.) Boxed areas are shown at higher magnification and colocalization in yellow. (F) HeLa cells expressing RNF167-YFP (green) counterstained for endogenous Lamp-1 (red). Colocalization is shown (yellow) in the merge image. Boxed areas are shown at higher magnification. (G) (Left) A representative image of a hippocampal neuron (DIV15) expressing HA-tagged RNF167 (green) and stained for endogenous Lamp-2 (red) and the neuronal marker MAP2 (blue). (Scale bar: 10 µm.) The boxed area is shown at higher magnification (Right). Colocalization between HA-RNF167 and the lysosomal marker Lamp-2 is shown (yellow) in the merge image. (Scale bar: 5 µm.)
As we mentioned previously, RNF167 was initially characterized as a vesicular endosomal protein. To examine the subcellular localization of RNF167, we cotransfected HeLa cells with HA-tagged RNF167 and GFP-tagged Rab proteins (GFP-Rab5, GFP-Rab7, GFP-Rab9) and used immunofluorescence microscopy. RNF167 does not colocalize with the early-endosome marker GFP-Rab5 (Fig. 2C), but only modestly overlaps with the late endosomal markers Rab7 and Rab9, suggesting that RNF167 is predominantly localized in late endocytic compartments (Fig. 2 D and E). This partial colocalization might be explained by the fact that Rab GTPases are relatively small proteins cycling between active and inactives states, which also regulate their association with endosomal membranes (19). Therefore, to better resolve the intracellular localization of the membrane protein RNF167, we transfected HeLa cells with YFP-tagged RNF167 and immunostained for the endogenous integral lysosomal membrane protein Lamp-1. We found an extensive overlapping of RNF167 and Lamp-1, consistent with RNF167 being localized in lysosomes at steady state (Fig. 2F). The subcellular localization of RNF167 expressed in hippocampal neurons also displayed a robust colocalization with the lysosomal marker Lamp-2 (Fig. 2G), demonstrating its enrichment in lysosomes, consistent with the findings in HeLa cells.
Because RNF167 ubiquitinates GluA2 in HEK293T cells, we investigated whether RNF167 activity might regulate the total protein expression of AMPARs in neurons. Neither the expression of WT nor mutant RNF167 in hippocampal neurons changed the total expression of endogenous GluA1, GluA2, or the NMDAR subunit GluN1 (Fig. S2). Similarly, WT or mutant RNF167 did not affect AMPAR subunits expression in HEK293T cells. Therefore, we next examined whether the expression of mutant RNF167 in hippocampal neurons would affect endogenous AMPAR surface expression. Hippocampal neurons were infected with lentiviruses expressing WT or mutant HA-tagged RNF167, and an antibody-based confocal imaging assay was performed. We found that mutant RNF167 significantly increased GluA2 surface expression compared with WT RNF167 or uninfected cultures (133.5 ± 7.9%, 109.4 ± 4.8%, and 100.0 ± 3.9%, respectively; Fig. 3 A and B). These results are consistent with our FACS screen analysis showing that mutant RNF167 increases GluA2 surface expression (Fig. 1B).
Fig. 3.
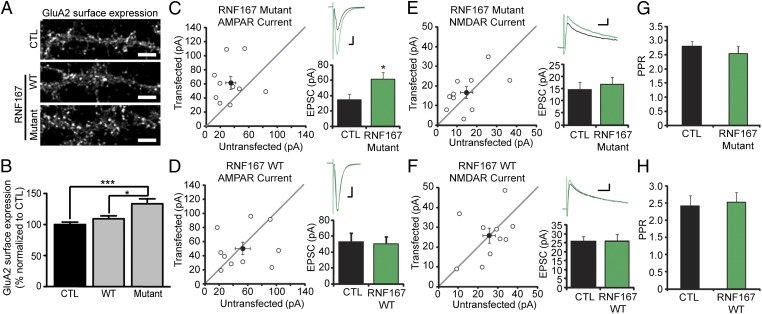
RNF167 regulates synaptic AMPARs. (A and B) Hippocampal neurons were transduced with WT or mutant RNF167 lentiviruses and the surface expression of AMPARs was evaluated by using confocal microscopy. Surface-expressed endogenous GluA2 was labeled under nonpermeabilized conditions. (A) Representative images are shown. (Scale bar: 5 µm.) (B) Quantitation of GluA2 surface expression in A. Bar graph shows that mutant but not WT RNF167 increases surface expression of GluA2 and is presented as mean ± SEM. Each group contains 30 cells from three independent experiments (*P < 0.05 and ***P < 0.005 using ANOVA one-way analysis and Tukey’s multiple comparison test). See also Fig. S2. (C–F) Scatter plots show amplitude of EPSCs for single pair (○) and mean (●). Representative superimposed traces are shown [black, control (CTL); green, WT or mutant RNF167]. (Scale bars: C and D, 20 pA and 10 ms; E and F, 20 pA and 50 ms.) Bar graphs show that expression of mutant RNF167 affects AMPAR currents (C; 77% increase, n = 10, *P = 0.037) but not NMDAR currents (E; 14.5% increase, n = 10, P = 0.80), and that WT RNF167 expression neither affects AMPAR (D; 5.4% increase, n = 10, P = 1.0) nor NMDAR currents (F; 1.6% increase, n = 10, P = 0.91). Paired-pulse ratios (PPR) show no differences when WT (G; P = 0.22) or mutant RNF167 (H; P = 0.65) is expressed. Errors bars represent mean ± SEM.
We next examined the functional effect of RNF167. We made dual whole-cell recordings from rat organotypic hippocampal slice cultures biolistically transfected with exogenous RNF167-HA and GFP. This strategy allowed us to simultaneously compare evoked excitatory postsynaptic currents (EPSCs) at Schaffer collateral/CA1 synapses in transfected and nearby control cells. Expression of mutant RNF167 significantly increased evoked AMPAR EPSC amplitude by 77% (Fig. 3C), whereas WT RNF167 had no effect (Fig. 3D). In contrast, NMDAR EPSCs were unaffected by mutant or WT RNF167 expression (Fig. 3 E and F). Expression of mutant or WT RNF167 did not affect paired-pulse facilitation (Fig. 3 G and H), indicating that presynaptic function is unaltered by RNF167 expression. Taken together, our measurements of synaptic currents show that RNF167 specifically regulates AMPARs, not NMDARs.
Our data thus far demonstrated that mutant RNF167 greatly increases AMPAR surface and synaptic expression. Because WT, but not mutant, RNF167 promotes GluA2 ubiquitination in HEK293T cells (Fig. 1C), we investigated the possibility that RNF167 regulates ubiquitination of endogenous AMPARs. In neurons, endogenous AMPAR ubiquitination is low at steady state unlike ubiquitination of exogenous AMPARs expressed in HEK293T cells (Fig. 1C). However, recent reports demonstrate that endogenous AMPARs, GluA1 and GluA2, undergo activity-dependent ubiquitination (11, 13). Specifically, we found that the GluA2 subunit is rapidly and selectively ubiquitinated upon bicuculline treatment (13). To evaluate the importance of endogenous RNF167 in activity-dependent AMPAR ubiquitination, we used lentiviruses harboring shRNAs for RNF167 to knock down endogenous RNF167. Cultured cortical neurons expressing control or RNF167-specific shRNA (shRNF167) were shortly treated with bicuculline, lysed, GluA2 immunoprecipitated, and immunoblotted for ubiquitin. Although bicuculline induced robust GluA2 ubiquitination in control neurons, we found that RNF167 knockdown cultures show reduced GluA2 ubiquitination (Fig. 4A). In addition, a control experiment showed that RNF167 knockdown doesn’t promote bicuculline-induced GluA1 ubiquitination (Fig. S3). Therefore, RNF167 appears to be involved in activity-dependent GluA2 ubiquitination.
Fig. 4.
Reduced RNF167 expression in cultured neurons decreases the activity-dependent ubiquitination of GluA2 and increases surface expression of AMPARs subunits. (A) DIV17 cortical neurons expressing shCTL or shRNF167 were treated with bicuculline (Bic; 40 µM, 7 min) or its control (CTL, DMSO). GluA2 was immunoprecipitated from cell lysate and immunoblotted for ubiquitin. Total expression of GluA2 and RNF167 were monitored by using specific antibodies as indicated. The asterisk (*) represents a nonspecific band found in total cell lysate. See also Fig. S3. (B) At DIV17, cortical neurons expressing shCTL or shRNF167 were incubated with NHS-SS-biotin to label surface-expressed proteins. Biotinated proteins were precipitated from cell lysate and immunoblotted by using specific antibodies as indicated. Lysate represents 25% of input used for streptavidin precipitation. (C) Hippocampal neurons were transfected with shCTL or shRNF167 and stained live for endogenous surface GluA1 and GluA2 under nonpermeabilized conditions before being evaluated using confocal microscopy. Representative images show AMPAR surface expression and EGFP from transfected cells. (Scale bar: 5 µm.) (D) Quantitation of AMPAR surface expression in C. Bar graph shows that shRNF167 but not shCTL increases surface expression of GluA1 and GluA2 and is presented as mean ± SEM. Each group contains 44–45 cells from three independent experiments (*P < 0.0001 using Student’s unpaired t test). (E) Hippocampal neurons were transduced with shCTL or shRNF167 lentiviruses, transfected with Flag-AMPAR subunits, stained under nonpermeabilized (surface) and permeabilized (intracellular) conditions, and the expression of Flag-AMPARs was evaluated by using confocal microscopy. Representative images show surface and intracellular expression of AMPARs. (Scale bar: 5 µm.) Quantitation of AMPAR surface (F) and intracellular (G) expression. Bar graphs show that shRNF167 but not shCTL increases surface expression of Flag-AMPARs and are presented as mean ± SEM (each group contains 30–40 cells from three to four independent experiments, *P < 0.0001 using Student’s unpaired t test).
Based on the findings that RNF167 knockdown affects bicuculline-induced GluA2 ubiquitination and that expression of mutant RNF167 increases surface and synaptic AMPAR, it is conceivable that a small but significant subpopulation of RNF167 is expressed at the plasma membrane. We reasoned that RNF167 is positioned to recruit the E2 ubiquitin-conjugating enzymes involved in the ubiquitination of surface AMPARs and then regulates their commitment to the endosomal/lysosomal pathway. Therefore, we investigated the possibility that RNF167 might also be expressed at the cell surface by using a cell surface biotination assay on cultured cortical neurons. We found that a substantial fraction of RNF167 is surface-expressed, consistent with a role in the regulation of surface-expressed proteins (Fig. 4B). The immunoblot analysis of the RNF167 knockdown shows the specificity of the RNF167 signal in both surface and total fractions. Our data are consistent with both a surface and an endosomal pool of RNF167 that regulate surface/synaptic AMPARs through ubiquitination.
Ubiquitination is a posttranslational modification that can modify the composition of the PSD, consequently altering plasticity and synaptic activity (20). Because our results demonstrate that GluA2 ubiquitination is reduced by RNF167 knockdown under intense synaptic activity (Fig. 4A), we evaluated the consequence of reducing endogenous RNF167 expression on the surface expression of AMPARs. Hippocampal neurons transfected with a construct expressing EGFP and a control (shCTL) or RNF167 specific (shRNF167) shRNA were subjected to an antibody-based confocal imaging assay. We found that endogenous surface expression of GluA1 and GluA2 AMPAR subunits was significantly increased in hippocampal neurons transfected with RNF167 shRNA compared with shCTL (Fig. 4 C and D). These results were confirmed in a second assay in which Flag-tagged AMPAR subunits were transfected in hippocampal neurons previously infected with lentiviruses expressing a control (shCTL) or RNF167-specific (shRNF167) shRNAs. We found that surface and total expression of the three AMPAR subunits showed a small but significant increase in RNF167 knocked-down hippocampal neurons compared with shCTL (Fig. 4 E–G).
Finally, to determine the functional effect of endogenous RNF167, we performed dual whole-cell recordings from rat organotypic hippocampal slice cultures biolistically transfected with shRNF167. We found that evoked excitatory postsynaptic currents (EPSCs) at Schaffer collateral/CA1 synapses in shRNF167-expressing cells, like mutant RNF167 (Fig. 3C), resulted in an enhancement of synaptic AMPAR, but not NMDAR, function (Fig. 5 A and C). Importantly, expressing shRNF167 with a resistant RNF167 construct rescues the AMPA-mediated current to an amplitude comparable to a control cell, and had no effect on NMDAR EPSCs (Fig. 5 B and D).
Fig. 5.
RNF167 knockdown affects AMPAR but not NMDAR currents in hippocampal neurons. Scatter plots show amplitude of EPSCs for single pairs (○) and the mean (●). Representative superimposed traces are shown [black, control (CTL); green, shRNF167 or shRNF167 + RNF167*]. (Scale bars: A and B, 20 pA and 20 ms; C and D, 20 pA and 50 ms.) Bar graphs show that expression of shRNF167 affects AMPAR currents (A; 105% increase, n = 8, *P = 0.04) but not NMDAR currents (C; 22.5% increase, n = 9, P = 0.91). The expression of shRNF167 and RNF167 resistant (RNF167*) rescued AMPAR currents to level similar to control (B; 29.1% increase, n = 9, P = 0.43) without affecting NMDAR currents (D; 12.2% increase, n = 9, P = 0.69). Errors bars represent mean ± SEM. See also Fig. S1D for rescue construct expression.
Discussion
Ubiquitination is an important regulator of synaptic activity and plasticity (20–22); however, many synaptic substrates and their specific E3 ubiquitin ligases remain undefined. Identifying the relevant E3 enzymes that target synaptic proteins is particularly difficult because of the sheer number of E3 ubiquitin ligases. Therefore, we reasoned that transmembrane E3 ligases might be specifically positioned to regulate transmembrane proteins in various organelles (i.e., mitochondria, endoplasmic reticulum, Golgi, endosome). By combining an extensive bioinformatic analysis with molecular and cell biology approaches, 49 transmembrane RING domain E3 ligases have been localized to discrete subcellular compartments (14). We then performed a screen from a pool of endosomal E3 candidates for potential modulators of trafficking or surface expression of AMPARs. In the current study, we found that the endosomal/lysosomal E3 ligase RNF167 modulates AMPAR surface expression. Here, we show that RNF167 influences the activity-dependent ubiquitination of neuronal AMPARs and regulates AMPA receptor-mediated synaptic transmission.
Ubiquitination can regulate both the internalization and the postendocytic sorting of plasma membrane receptors. Indeed, ubiquitination of plasma membrane proteins has been reported to trigger endocytosis (23), but has also been shown to be a molecular signal to sort proteins from endosomes into the multivesicular-body/lysosomal degradative pathway (24, 25). Although multiple studies report that ubiquitination is an important process regulating AMPAR turnover (26–29), it is only recently that mammalian AMPARs were shown to be directly ubiquitinated through an activity-dependent process (11, 13).
We now show that RNF167 is an E3 ubiquitin ligase involved in AMPAR ubiquitination (Figs. 1C and 4A). Because RING-type E3 ubiquitin ligases transiently interact with the E2 ubiquitin-conjugating enzymes through their RING domains, catalyzing the ubiquitin transfer from the E2 to the substrate (30), it is possible that the mutations in the RNF167 RING domain reduce the capability of RNF167 to recruit E2s. RNF167 is reported to interact with many E2 ubiquitin-conjugating enzymes, but the function of these interactions has yet to be elucidated (31, 32).
In their study, Yamada and Gorbsky (31) demonstrated that RNF167/RING105 interacts with and ubiquitinates TSSC5/SLC22A1L, a poorly understood transmembrane protein. As with our findings, multiple species of RNF167 were revealed by using immunoblotting. Interestingly, they found that only the fast migrating species of RNF167 coimmunoprecipitated with TSSC5. Importantly, we found that RNF167 is expressed at the surface of neurons, and in endosomes, consistent with a role in trafficking. Interestingly, surface RNF167 in neurons also has a smaller apparent molecular mass than the migration pattern found in the total cell lysate (Fig. 4B). Therefore, the best interpretation of the data is that the fast migrating species of RNF167 is specific to surface RNF167 and may be RNF167 associated with AMPARs. However, the transient nature of E3 ubiquitin ligase interactions with substrates makes it difficult to study in neurons.
Our study demonstrates that RNF167 E3 ligase activity regulates AMPAR surface/synaptic expression. Indeed, the expression of mutant RNF167 greatly increases surface expression of AMPARs, which is unaffected by WT-RNF167 (Fig. 3B). This result contrasts with recent reports showing that the expression of the HECT-domain ubiquitin ligase Nedd4-1 reduces GluA1 surface expression through ubiquitination (11, 12). Therefore, our findings suggest that endogenous RNF167 in neurons is not rate limiting, but has a profound effect in regulating the trafficking of surface/synaptic receptors with specificity for AMPARs over NMDARs (Figs. 3–5). Additionally, knocking down RNF167 expression results in increased AMPAR surface expression and synaptic strength (Figs. 4 and 5), the latter being rescued by the expression of a shRNF167-resistant RNF167 construct. Taken altogether, our results show that RNF167 is an important regulator of the synaptic expression of AMPARs.
Lysosomes are seen as the end point of the endocytic pathway and localize predominantly in the soma of neurons. Lysosomal proteins typically traffic through many compartments within the secretory pathway before accumulating at steady state in lysosomes. It is likely that RNF167, which shares many characteristics with resident lysosomal proteins such as the presence of N-glycan and at least one transmembrane domain, also uses a similar route as it traffics to lysosomes. In addition, N-glycans have been shown to protect integral lysosomal membrane proteins such as Lamp-1 and Lamp-2 from proteolysis (33). Therefore, it is plausible that the glycosylation state of RNF167 offers a similar protective mechanism against lysosomal degradation and might be a regulatory mechanism for its sorting and proper intracellular trafficking and localization. Thus, it is quite possible that the effect of RNF167 on AMPARs take place in another compartment of the endocytic pathway rather than in the lysosome itself where RNF167 accumulates.
Our results show that RNF167 also colocalizes with late endosomal/lysosomal markers (Fig. 2 D–G), but how do we explain RNF167 localization and its function on synaptic AMPARs? We believe that the explanation is provided by the discovery of a pool of RNF167 localized at plasma membrane (Fig. 4B). We have shown that expressing a specific shRNA not only increases the surface/synaptic expression of AMPARs (Figs. 4 and 5) but also reduces bicuculline/activity-induced GluA2 ubiquitination (Fig. 4A). Although a reduction of lysosomal degradation might explain the increase in AMPARs observed in both surface and intracellular compartments (Figs. 3–5), an exclusive lysosomal localization of RNF167 will not explain the difference detected by using an acute bicuculline treatment promoting GluA2 ubiquitination. Consequently, our current model is that surface RNF167 controls surface AMPARs and their commitment to the endosomal/lysosomal path, either through direct ubiquitination or possibly as a scaffold accessory protein. Further studies are required to clarify the RNF167-dependent mechanism regulating synaptic expression of AMPARs. Importantly, our study extends the repertoire of proteins regulating AMPAR function and illuminates the importance of expanding our understanding of the ubiquitin enzymes and the sorting pathways regulating synaptic AMPARs.
Materials and Methods
Dissociated cultured neurons were prepared from embryonic day 18 Sprague–Dawley rats (Harlan) by following the guidelines of the National Institutes of Health Animal Research Advisory Committee. When required, transfections were performed at day in vitro (DIV)11 for 3 d by using LipofectAMINE 2000 (Invitrogen), or lentiviruses were added for 3–7 d to the cultures. Organotypical hippocampal slice cultures were prepared from postnatal days 6–8 rat, transfected biolistically after 3–4 d in culture, and kept for a total of 7 d in culture. Immunocytochemistry was used to study expression of endogenous and Flag-tagged AMPAR subunits in dissociated hippocampal cultures, and for RNF167 localization in HeLa cells and hippocampal neurons. Details for constructs, antibody production, biochemical experiments, immunostaining, and electrophysiological recordings are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank John D. Badger II for technical assistance, Dr. Dragan Maric for advice and expertise with the FACS analysis, and the National Institute of Neurological Disorders and Stroke (NINDS) DNA sequencing facility. The NINDS Intramural Research Program (K.W.R. and R.J.Y.) and the National Institute of Mental Health (R.A.N.) supported this research. M.P.L. is the recipient of a postdoctoral fellowship from Le Fond de la Recherche en Santé du Québec, and B.E.H. receives support from the Brain and Behavior Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217477109/-/DCSupplemental.
References
- 1.Lu W, et al. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62(2):254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenthold RJ, Petralia RS, Blahos J II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16(6):1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciechanover A, Heller H, Elias S, Haas AL, Hershko A. ATP-dependent conjugation of reticulocyte proteins with the polypeptide required for protein degradation. Proc Natl Acad Sci USA. 1980;77(3):1365–1368. doi: 10.1073/pnas.77.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 5.Arancibia-Cárcamo IL, et al. Ubiquitin-dependent lysosomal targeting of GABA(A) receptors regulates neuronal inhibition. Proc Natl Acad Sci USA. 2009;106(41):17552–17557. doi: 10.1073/pnas.0905502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciechanover A, Iwai K. The ubiquitin system: From basic mechanisms to the patient bed. IUBMB Life. 2004;56(4):193–201. doi: 10.1080/1521654042000223616. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28(2):511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 8.Pryor PR, Luzio JP. Delivery of endocytosed membrane proteins to the lysosome. Biochim Biophys Acta. 2009;1793(4):615–624. doi: 10.1016/j.bbamcr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Saliba RS, Gu Z, Yan Z, Moss SJ. Blocking L-type voltage-gated Ca2+ channels with dihydropyridines reduces gamma-aminobutyric acid type A receptor expression and synaptic inhibition. J Biol Chem. 2009;284(47):32544–32550. doi: 10.1074/jbc.M109.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saliba RS, Michels G, Jacob TC, Pangalos MN, Moss SJ. Activity-dependent ubiquitination of GABA(A) receptors regulates their accumulation at synaptic sites. J Neurosci. 2007;27(48):13341–13351. doi: 10.1523/JNEUROSCI.3277-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz LA, Hall BJ, Patrick GN. Activity-dependent ubiquitination of GluA1 mediates a distinct AMPA receptor endocytosis and sorting pathway. J Neurosci. 2010;30(49):16718–16729. doi: 10.1523/JNEUROSCI.3686-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin A, et al. Nedd4-mediated AMPA receptor ubiquitination regulates receptor turnover and trafficking. J Neurochem. 2011;119(1):27–39. doi: 10.1111/j.1471-4159.2011.07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lussier MP, Nasu-Nishimura Y, Roche KW. Activity-dependent ubiquitination of the AMPA receptor subunit GluA2. J Neurosci. 2011;31(8):3077–3081. doi: 10.1523/JNEUROSCI.5944-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neutzner A, et al. A systematic search for endoplasmic reticulum (ER) membrane-associated RING finger proteins identifies Nixin/ZNRF4 as a regulator of calnexin stability and ER homeostasis. J Biol Chem. 2011;286(10):8633–8643. doi: 10.1074/jbc.M110.197459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanley JG. Endosomal sorting of AMPA receptors in hippocampal neurons. Biochem Soc Trans. 2010;38(2):460–465. doi: 10.1042/BST0380460. [DOI] [PubMed] [Google Scholar]
- 16.Hirling H. Endosomal trafficking of AMPA-type glutamate receptors. Neuroscience. 2009;158(1):36–44. doi: 10.1016/j.neuroscience.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 17.Bottomley MJ, et al. NMR structure of the first PHD finger of autoimmune regulator protein (AIRE1). Insights into autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) disease. J Biol Chem. 2005;280(12):11505–11512. doi: 10.1074/jbc.M413959200. [DOI] [PubMed] [Google Scholar]
- 18.Karbowski M, Neutzner A, Youle RJ. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol. 2007;178(1):71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeffer SR, Dirac-Svejstrup AB, Soldati T. Rab GDP dissociation inhibitor: Putting rab GTPases in the right place. J Biol Chem. 1995;270(29):17057–17059. doi: 10.1074/jbc.270.29.17057. [DOI] [PubMed] [Google Scholar]
- 20.DiAntonio A, Hicke L. Ubiquitin-dependent regulation of the synapse. Annu Rev Neurosci. 2004;27:223–246. doi: 10.1146/annurev.neuro.27.070203.144317. [DOI] [PubMed] [Google Scholar]
- 21.Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6(3):231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- 22.Mabb AM, Ehlers MD. Ubiquitination in postsynaptic function and plasticity. Annu Rev Cell Dev Biol. 2010;26:179–210. doi: 10.1146/annurev-cellbio-100109-104129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 24.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3(12):893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 25.Urbanowski JL, Piper RC. Ubiquitin sorts proteins into the intralumenal degradative compartment of the late-endosome/vacuole. Traffic. 2001;2(9):622–630. doi: 10.1034/j.1600-0854.2001.20905.x. [DOI] [PubMed] [Google Scholar]
- 26.Dreier L, Burbea M, Kaplan JM. LIN-23-mediated degradation of beta-catenin regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Neuron. 2005;46(1):51–64. doi: 10.1016/j.neuron.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 27.Greer PL, et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140(5):704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juo P, Kaplan JM. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr Biol. 2004;14(22):2057–2062. doi: 10.1016/j.cub.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer H, Rongo C. KEL-8 is a substrate receptor for CUL3-dependent ubiquitin ligase that regulates synaptic glutamate receptor turnover. Mol Biol Cell. 2006;17(3):1250–1260. doi: 10.1091/mbc.E05-08-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ardley HC, Robinson PA. E3 ubiquitin ligases. Essays Biochem. 2005;41:15–30. doi: 10.1042/EB0410015. [DOI] [PubMed] [Google Scholar]
- 31.Yamada HY, Gorbsky GJ. Tumor suppressor candidate TSSC5 is regulated by UbcH6 and a novel ubiquitin ligase RING105. Oncogene. 2006;25(9):1330–1339. doi: 10.1038/sj.onc.1209167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Wijk SJ, et al. A comprehensive framework of E2-RING E3 interactions of the human ubiquitin-proteasome system. Mol Syst Biol. 2009;5:295. doi: 10.1038/msb.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kundra R, Kornfeld S. Asparagine-linked oligosaccharides protect Lamp-1 and Lamp-2 from intracellular proteolysis. J Biol Chem. 1999;274(43):31039–31046. doi: 10.1074/jbc.274.43.31039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.