Abstract
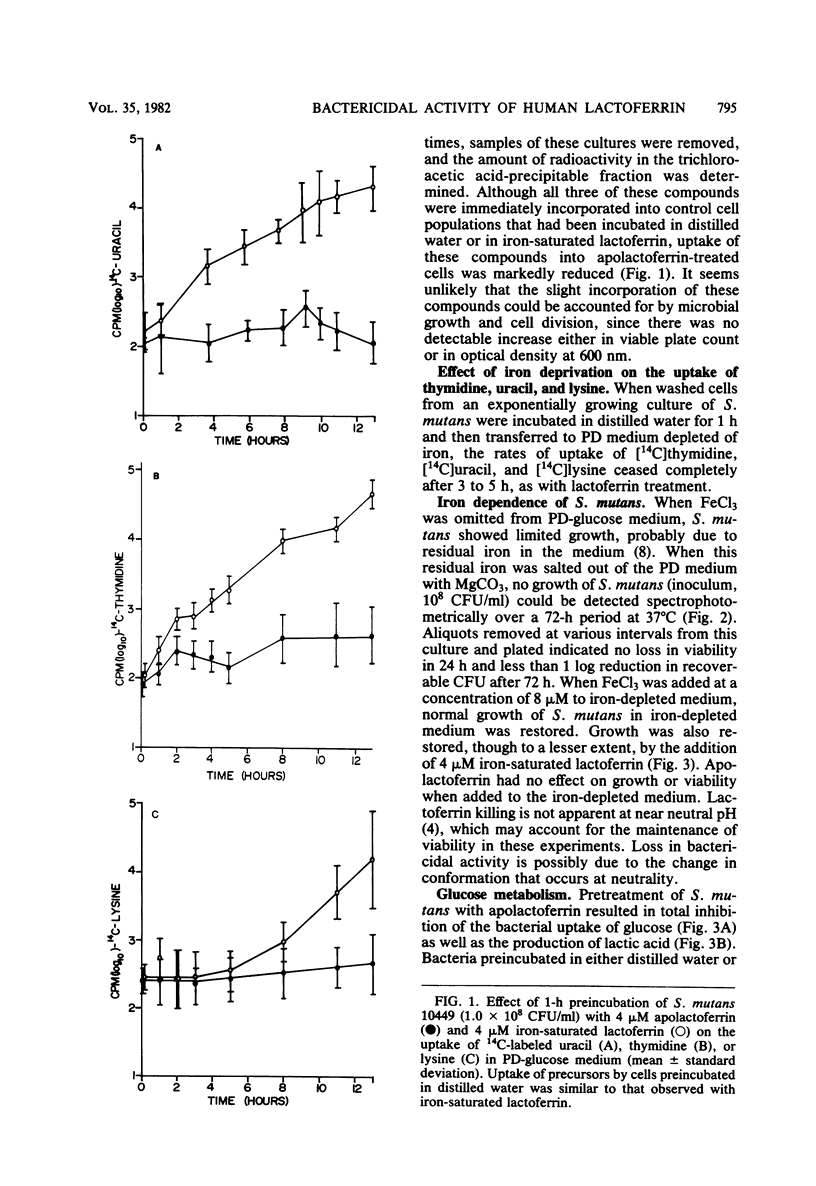
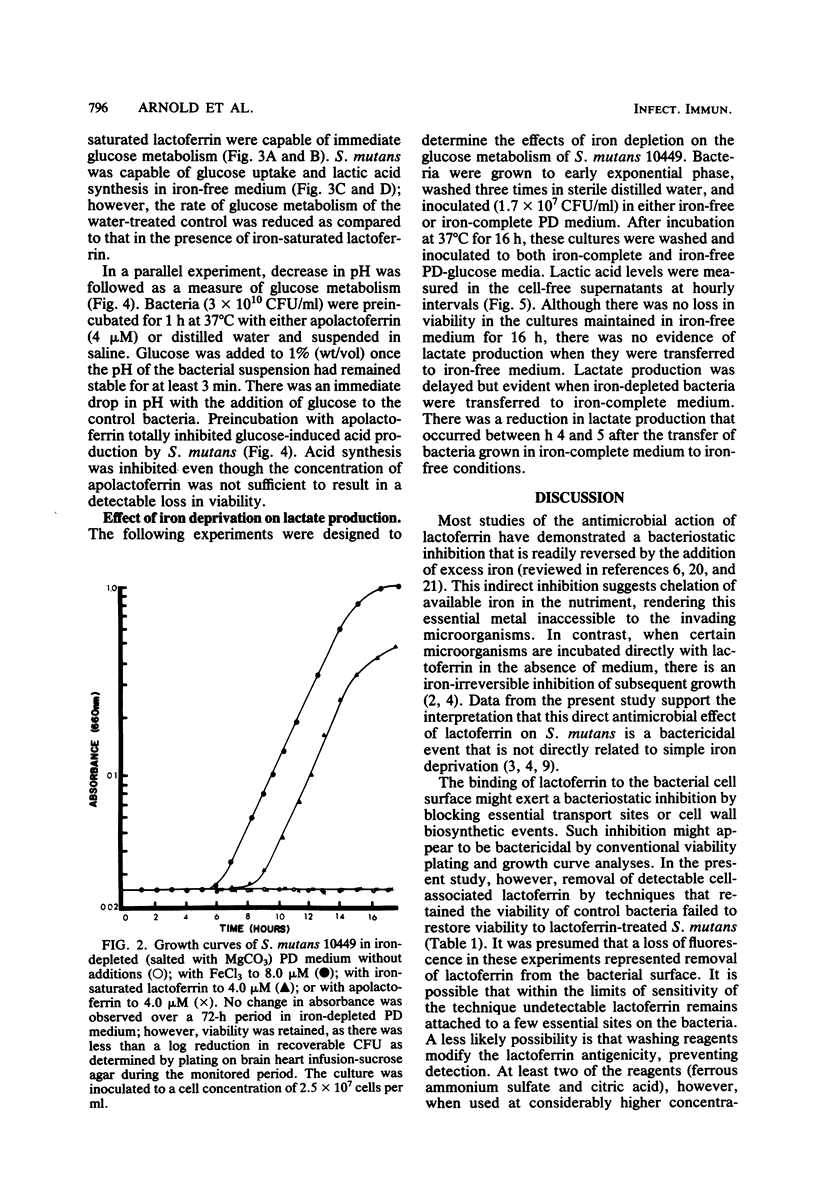
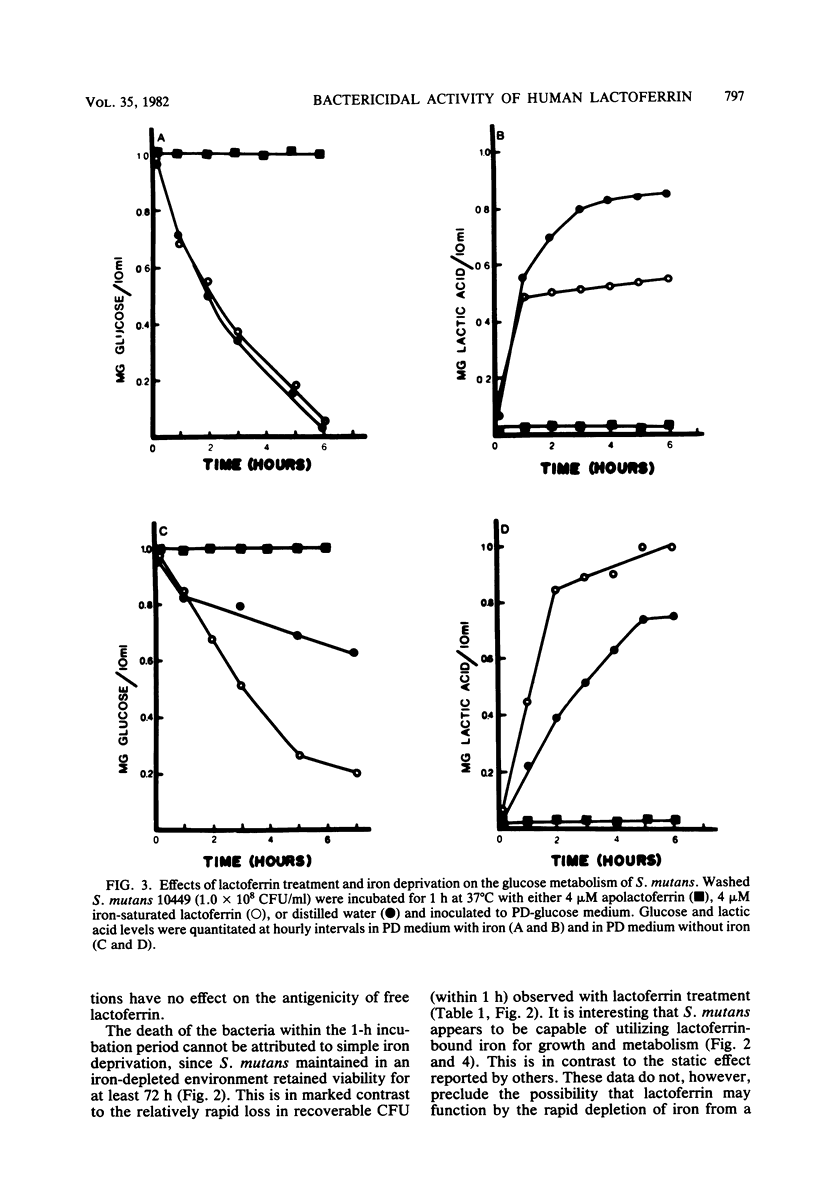
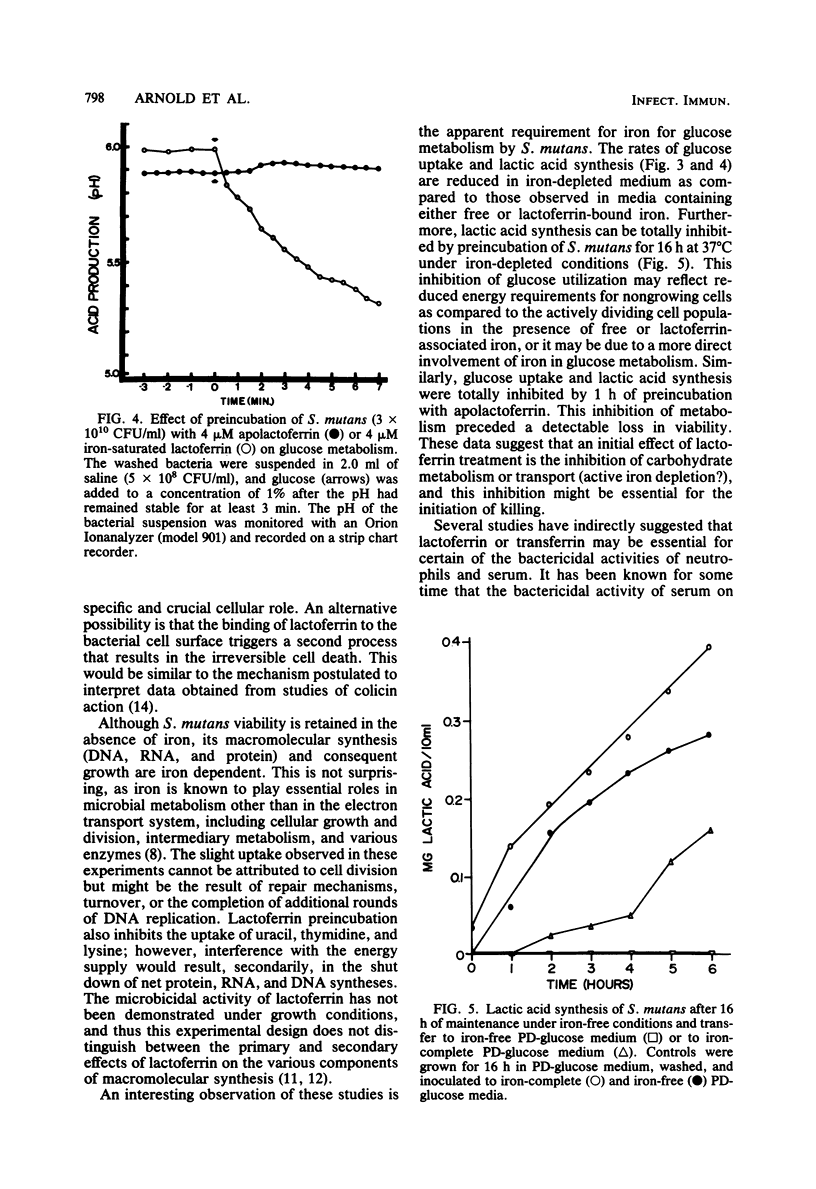
Previous studies have demonstrated a direct iron-irreversible inhibition of a variety of microorganisms by human apolactoferrin. The present study compared the bactericidal effects of lactoferrin on Streptococcus mutans with the bacteriostatic effects of iron deprivation. Growth (as determined by change in optical density) and macromolecular synthesis, as determined by incorporation of 14C-labeled uracil, thymidine, and lysine, were inhibited by incubation of washed exponential-phase S. mutans NCTC 10449 with purified human apolactoferrin. Similarly, apolactoferrin inhibited glucose uptake and metabolism. Iron-saturated lactoferrin had no effect on bacterial growth or metabolism and was capable of serving as a source of iron in iron-depleted medium. S. mutans failed to grow, and there was no indication of macromolecular synthesis in iron-depleted partially defined medium; however, glucose metabolism continued, though at a reduced rate, and viability was retained for 72 h. There was no detectable metabolism of glucose by cells maintained for 18 h in iron-free medium. Metabolism was restored by transfer of iron-depleted S. mutans to iron-complete medium. This was in contrast to the irreversible inhibition by lactoferrin after 1 h of incubation. Inhibition could not be reversed by removal of cell surface-associated lactoferrin as detected by rhodamine isothiocyanate-labeled antilactoferrin. This inhibition of metabolism and rapid loss in viability observed with lactoferrin treatment suggest that lactoferrin has a direct bactericidal effect on S. mutans that cannot be attributed to simple iron deprivation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Brewer M., Gauthier J. J. Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect Immun. 1980 Jun;28(3):893–898. doi: 10.1128/iai.28.3.893-898.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Cole M. F., McGhee J. R. A bactericidal effect for human lactoferrin. Science. 1977 Jul 15;197(4300):263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Arnold R. R., Russell J. E., Champion W. J., Gauthier J. J. Bactericidal activity of human lactoferrin: influence of physical conditions and metabolic state of the target microorganism. Infect Immun. 1981 May;32(2):655–660. doi: 10.1128/iai.32.2.655-660.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Armstrong J. A. The role of lactoferrin in the bactericidal function of polymorphonuclear leucocytes. Immunology. 1979 Apr;36(4):781–791. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Griffiths E. Effect of pH and haem compounds on the killing of Pasteurella septica by specific antiserum. J Gen Microbiol. 1975 Jun;88(2):345–354. doi: 10.1099/00221287-88-2-345. [DOI] [PubMed] [Google Scholar]
- Griffiths E. Mechanism of action of specific antiserum on Pasteurella septica. Selective inhibition of net macromolecular synthesis and its reversal by iron compounds. Eur J Biochem. 1971 Nov 11;23(1):69–76. doi: 10.1111/j.1432-1033.1971.tb01593.x. [DOI] [PubMed] [Google Scholar]
- Griffiths E. Selective inhibition of macromolecular synthesis in Pasteurella septica by antiserum and its reversal by iron. Nat New Biol. 1971 Jul 21;232(29):89–90. doi: 10.1038/newbio232089a0. [DOI] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Association of lactoferrin with lysozyme in granules of human polymorphonuclear leukocytes. Infect Immun. 1972 Nov;6(5):761–765. doi: 10.1128/iai.6.5.761-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Siderophore production by Vibrio cholerae. Infect Immun. 1978 Apr;20(1):310–311. doi: 10.1128/iai.20.1.310-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt K. M., Adamson M., Arnold R. Lactoperoxidase binding to streptococci. Infect Immun. 1979 Jul;25(1):304–309. doi: 10.1128/iai.25.1.304-309.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]


