Abstract
The transport, compartmentation, and metabolism of homoserine was characterized in two strains of meristematic higher plant cells, the dicotyledonous sycamore (Acer pseudoplatanus) and the monocotyledonous weed Echinochloa colonum. Homoserine is an intermediate in the synthesis of the aspartate-derived amino acids methionine, threonine (Thr), and isoleucine. Using 13C-nuclear magnetic resonance, we showed that homoserine actively entered the cells via a high-affinity proton-symport carrier (Km approximately 50–60 μm) at the maximum rate of 8 ± 0.5 μmol h−1 g−1 cell wet weight, and in competition with serine or Thr. We could visualize the compartmentation of homoserine, and observed that it accumulated at a concentration 4 to 5 times higher in the cytoplasm than in the large vacuolar compartment. 31P-nuclear magnetic resonance permitted us to analyze the phosphorylation of homoserine. When sycamore cells were incubated with 100 μm homoserine, phosphohomoserine steadily accumulated in the cytoplasmic compartment over 24 h at the constant rate of 0.7 μmol h−1 g−1 cell wet weight, indicating that homoserine kinase was not inhibited in vivo by its product, phosphohomoserine. The rate of metabolism of phosphohomoserine was much lower (0.06 μmol h−1 g−1 cell wet weight) and essentially sustained Thr accumulation. Similarly, homoserine was actively incorporated by E. colonum cells. However, in contrast to what was seen in sycamore cells, large accumulations of Thr were observed, whereas the intracellular concentration of homoserine remained low, and phosphohomoserine did not accumulate. These differences with sycamore cells were attributed to the presence of a higher Thr synthase activity in this strain of monocot cells.
Higher plants are composed of many heterotrophic organs, such as roots and young, expanding leaves, that are dependent on sugar and amino acid import for growth and development. Experiments with plasma membrane vesicles, intact tissues, and suspension-cultured cells provided strong evidence for at least four amino acid carriers: a basic amino acid symporter, an acidic amino acid symporter, and two distinct symporters for the neutral amino acids (for review, see Bush, 1993; Frommer et al., 1994; Bush et al., 1996). The transport of amino acids by proton-coupled symporters across the plasma membrane is driven by a trans-membrane proton concentration difference generated by a P-type, H+-pumping ATPase. The occurrence of specific tonoplast carriers involved in the concentration of amino acids into the vacuole is unclear. Likewise, if and how amino acids are compartmented in plant cells is largely unknown.
Although homoserine is not incorporated into proteins, this amino acid could represent a form of transported nitrogen via the sieve tube to the heterotrophic tissues. In bacteria and yeast, homoserine is the branch-point metabolite for Thr, Ile, and Met synthesis. Thr and Ile synthesis involves homoserine kinase (EC 2.7.1.39) and Thr synthase (EC 4.2.99.2), which convert homoserine to Thr via phosphohomoserine. In this case, homoserine kinase is feedback inhibited by Thr (Ramos et al., 1991). Met derives from succinyl or acetyl homoserine. In contrast, the branch point for synthesis of Thr, Ile, and Met in plants occurs at the level of phosphohomoserine (for review, see Bryan, 1980). Thus, homoserine phosphorylation by homoserine kinase is a common step for Thr, Ile, and Met synthesis in plants. This enzyme has been discussed as a potential control point for the regulation of the synthesis of these amino acids (Bryan, 1990).
In the present experiments we used 31P- and 13C-NMR to investigate homoserine transport and metabolism in cells of sycamore (Acer pseudoplatanus L.) and the weed Echinochloa colonum. Our results shed new light on the transport and subcellular compartmentation of homoserine and other amino acids, on the metabolism of homoserine, and on the in vivo regulation of the synthesis of aspartate-derived amino acids in higher plant cells.
MATERIALS AND METHODS
Cell suspensions were chosen in preference to dense tissues to improve the homogeneity of the incubation conditions (particularly the extracellular pH and the O2 supply). Furthermore, with dense tissues such as maize root tips, the external medium does not have ready access to the internal cells. In other words, the free diffusion of homoserine and various amino acids, including Ile, homoalanine, and Thr, is hampered by the compactness of the tissue, which could cause misleading results. Sycamore (Acer pseudoplatanus L.) and Echinochloa colonum cells used in the present study were grown at 20°C as a suspension in a liquid nutrient medium according to the methods of Bligny and Leguay (1987) and Murashige and Skoog (1962), respectively. The culture medium was kept at a volume of 0.3 L and stirred continuously at 60 rpm. Under these conditions the cell number doubling time was 40 to 48 h after a lag phase of approximately 2 d, and the maximum cell density was attained after 7 to 8 d of growth, when the stationary phase was attained. The cell suspensions were maintained in exponential growth by subculture every 7 d. The cell wet weight was measured after straining culture aliquots onto a glass-fiber filter.
In Vitro NMR Measurements
Perchloric Acid Extract Preparation
For perchloric acid extraction, cells (9 g wet weight) were quickly frozen in liquid N2 and ground to a fine powder with a mortar and pestle and 1 mL of 70% (v/v) perchloric acid. The frozen powder was then placed at −10°C and thawed. The thick suspension thus obtained was centrifuged at 15,000g for 10 min to remove particulate matter, and the supernatant was neutralized with 2 m KHCO3 to about pH 5.0. The supernatant was then centrifuged at 10,000g for 10 min to remove KClO4; the resulting supernatant was lyophilized and stored in liquid N2. This freeze-dried material was redissolved in 2.5 mL of water containing 10% 2H2O, neutralized to pH 7.5, and buffered with 50 mm Hepes. Divalent cations (particularly Mn2+ and Mg2+) were chelated by the addition of sufficient amounts of 1,2-cyclohexylenedinitrilotetraacetic acid ranging from 50 to 100 μmol depending on the sample.
NMR Measurements
Spectra of neutralized perchloric acid extracts were recorded on an NMR spectrometer (AMX 400, Bruker, Billerica, MA) equipped with a 10-mm multinuclear probe tuned at 162 MHz for 31P-NMR studies and at 100.6 MHz for 13C-NMR studies. The deuterium resonance of 2H2O was used as a lock signal.
31P-NMR acquisition conditions used were: 70° radio-frequency pulses (15 μs) at 3.6-s intervals; spectral width 8200 Hz; 1024 scans; and Waltz-16 1H decoupling sequence (with two levels of decoupling: 1 W during acquisition time, 0.5 W during delay). Free induction decays were collected as 8K data points, zero filled to 16K, and processed with a 0.2-Hz exponential line broadening. 31P-NMR spectra are referenced to methylene diphosphonic acid, pH 8.9, at 16.38 ppm.
13C-NMR acquisition conditions used were: 90° radio-frequency pulses (19 μs) at 6-s intervals; spectral width 20,000 Hz; 900 scans; and Waltz-16 1H decoupling sequence (with two levels of decoupling: 2.5 W during acquisition time, 0.5 W during delay). Free induction decays were collected as 16K data points, zero filled to 32K, and processed with a 0.2-Hz exponential line broadening. 13C-NMR spectra are referenced to hexamethyldisiloxane at 2.7 ppm.
Identification and Quantification of Metabolites
Spectra of standard solutions of known compounds at pH 7.5 were compared with the spectrum of a perchloric acid extract of sycamore cells. The definitive assignments were made after running a series of spectra obtained by the addition of the authentic compounds to the perchloric acid extracts, according to the methods described in previous publications (for 31P-NMR, see Roby et al., 1987; Aubert et al., 1996b; for 13C-NMR, see Gout et al., 1993). To determine accurately the total amount of phosphohomoserine, homoserine, and various amino acids in perchloric extracts, we proceeded as follows: a 20-s recycling time was used to obtain fully relaxed spectra, and the calibration of the peak intensities by the addition of known amounts of the corresponding authentic compounds. The possible errors in the measurements caused by perchloric acid extraction were estimated by adding known amounts of authentic compounds to frozen cells before grinding. We observed that for all of the compounds studied, the overall yield of recovery was about 80%.
Finally, when given, intracellular concentrations were calculated on the following basis: 1 g cell wet weight corresponds to 1 mL of cell volume and roughly 0.13 mL of cytoplasm and 0.78 mL of vacuole volume.
In Vivo NMR Measurements
To get a better signal-to-noise ratio, an experimental arrangement was devised to analyze the maximum cell volume and to optimize the homogeneity of the cell incubation conditions (Aubert et al., 1996b). Such a system prevents gas bubbles within the cell mass and subsequent magnetic field inhomogeneity, as well as the channeling observed with our previous device using slightly compressed cells between two porous glass plates (Roby et al., 1987). The perfusion medium contained 5 mm Suc, 10 mm KNO3, 1 mm KCl, 0.5 mm MgSO4, 0.5 mm Ca(NO3)2, and 100 μm Pi. Spectra were recorded on a spectrometer (AMX 400, Bruker) equipped with a 25-mm probe tuned at 162 MHz or 100.6 MHz for 31P- or 13C-NMR studies, respectively.
Acquisition conditions used for 31P-NMR were: 50° radio-frequency pulses (70 μs) at 0.6-s intervals; spectral width 9800 Hz; 6000 scans; Waltz-16 1H decoupling sequence (with two levels of decoupling: 2.5 W during acquisition time, 0.5 W during delay). Free induction decays were collected as 4K data points, zero filled to 8K, and processed with a 2-Hz exponential line broadening. Spectra are referenced to a solution of 50 mm methylene diphosphonic acid, pH 8.9, in 30 mm Tris contained in a 0.8-mm capillary inserted inside the inlet tube along the symmetry axis of the cell sample (see Roby et al., 1987). The assignment of Pi, phosphate esters, phosphate diesters, and nucleotides to specific peaks was carried out according to the method of Roberts and Jardetzky (1981), Roby et al. (1987), Aubert et al. (1996b), and from spectra of the perchloric acid extracts that contained the soluble, low-molecular-weight constituents.
13C-NMR acquisition conditions were: 90° radio-frequency pulses (70 μs) at 5.6-s intervals; spectral width 20,730 Hz; 900 scans; and Waltz-16 1H decoupling sequence (with two levels of decoupling: 4 W during acquisition time, 0.5 W during delay). Free induction decays were collected as 16K data points, zero filled to 32K, and processed with a 2-Hz exponential line broadening. Spectra are referenced to hexamethyldisiloxane contained in a 0.8-mm capillary inserted inside the inlet tube along the symmetry axis of the cell sample (see Roby et al., 1987). The assignments of resonance peaks were carried out according to the methods described in previous publications (Gout et al., 1993, and refs. therein) and from spectra of the perchloric acid extracts that contained the soluble, low-molecular-weight constituents.
Thr Synthase Assays
For crude extract preparation, cells (5 g wet weight) were harvested and ground in liquid N2 using a mortar and pestle. The powder was then homogenized with 18 mL of buffer A containing 50 mm Na-Hepes, pH 7.5, 1 mm EDTA, 5 mm DTT, 0.1% (w/v) streptomycin sulfate, 0.5 mm ε-aminocaproic acid, and 1 mm benzamidine. The homogenate was centrifuged at 40,000g for 20 min. Soluble protein from the supernatant was then precipitated with (NH4)2SO4 (80% saturation). After centrifugation at 40,000g for 30 min, the protein was dissolved in 2.5 mL of buffer A and desalted on a G 25 M (Pharmacia) column. All procedures were carried out at 4°C.
Thr synthase activity was measured in a volume of 100 μL containing 100 mm Na-Hepes, pH 8.0, 1 mm phosphohomoserine, 10 mm NaF, and 50 μm pyridoxal 5-phosphate, in the absence or presence of 200 μm SAM (Curien et al., 1996). After incubation at 30°C for 5 to 60 min, reactions were stopped by the addition of 50 μL of 20% (w/v) TCA, and the precipitated protein was removed by centrifugation. l-Thr formation was determined by HPLC after derivatization of 2 to 20 μL of the TCA supernatants with O-phthaldialdehyde (Lindroth and Mopper, 1979). The samples (10–50 μL) were injected onto a C18 column (3.9 × 150 mm, 4-μm particle size; Novapak, Millipore) connected to an HPLC system. O-Phthaldialdehyde derivatives were eluted in isocratic conditions (67.2 mm sodium acetate, pH 4.5, 16.8% [v/v] acetonitrile, flow rate 1 mL/min), and detected by fluorescence measurement at 455 nm after excitation at 340 nm using a fluorimeter (model SFM 25, Kontron, Everett, MA). Quantitative analysis of amino acids was carried out by measuring peak areas using a chromatography data system with 450-MT2 software (Kontron) and solutions of amino acids of known concentrations as the standards.
RESULTS
Accumulation and Compartmentation of Homoserine in Sycamore Cells
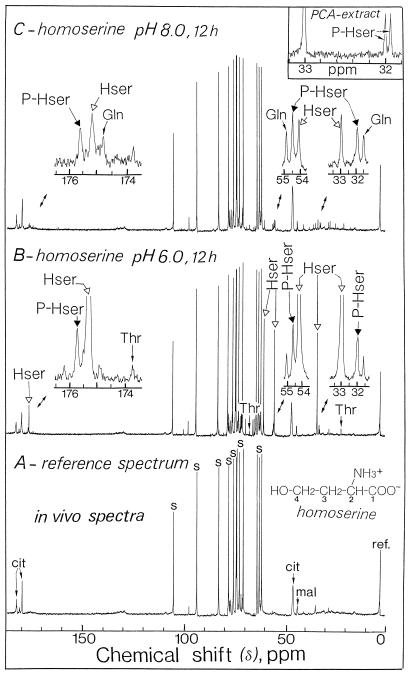
13C-NMR spectroscopy was performed on intact sycamore cells. Figure 1 illustrates the changes that occurred when the cells were incubated for 12 h in a nutrient medium containing 100 μm homoserine and maintained at two different pHs (6.0 and 8.0). In the absence of exogenous homoserine (Fig. 1A; pH 6.0), the strongest signals were from glucosyl and fructosyl moieties of Suc and corresponded to an intracellular level of approximately 75 to 80 μmol g−1 wet weight, which is in good agreement with previous biochemical determinations (Journet et al., 1986). The other major resonances in the chemical shift range of 40 to 50 ppm and those around 180 ppm (carboxyl groups) arose from citrate and malate (see Gout et al., 1993).
Figure 1.
Representative in vivo 13C-NMR spectra obtained from sycamore cells. The spectra, recorded at 20°C, are the result of 900 transients (1 h). A, Control cells at pH 6.0; B, cells incubated for 12 h with 100 μm homoserine at pH 6.0; C, cells incubated for 12 h with 100 μm homoserine at pH 8.0. Note that the C1 resonance of homoserine (carboxyl group) is smaller than those of C2 to C4. This is attributable to the fact that the relaxation time of the carboxyl groups exceeds the duration of induction decays, resulting in a saturation of the signal. In addition, the C1 peak is broadened because of interaction of the carboxyl group with divalent cations. cit, Citrate; Hser, homoserine; mal, malate; PCA, perchloric acid; P-Hser, phosphohomoserine; ref., reference signal; and s, Suc.
After 12 h of incubation in the presence of 100 μm homoserine at pH 6.0, significant changes in the spectra of cells were evident (Fig. 1B). Of particular interest was the huge accumulation of homoserine, characterized by its four resonances centered at 33.1, 54.2, 59.5, and 175.3 ppm, coinciding with C3, C2, C4, and C1, respectively. Note that the C1 resonance is smaller than the resonances of other Cs because of a slower relaxation of the carboxyl group (see legend to Fig. 1). The total amount of homoserine accumulated in the cells, estimated as indicated in “Materials and Methods,” was 60 to 65 μmol g−1 cell wet weight. An increase in the amount of Thr was also observed (resonances centered at 20.2, 66.7, and 173.7 ppm). The resonances at 54.4 and 32.0 ppm (Fig. 1B, expanded scale) coincided with those of C2 and C3 of phosphohomoserine, respectively.
At higher resolution using cell extracts, the resonance centered at 32.0 ppm appeared as two distinct peaks (coupling constant, 6.2 Hz; inset of Fig. 1C), due to the splitting of the C peak by coupling P of the phosphate group bonded to this C. In addition, the examination of the resonances in the chemical shift range from 10 to 60 ppm indicated that feeding homoserine to these cells did not lead to detectable accumulation of either S amino acids (cystathionine, homocysteine, and Met) or branched-chain amino acids (Ile). The intracellular levels of these amino acids remained lower than 1 to 2 μmol g−1 wet weight (the detection limit of in vivo 13C-NMR).
The spectra shown in Figure 1 did not permit distinction between the vacuolar homoserine pool at acidic pH (5.7) and the cytoplasmic homoserine pool at the slightly alkaline pH (7.5). This is due to the fact that homoserine in both compartments has the same chemical shift, because the ionization state of this amino acid remains essentially unchanged at either pH (the dissociation constant for the α-NH3+ group of homoserine is around 9). To overcome this limitation, and to resolve vacuolar and cytoplasmic amino acid signals, we raised the cytoplasmic pH in these cells by up to 1 pH unit. This was accomplished by adjusting the pHe from 6.0 to 9.0 in the presence of 0.5 mm NH4+. Within 30 min the cytoplasmic pH increased from 7.5 to 8.5 and the vacuolar pH increased from 5.7 to 6.5 (see Gout et al., 1992). Such a situation is fully reversible; the cytoplasmic pH returned to its initial value (7.5) less than 15 min after decreasing the pHe to 6.0.
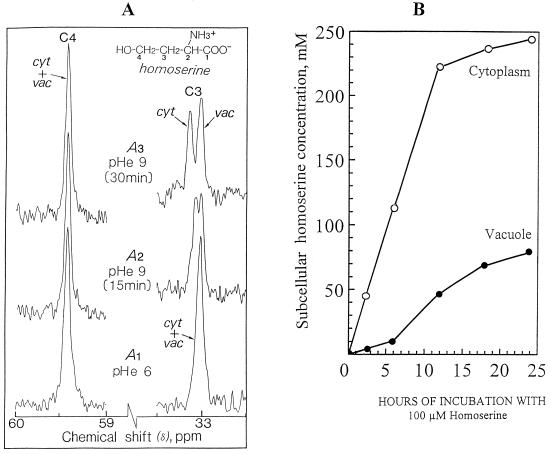
Under these conditions a close examination of an expanded scale of the spectra between 33.5 and 32.5 ppm (Fig. 2, A2 and A3) showed two distinct peaks of intracellular C3 of homoserine at approximately 33.05 and 33.15 ppm, corresponding to pH 8.5 and 6.5, respectively (note that the sum of the two peak areas observed at pHe 9.0 is almost identical to that of the single peak observed at pHe 6.0, Fig. 2, A1). These values reflect the presence of the vacuolar homoserine pool at the acidic pH and the cytoplasmic pool at alkaline pH. It was therefore possible to observe directly, for the first time to our knowledge, the compartmentation of an amino acid (homoserine) between cytoplasmic and vacuolar compartments.
Figure 2.
Compartmentation of homoserine between cytoplasm and vacuole, determined by in vivo 13C-NMR after rapid alkalization. Sycamore cells were incubated with 100 μm homoserine for 12 h at pH 6.0 as in Figure 1. Magnifications of the resonance peaks of C3 and C4 of homoserine are shown (A). Each spectrum is the result of 225 transients (15 min). A1, Spectrum obtained before NH4+ addition, pH 6.0; A2 and A3, spectra obtained 15 and 30 min, respectively, after alkalization of the perfusion medium (pHe) from 6.0 to 9.0 in the presence of 0.5 mm NH4+. Under these conditions C4 from homoserine was not affected by pH, whereas C3 was split into two distinct peaks corresponding to the vacuolar and cytoplasmic pools of homoserine. B, Curves show kinetics of accumulation of homoserine in the cytoplasm and in the vacuole. Each time point was obtained from a separate set of cells incubated with 100 μm homoserine at pHe 6.0 during various times and then subjected to NH4+ addition. cyt, Cytoplasm; vac, vacuole.
The signal from the carboxyl group (C1) of homoserine was also split in two peaks. The pH calibration curves done with perchloric acid extracts confirm that the chemical shift of C1 and C3 of homoserine moves downfield when the pH increases above 7.5 (not shown). Under the same conditions, the chemical shift of C4 remained nearly constant (Fig. 2A, at 59.4 ppm). The signal from C2 (at 54.2 ppm), which is close to the ammonium and carboxyl groups of the molecule, was not significantly influenced by pH. The influence of the pH on the position of the signal from C3 of homoserine could be attributable to a potential folding of the molecule, permitting interactions with the ammonium group at alkaline pH. In this case the steric effect would be predominant compared with the inductive effect (Deslauriers and Smith, 1980; Wehrli et al., 1995).
After 12 h of incubation with 100 μm homoserine, the distribution of homoserine between the cytoplasm and the vacuole was nearly 45 and 55%, respectively (Fig. 2, A and B). Assuming a vacuole-to-cytoplasm ratio of 6 (Aubert et al., 1996c), we calculated that cytoplasmic and vacuolar concentrations were approximately 210 and 42 mm, respectively, when the total intracellular concentration was about 66 mm. Figure 2B indicates that homoserine first accumulated in the cytoplasmic compartment. The concentration of homoserine remained much higher in the cytoplasm than in the vacuole throughout its accumulation, suggesting that homoserine molecules were slowly expelled into the vacuole. After 12 h the concentration ratio of homoserine between cytoplasm and vacuole stabilized at 4 to 5. When the perfusion medium contained 100 μm homoserine at pH 6.0 the final concentration of cytosolic homoserine (exceeding 200 mm) was more than 1000 times higher than the concentration of external homoserine.
In contrast, perfusion of intact cells with 100 μm homoserine at pH 8.0 instead of 6.0 led to a marked reduction of intracellular signals deriving from homoserine (Fig. 1C, expanded scale). However, under the same conditions the intracellular signals deriving from phosphohomoserine did not decline, suggesting that the intracellular concentration of homoserine was sufficient to sustain the full rate of phosphohomoserine synthesis (see below). Because cytoplasmic pH (7.5) is independent of pHe over the range 5.0 to 8.0 (Gout et al., 1992), these results suggest that homoserine enters plant cells via an active process involving or regulated by the pH gradient across the plasmalemma. In the next section, we describe the kinetic parameters of this active transport process.
Characterization of Homoserine Uptake by Sycamore Cells
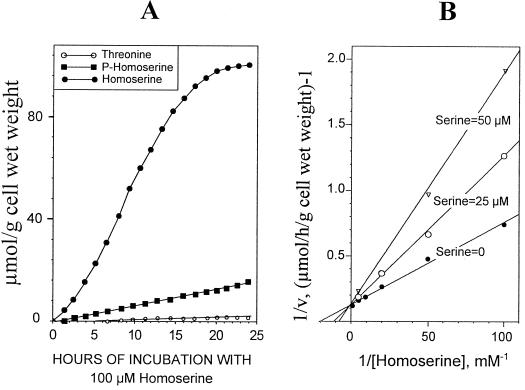
Cells were perfused at a flow rate of 50 mL min−1 with a well-oxygenated (O2 bubbling) nutrient medium containing 100 μm phosphate (see Methods) and adjusted to pH 6.0. Incubations were commenced at time 0 by the addition of known amounts of homoserine to the perfusion medium. The intracellular accumulation of free homoserine, phosphohomoserine, and Thr were recorded continuously with 13C-NMR. When 100 μm homoserine was added, free homoserine accumulated very rapidly in the cell (Fig. 3A), where its final cellular level attained approximately 95 to 100 μmol g−1 cell wet weight. This experiment also shows that the accumulation of phosphohomoserine and Thr exhibited a linear increase over a very long period of time.
Figure 3.
Characterization of homoserine transport in sycamore cells. A, Evolution of intracellular homoserine, phosphohomoserine, and Thr in sycamore cells incubated with 100 μm homoserine at pH 6.0, determined from in vivo 13C-NMR spectra. The values, expressed as μmol g−1 cell wet weight, are from a representative experiment chosen from a series of five. B, Double-reciprocal plot of the initial rates of homoserine uptake (rate of homoserine accumulation plus the rate of phosphohomoserine accumulation) in the absence or presence of Ser (25 and 50 μm).
When the maximal rates of homoserine uptake (the rate of homoserine accumulation plus the rate of phosphohomoserine accumulation determined by 13C-NMR, assuming that the rate of phosphohomoserine utilization was negligible; see below and Fig. 6) were presented as a double-reciprocal plot, Michaelis-Menten kinetics were observed. For six experiments the apparent Km was 50 to 60 μm and the Vm was 8 ± 0.5 μmol homoserine incorporated h−1 g−1 cell wet weight at 20°C, suggesting that a carrier was involved in the transport of homoserine through the plasma membrane (Fig. 3B). The activity of this carrier was determined as a function of pHe from 5.0 to 8.0. Table I indicates that the transport of homoserine declined as the pH of the external medium increased. For example, at pH 8.0, Vm was 4 to 4.5 times lower than at pH 6.0. Figure 3B also shows that Ser behaved as a competitive inhibitor of homoserine carrier (Ki, approximately 25–30 μm). Similar results were observed with Thr and Ile (not shown). On the other hand, other amino acids, including glutamate and Lys, had no effect on the transport of homoserine through the plasma membrane.
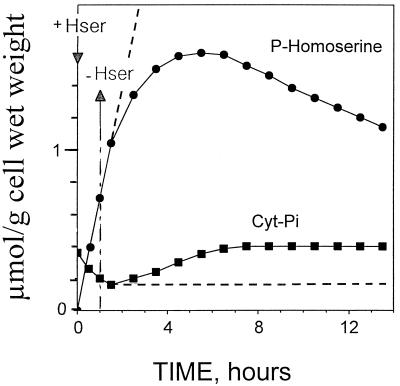
Figure 6.
Evolution of cytoplasmic Pi (Cyt-Pi) and phosphohomoserine in sycamore cells. In vivo 31P-NMR was used to determine Cyt-Pi and phosphohomoserine. At time 0, 100 μm homoserine was added to the perfusion medium at pH 6.0. After 1 h, cells were maintained in the medium (dashed lines) or perfused with a culture medium devoid of homoserine to remove extracellular homoserine (unbroken lines). The values, expressed as μmol g−1 cell wet weight, are from a representative experiment chosen from a series of five. Hser, Homoserine.
Table I.
Influence of pH on the maximal activity (Vm) of homoserine transport in sycamore cells
| Homoserine Transport Vm
| |||
|---|---|---|---|
| pH 5.0 | pH 6.0 | pH 7.0 | pH 8.0 |
| μmol h−1 g−1 cell wet wt | |||
| 6.5 ± 0.4 | 8 ± 0.5 | 3.8 ± 0.3 | 1.8 ± 0.2 |
Cells were incubated under the experimental conditions described in the legend to Figure 3, at various external pHs. The values were obtained from the analysis of 13C-NMR spectra (five independent experiments). The results are means ± sd of five experiments.
Accumulation and Metabolism of Phosphohomoserine in Sycamore Cells
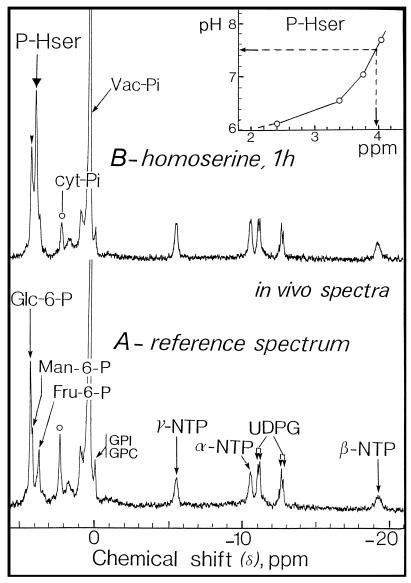
Figure 4 illustrates the changes that occurred in the 31P-NMR spectra from cells incubated with 100 μm homoserine in the perfusion medium at pH 6.0. After 1 h a marked increase in the resonance centered at 4.1 ppm was observed. This peak was attributed to phosphohomoserine after the analysis of perchloric acid extracts (see Fig. 5). Signals from hexose 6-P (especially Man 6-P and Fru 6-P) and phosphohomoserine partially overlapped on in vivo spectra (Fig. 4). However, these signals were clearly separated on the corresponding spectra (Fig. 5) obtained from perchloric acid extracts, thus permitting an accurate identification and quantification of the resonances.
Figure 4.
Representative in vivo 31P-NMR spectra of sycamore cells. The spectra, recorded at 20°C, are the result of 6000 transients (1 h). A, Control cells; B, cells incubated with 100 μm homoserine for 1 h at pH 6.0. cyt, Cytoplasm; GPC, glycerylphosphorylcholine; GPI, glycerylphosphorylinositol; NTP, nucleoside triphosphate; P-Hser, phosphohomoserine; UDPG, UDP-α-d-Glc; and Vac, vacuole.
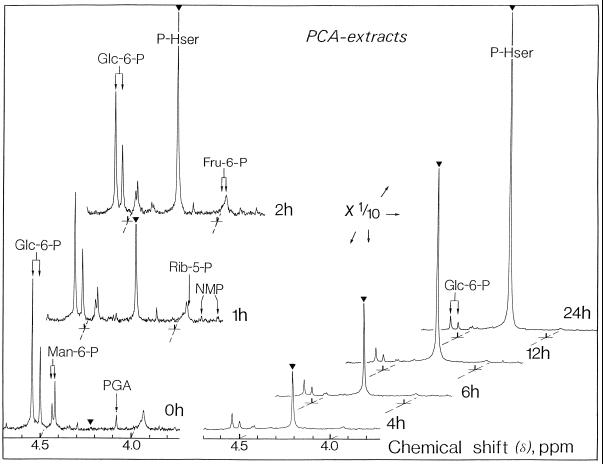
Figure 5.
Representative in vitro 31P-NMR spectra (perchloric acid extracts, expanded scale from 3.7 to 4.7 ppm) of sycamore cells. Cells were incubated at various times with 100 μm homoserine at pH 6.0. Perchloric acid extracts were prepared according to the procedure described in Methods. The spectra, recorded at 20°C, are the result of 1024 transients (1 h). NMP, Nucleoside monophosphate; PCA, perchloric acid; PGA, 3-phosphoglycerate; and P-Hser, phosphohomoserine.
Titration curves (Fig. 4) plotting chemical shift versus pH for phosphohomoserine in crude cell extracts indicated that the position of the phosphoryl group centered at 4.1 ppm corresponded to that of phosphohomoserine at pH > 7.0. This suggests that this phosphorylated compound derived from exogenously added homoserine accumulated in the cytoplasmic compartment (pH 7.5) but not in the vacuole (pH 5.7). This was confirmed by the fact that Mn2+ added to the perfusion medium did not eclipse the peak of cytoplasmic phosphohomoserine (not shown). We have previously established that this paramagnetic ion accumulated specifically in the vacuole (Roby et al., 1988) and suppressed all of the resonances arising from vacuolar compounds (including phosphate).
Phosphohomoserine steadily accumulated at the constant rate of approximately 0.7 μmol h−1 g−1 cell wet weight, as determined from 31P-NMR (Figs. 5 and 6) and from 13C-NMR (Fig. 3A). After a 24-h incubation with 100 μm homoserine, the concentration of phosphohomoserine attained in the cytoplasmic compartment was considerable (>0.1 m). During the course of rapid phosphohomoserine accumulation in the cytoplasmic compartment, hexose-6-P peak intensities, including Glc-6-P, Man-6-P, and Fru-6-P, did not change significantly. Likewise, the cytoplasmic pH remained constant throughout phosphohomoserine accumulation.
Experiments were undertaken to follow the rate of phosphohomoserine utilization. The cells were first maintained in a medium containing homoserine for 60 min (pulse), and were then perfused with a culture medium devoid of homoserine to remove extracellular homoserine (Fig. 6, unbroken lines). The cytoplasmic phosphohomoserine peak, which had built up in the presence of 100 μm external homoserine, decreased steadily (Fig. 6). A comparison of Figures 3 and 6 indicates that the rate of phosphohomoserine utilization (approximately 0.06 μmol h−1 g−1 cell wet weight) was much lower than its rate of accumulation (approximately 0.7 μmol h−1 g−1 cell wet weight) and perfectly matched the rate of Thr accumulation (approximately 0.05–0.06 μmol h−1 g−1 cell wet weight; see below and Fig. 3A). The lag phase observed before the steady utilization of phosphohomoserine (Fig. 6) was attributable to the progressive phosphorylation of the endogenous pool of homoserine previously accumulated during the pulse experiment (see above).
During the course of homoserine phosphorylation the total amount of cytoplasmic phosphate declined (see Fig. 4) to a new steady-state equilibrium insofar as the medium contained homoserine (Fig. 6, dashed lines). After removal of external homoserine, the concentration of cytoplasmic phosphate increased progressively up to its original value as the rate of homoserine phosphorylation decreased, due to a progressive decrease in the concentration of intracellular homoserine (Fig. 6, unbroken lines). In fact, in a previous article Rébeillé et al. (1982) demonstrated that the rate of phosphate transport across the plasma membrane in sycamore cells can fluctuate from a low value (0.3 μmol h−1 g−1 cell wet weight) in normal cells to a high value (3 μmol h−1 g−1 cell wet weight) in phosphate-starved cells, and that the cytoplasmic phosphate concentration controls its rate of transport through the plasma membrane.
Consequently, at the very beginning of homoserine phosphorylation the phosphate uptake rate (0.3 μmol h−1 g−1 cell wet weight) was not sufficient to sustain the full rate of homoserine phosphorylation (0.7 μmol h−1 g−1 cell wet weight), and the phosphate necessary for phosphohomoserine synthesis was derived partly from the cytoplasmic phosphate pool. The total amount of cytoplasmic phosphate therefore decreased, leading to a progressive acceleration in the rate of phosphate import. The new steady-state equilibrium in cytoplasmic phosphate concentration was attained when the rate of phosphate transport perfectly matched the rate of homoserine phosphorylation. On the other hand, in the absence of phosphate in the perfusion medium the phosphate necessary for phosphohomoserine synthesis was derived first from cytoplasmic phosphate. When a threshold of cytoplasmic phosphate was attained, the phosphorylation of homoserine was sustained by the continuous release of phosphate from the vacuole, at a rate that decreased from 0.7 μmol h−1 g−1 cell wet weight to 30 nmol h−1 g−1 cell wet weight, reflecting the low rate of vacuolar phosphate efflux that occurs in response to the depletion of phosphate from the cytoplasmic compartment (not shown). This is consistent with the results obtained with spinach leaves incubated with choline as a Pi-sequestering reagent (see Bligny et al., 1990).
Finally, we observed that at low external concentrations of homoserine (<10 μm, at pH 6.0) homoserine did not accumulate because the rate of phosphorylation (maximal rate, approximately 0.7 μmol h−1 g−1 cell wet weight) was higher than the initial uptake rate of homoserine. Consequently, under these conditions homoserine transported through the membrane was instantly converted into phosphohomoserine by homoserine kinase. However, at higher external homoserine concentrations (>10 μm, at pH 6.0), homoserine accumulated because the maximal rate of phosphohomoserine formation was not sufficient to cope with the massive influx of homoserine. Homoserine was therefore increasingly recovered as free homoserine sequestered in the cell (cytoplasm plus vacuole; see above).
Effect of Homoserine on E. colonum Cells
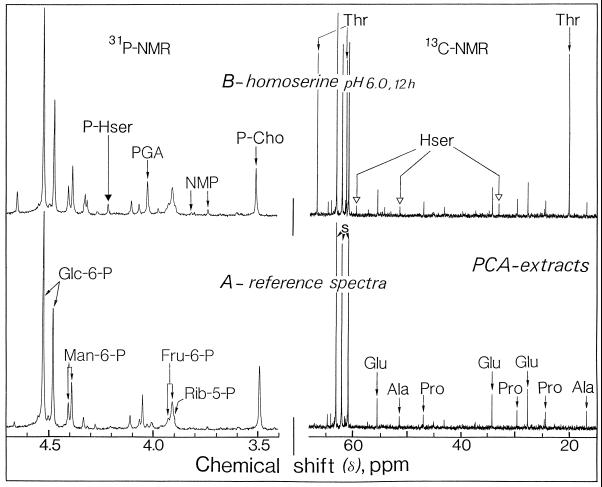
Figure 7 illustrates the changes that occurred in the 31P- and 13C-NMR spectra of perchloric acid extracts obtained from cells of the weed monocot E. colonum maintained at pH 6.0 for 12 h in a nutrient medium containing 100 μm homoserine. The perchloric acid extracts were prepared from 9 g (wet weight) of E. colonum cells and neutralized at pH 7.5 in the presence of 60 mm 1,2-cyclohexylenedinitrilotetraacetic acid. In the 13C-NMR reference spectra from E. colonum cells (Fig. 7A, 13C-NMR) (homoserine was omitted from the nutrient medium) the major resonances in the chemical shift range of 10 to 80 ppm arose from glutamate (at 27.8, 34.2, and 55.6 ppm), Pro (at 24.5, 29.6, and 47.0 ppm), and Ala (at 17.0 and 51.4 ppm). Under these conditions resonances from homoserine and Thr were undetectable. In marked contrast to the situation observed with sycamore cells, E. colonum cells maintained in a medium containing homoserine (100 μm for 12 h at pH 6.0) accumulated Thr in place of homoserine. The resonances of highest intensity in the chemical range from 10 to 80 ppm corresponded to those of C2, C3, and C4 of Thr at 20.2, 61.3, and 66.7 ppm, respectively (Fig. 7B). The intracellular Thr content attained after 12 h was considerable, approximately 55 to 60 μmol g−1 cell wet weight.
Figure 7.
Representative in vitro 13C- (expanded scale from 15 to 65 ppm) and 31P- (expanded scale from 3.4 to 4.7 ppm) NMR spectra from E. colonum cells. A, Control cells; B, cells incubated for 12 h with 100 μm homoserine at pH 6.0. Hser, Homoserine; NMP, nucleoside monophosphate; PCA, perchloric acid; P-Cho, phosphorylcholine; PGA, 3-phosphoglycerate; P-Hser, phosphohomoserine; and s, Suc.
Using NH4+ to localize Thr (see Fig. 2), we observed that the distribution of Thr between cytoplasm and vacuole was nearly 40 and 60%, respectively (data not shown). Assuming a vacuole-to-cytoplasm ratio of 6, the calculated concentration of Thr in the cytoplasm was nearly 180 mm, 4 to 5 times higher than in the vacuole (nearly 40 mm). The other major resonances in the chemical shift range of 10 to 80 ppm arose from homoserine and from the free amino acids previously observed in the control cells (glutamate, Pro, and Ala). The activity of homoserine transport (estimated by adding the rates of homoserine and Thr accumulations) were similar to those observed in sycamore cells (Vm approximately 8–9 μmol h−1 g−1 cell wet weight). On the other hand, the 31P-NMR spectra of perchloric acid extracts indicate that homoserine did not trigger massive accumulation of phosphohomoserine in E. colonum cells. Again, such behavior contrasted markedly to the situation observed in sycamore cells, in which phosphohomoserine accumulated in the cytoplasmic compartment to very high levels.
To understand these differences between sycamore and E. colonum cells, we analyzed the activity of Thr synthase, the enzyme involved in the conversion of phosphohomoserine to Thr. The activities were assayed in the presence of the activator SAM (Table II). The maximal activity of E. colonum Thr synthase assayed in the presence of SAM (4.3 ± 0.3 μmol phosphohomoserine converted h−1 g−1 cell wet weight) roughly matched the large accumulations of Thr (55–60 μmol g−1 cell wet weight after 12 h of incubation with homoserine, see Fig. 7). On the other hand, Thr synthase activity was much lower in sycamore cells, consistent with the relatively low accumulations of Thr and the high accumulations of phosphohomoserine previously observed. Finally, western-blot analysis indicated that E. colonum cells contained 6 to 8 times more Thr synthase than did sycamore cells (not shown).
Table II.
Thr synthase activities in crude extracts from sycamore and E. colonum cells
| Thr Synthase Activity
| |
|---|---|
| Sycamore | E. colonum |
| μmol g−1 cell wet wt | |
| 0.54 ± 0.07 | 4.3 ± 0.3 |
Activities were assayed at 20°C in the presence of 200 μm SAM, and expressed as phosphohomoserine converted to Thr. The results are means of five independent experiments.
DISCUSSION
The results presented in this article demonstrate that a carrier is involved in the transport of homoserine into plant cells based on the observations that it was saturable and that homoserine accumulated within the cell cytoplasm against a large concentration gradient. Experiments with purified plasma membrane vesicles obtained from plant tissues provided evidence for at least four proton-amino acid symporters: an acidic amino acid symporter, a basic amino acid symporter, and two symporters for the neutral amino acids (for review, see Bush, 1993; Frommer et al., 1994). Our results strongly suggest that homoserine enters the cell via a proton-Ser (Thr) symport because its rate of entry was markedly decreased at alkaline pH and was competitively inhibited by hydroxyl-containing amino acids such as Ser and Thr. The patterns of inhibition observed in intact sycamore cells (see Fig. 3B) were similar to those described by Li and Bush (1991) using plasma membrane vesicles from sugar beet leaves.
The fact that plant cells exhibit a powerful carrier driving homoserine (and other amino acids) inside the cell is rather puzzling because sycamore and E. colonum cells used in this study contain in their plastids all of the enzymatic machinery for amino acid synthesis from NO2−. However, because the cytoplasmic compartment in various metabolic situations accumulates rather high concentrations of various amino acids, one may suppose that amino acids leaving cells by passive diffusion are immediately reimported by the proton-coupled symporters linking translocation across the plasma membrane to the ΔpH generated by a P-type, H+-pumping ATPase. In other words, in addition to their role in amino acid uptake from the sieve, one of the major functions of proton-coupled symporters would be to counteract the passive leakage of amino acids out of the cells.
Incubation of cells with homoserine or other amino acids resulted in large intracellular accumulations of these amino acids. This observation could account for the inhibitory effect of amino acids on the growth of cell suspensions previously reported (Bonner et al., 1992, and refs. therein) and verified here in sycamore and E. colonum cells (data not shown). The use of in vivo 13C-NMR led us to visualize, for the first time to our knowledge, the compartmentation of an amino acid such as homoserine between cytoplasm and vacuole when the medium contained NH4+, which induces a rapid alkalization of cell cytoplasm and vacuole. Using nonaqueous fractionation, Riens et al. (1991) reported that amino acids found in spinach leaves were located mainly in the cytoplasm (chloroplast stroma and cytosol), whereas the amino acid concentrations in the vacuole were 1 order of magnitude lower. In contrast, using vacuoles isolated from barley leaf protoplasts, Dietz et al. (1990) showed that the major part of amino acids was sequestered inside the vacuolar compartment (except glutamate, which was predominantly localized in the cytoplasm) (see also Homeyer et al., 1989; Goerlach and Willms-Hoff, 1992; Martinoia, 1992).
Our results show that homoserine concentration in the cytoplasmic compartment was much higher than in the vacuole, even at equilibrium after a long time of incubation. Similarly, we have observed that addition of various amino acids (including Ser, Thr, and Ile) to the perfusion medium (100 μm) also led to high intracellular concentrations of these amino acids (>50 μmol g−1 wet weight after 1 d of incubation). Again, the final concentrations of these amino acids attained in the vacuole were systematically much lower than those found in the cytoplasmic compartment. Our results, therefore, cast doubts on the presence of translocators in tonoplast membranes involved in the concentration of amino acids into the vacuole.
The data reported here also suggest that the conversion of homoserine to Thr via phosphohomoserine is not subjected to regulation in vivo by the end products Thr and Ile. Indeed, in sycamore cells fed with homoserine, both phosphohomoserine and Thr steadily accumulated to dramatic concentrations over more than 24 h. In addition, in E. colonum and sycamore cells, we observed that the flux of C flowing through Thr synthase is roughly governed by the maximal capacity of this enzyme, suggesting that in vivo the concentration of SAM is sufficient enough to sustain the full activity of Thr synthase.
Homoserine did not trigger an accumulation of cystathionine or Met, probably because cystathionine-γ synthase activity (the enzyme converting phosphohomoserine to cystathionine) is limited by the supply of Cys. On the other hand, the accumulations of Thr observed in both cell species did not trigger Ile accumulation. This observation is consistent with the suggestion that the Ile biosynthetic pathway from phosphohomoserine is strongly regulated after Thr. In this regard, we have recently reported that sulfometuron methyl, a potent inhibitor of acetolactate synthase (the enzyme that follows Thr dehydratase in the conversion of Thr to Ile), triggered the accumulation of α-oxobutyrate and its transamination product, α-aminobutyrate, in sycamore cells (Aubert et al., 1996a).
We also observed that incubation of sycamore cells with sulfometuron methyl plus homoserine resulted in dramatic accumulations of α-aminobutyrate (up to 35–40 μmol g−1 cell wet weight), whereas Ile fully prevented this accumulation. All of these data are consistent with an in vivo feedback inhibition of Thr dehydratase by Ile, as has been characterized in vitro (for review, see Singh and Shaner, 1995). Finally, the enzymatic steps involved in the conversion of α-oxobutyrate to Ile are likely not regulated in vivo because the incubation of sycamore cells with α-aminobutyrate results in dramatic accumulations of Ile (Aubert et al., 1997).
Little is known about the transport of amino acids through the plastidial envelope (for reviews, see Douce and Joyard, 1979; Heldt and Flügge, 1987). Our results raise the problem of the massive penetration of homoserine into the plastids. Indeed, because homoserine kinase and Thr synthase are confined to the stroma (Wallsgrove et al., 1983), the rate of homoserine import into the plastids must be at least comparable to the rate of phosphohomoserine plus Thr accumulation, i.e. approximately 0.8 and 5 μmol h−1 g−1 cell wet weight in sycamore and E. colonum cells, respectively. In addition, 31P-NMR indicated that phosphohomoserine accumulated in the cytoplasmic compartment of sycamore cells to a concentration up to 0.1 m. Assuming that phosphohomoserine remained sequestered in the plastids and that the volume of plastids in sycamore cells accounts for less than 10% of the cytoplasm (Aubert et al., 1996c), its concentration in the stroma would be >1 m. Such a huge accumulation could account for the toxicity of homoserine treatment, but a diffusion of this phosphorylated compound to the cytosol cannot be excluded.
Finally, concerning Thr accumulation in E. colonum cells, 13C-NMR showed that this amino acid synthesized in the plastids was partly found in the vacuolar compartment. This observation indicates that Thr was transported out of the plastids to the cytosol and the vacuole. This is consistent with the fact that most amino acids are synthesized exclusively in the stroma, and that transport systems must be present in the plastid envelope to sustain general metabolism such as cytosolic protein synthesis.
Abbreviations:
- pHe
external pH
- SAM
S-adenosylmethionine
LITERATURE CITED
- Aubert S, Alban C, Bligny R, Douce R. Induction of β-methylcrotonyl-coenzyme A carboxylase in higher plant cells during carbohydrate starvation: evidence for a role of MCCase in leucine catabolism. FEBS Lett. 1996a;383:175–180. doi: 10.1016/0014-5793(96)00244-x. [DOI] [PubMed] [Google Scholar]
- Aubert S, Bligny R, Day DA, Whelan J, Douce R. Induction of alternative oxidase synthesis by herbicides inhibiting branched-chain amino acid synthesis. Plant J. 1997;11:649–657. [Google Scholar]
- Aubert S, Bligny R, Douce R (1996b) NMR studies of metabolism in cell suspensions and tissue cultures. In Y Shachar-Hill, P Pfeffer, eds, Nuclear Magnetic Resonance in Plant Physiology. American Society of Plant Physiologists, Rockville, MD, pp 109–154
- Aubert S, Gout E, Bligny R, Mazars-Marty D, Barrieu F, Alabouvette J, Marty F, Douce R. Ultrastructural and biochemical characterization of autophagy in higher plant cells submitted to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol. 1996c;133:1251–1263. doi: 10.1083/jcb.133.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligny R, Gardestrom P, Roby C, Douce R. 31P NMR studies of spinach leaves and their chloroplasts. J Biol Chem. 1990;265:1319–1326. [PubMed] [Google Scholar]
- Bligny R, Leguay J-J. Techniques of cell cultures. Methods Enzymol. 1987;148:3–16. [Google Scholar]
- Bonner CA, Rodrigues JA, Miller JA, Jensen RA. Amino acids are general inhibitors of Nicotiana sylvestris in tissue culture. Physiol Plant. 1992;84:319–328. [Google Scholar]
- Bryan JK. Synthesis of the aspartate family and branched-chain amino acids. In: Stumpf PK, Conn EE, editors. The Biochemistry of Plants, Vol 5. San Diego, CA: Academic Press; 1980. pp. 403–453. [Google Scholar]
- Bryan JK. Advances in the biochemistry of amino acid biosynthesis. In: Stumpf PK, Conn EE, editors. The Biochemistry of Plants, Vol 16. San Diego, CA: Academic Press; 1990. pp. 161–195. [Google Scholar]
- Bush DR. Proton-coupled sugar and amino acid transporters in plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:513–542. [Google Scholar]
- Bush DR, Chiou T-J, Chen L. Molecular analysis of plant sugar and amino acid transporters. J Exp Bot. 1996;47:1205–1210. doi: 10.1093/jxb/47.Special_Issue.1205. [DOI] [PubMed] [Google Scholar]
- Curien G, Dumas R, Ravanel S, Douce R. Characterization of an Arabidopsis thaliana cDNA encoding an S-adenosylmethionine sensitive threonine synthase. FEBS Lett. 1996;390:85–90. doi: 10.1016/0014-5793(96)00633-3. [DOI] [PubMed] [Google Scholar]
- Deslauriers R, Smith ICP (1980) The multinuclear NMR approach to peptides: structures, conformations, and dynamics. In LJ Berliner, J Reuben, eds, Biological Magnetic Resonance, Vol 2. Plenum Press, New York, pp 243–344
- Dietz KJ, Jäger R, Kaiser G, Martinoia E. Amino acid transport across the tonoplast of vacuoles isolated from barley mesophyll protoplasts. Plant Physiol. 1990;92:123–129. doi: 10.1104/pp.92.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R, Joyard J. Structure and function of the plastid envelope. Adv Bot Res. 1979;7:1–116. [Google Scholar]
- Frommer WB, Kwart M, Hirner B, Fischer WN, Hummel S, Ninnemann O. Transporters for nitrogenous compounds in plants. Plant Mol Biol. 1994;26:1651–1670. doi: 10.1007/BF00016495. [DOI] [PubMed] [Google Scholar]
- Goerlach J, Willms-Hoff I. Glycine uptake into barley mesophyll vacuoles is regulated but not energized by ATP. Plant Physiol. 1992;99:134–139. doi: 10.1104/pp.99.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout E, Bligny R, Douce R. Regulation of intracellular pH values in higher plant cells: carbon-13 and phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1992;267:13903–13909. [PubMed] [Google Scholar]
- Gout E, Bligny R, Pascal N, Douce R. 13C nuclear magnetic resonance studies of malate and citrate synthesis and compartmentation in higher plant cells. J Biol Chem. 1993;268:3986–3992. [PubMed] [Google Scholar]
- Heldt HW, Flügge UI (1987) Subcellular transport of metabolites in plant cells. In DD Davies, ed, The Biochemistry of Plants, Vol 12. Academic Press, New York, pp 50–85
- Homeyer U, Litek K, Schulz G. Uptake of phenylalanine into isolated barley vacuoles is driven by both tonoplast adenosine triphosphatase and pyrophosphatase. Plant Physiol. 1989;89:1388–1393. doi: 10.1104/pp.89.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet E-P, Bligny R, Douce R. Biochemical changes during sucrose deprivation in higher plant cells. J Biol Chem. 1986;261:3193–3199. [PubMed] [Google Scholar]
- Li ZC, Bush DR. ΔpH-dependent amino acid transport into plasma membrane vesicles isolated from sugar beet (Beta vulgaris L.) leaves. Plant Physiol. 1991;96:1338–1344. doi: 10.1104/pp.96.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroth P, Mopper K. High performance liquid chromatographic determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization with O-phthaldialdehyde. Anal Biochem. 1979;51:1667–1674. [Google Scholar]
- Martinoia E. Transport processes in vacuoles of higher plants. Bot Acta. 1992;105:232–245. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Ramos C, Delgado MA, Calderon IL. Inhibition by different amino acids of the aspartate kinase and the homoserine kinase of the yeast Saccharomyces cerevisiae. FEBS Lett. 1991;278:123–126. doi: 10.1016/0014-5793(91)80098-n. [DOI] [PubMed] [Google Scholar]
- Rébeillé F, Bligny R, Douce R. Regulation of Pi uptake by Acer pseudoplatanus cells. Arch Biochem Biophys. 1982;219:371–378. doi: 10.1016/0003-9861(82)90168-0. [DOI] [PubMed] [Google Scholar]
- Riens B, Lohaus G, Heineke D, Heldt HW. Amino acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the phloem sap of spinach leaves. Plant Physiol. 1991;97:227–233. doi: 10.1104/pp.97.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Jardetzky O. Monitoring of cellular metabolism by NMR. Biochim Biophys Acta. 1981;639:53–76. doi: 10.1016/0304-4173(81)90005-7. [DOI] [PubMed] [Google Scholar]
- Roby C, Bligny R, Douce R, Tu SI, Pfeffer PE. Facilitated transport of Mn2+ in sycamore (Acer pseudoplatanus L.) cells and excised maize root tips. Biochem J. 1988;250:401–408. doi: 10.1042/bj2520401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby C, Martin J-B, Bligny R, Douce R. Biochemical changes during sucrose deprivation in higher plant cells: phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1987;262:5000–5007. [PubMed] [Google Scholar]
- Singh BK, Shaner DL. Biosynthesis of branched chain amino acids: from test tube to field. Plant Cell. 1995;7:935–944. doi: 10.1105/tpc.7.7.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove RM, Lea PJ, Miflin BJ. Intracellular localization of aspartate kinase and the enzymes of threonine and methionine synthesis. Plant Physiol. 1983;71:780–784. doi: 10.1104/pp.71.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli FW, Marchand AP, Wehrli S (1995) Interpretation of Carbon-13 NMR spectra, Ed 2. John Wiley & Sons, New York, pp 33–97