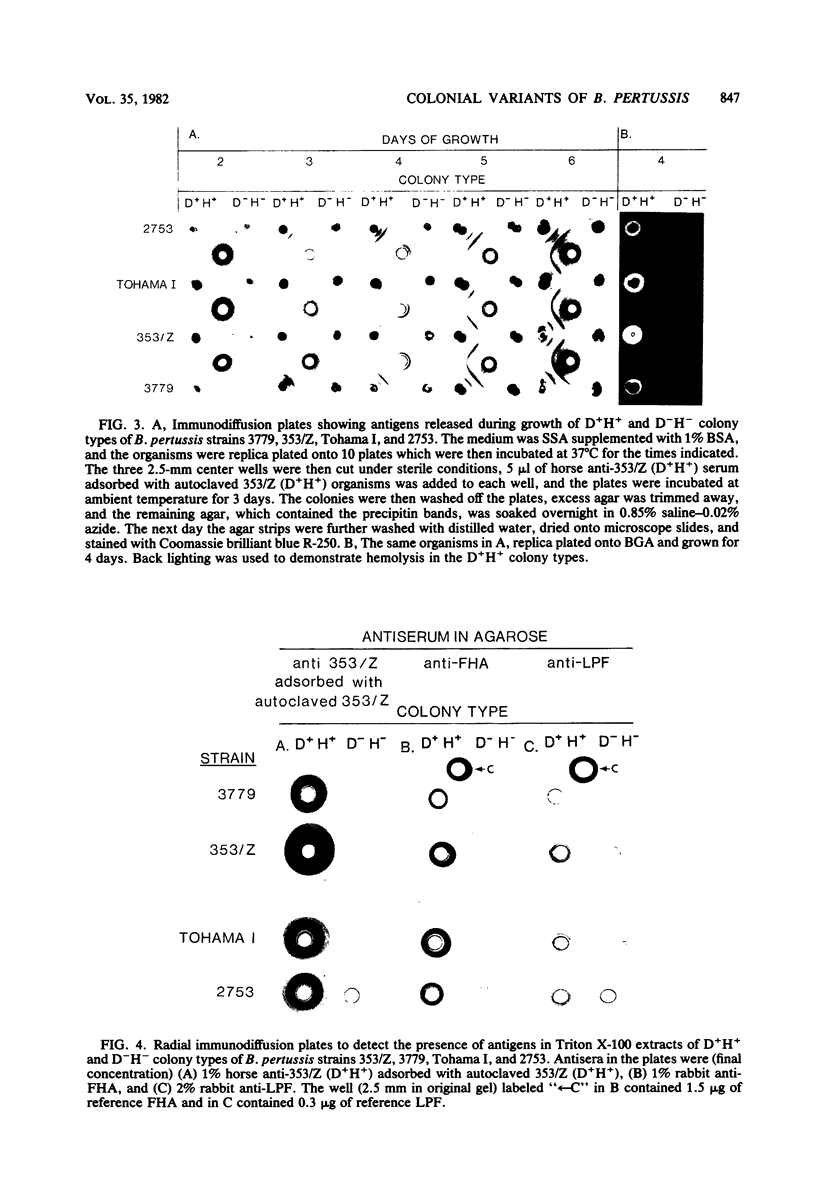
Abstract
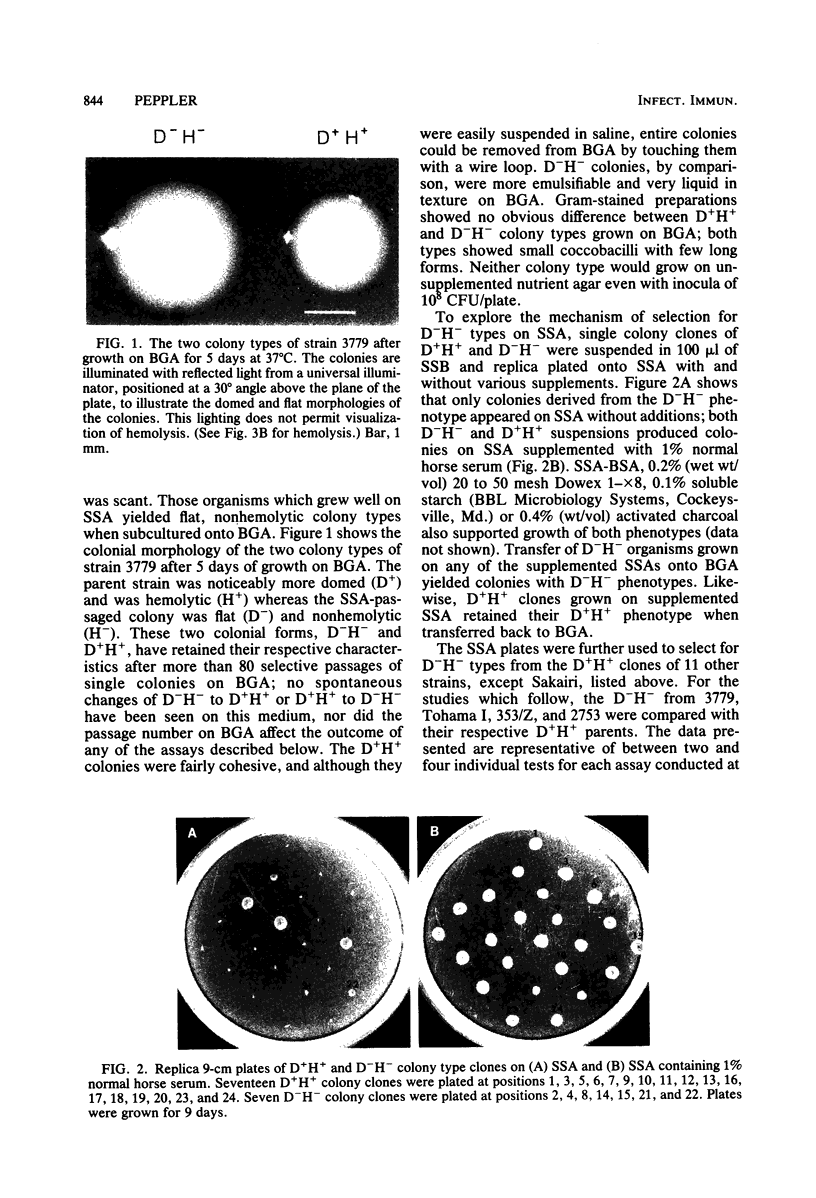
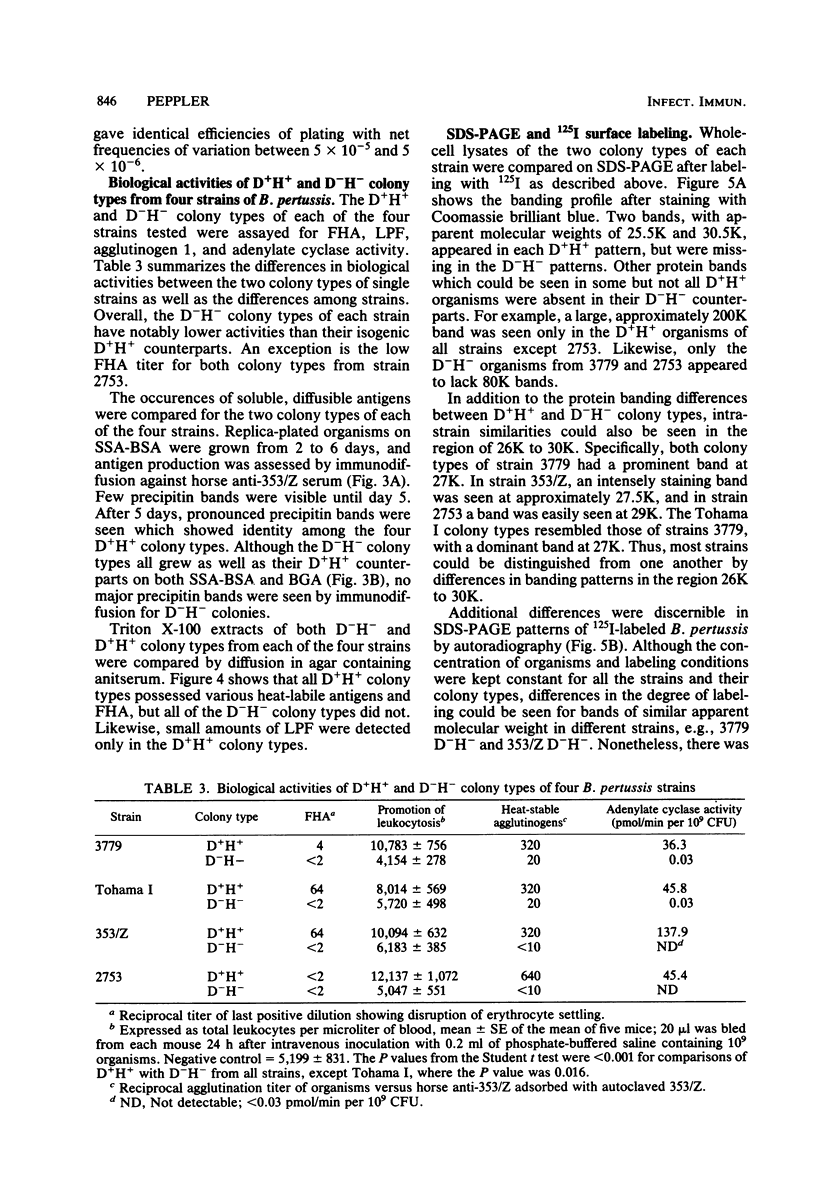
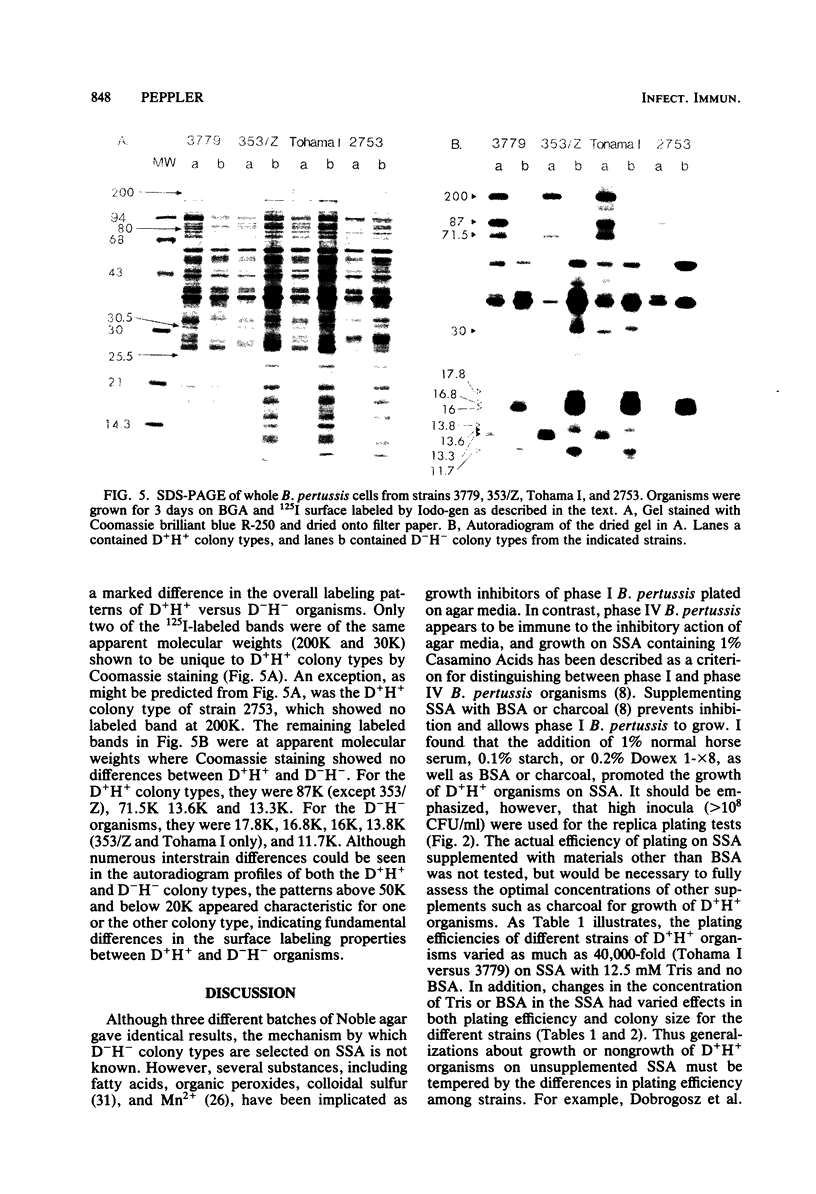
Four different serotype strains of Bordetella pertussis, 3779BL2S4, Tohama I, 353/Z, and 2753, were plated on Bordet-Gengou agar, where they grew as domed, hemolytic (D+H+) wild-type colonies. Cloned D+H+ colony types of all four strains were passed onto modified Stainer-Scholte medium solidified with 1% Noble Agar. Colonies were selected from Stainer-Scholte agar, and these subsequently grew as flat, nonhemolytic (D−H−) colonies when transferred back onto Bordet-Gengou agar. The frequency of D−H− organisms within a population of cloned D+H+ was determined to be between 5 × 10−5 and 5 × 10−6. The D−H− colony types maintained their flat, nonhemolytic characteristics for over 80 single-colony passages on Bordet-Gengou agar. The isogenic pairs of D+H+ and D−H− colony types from the four strains were compared for hemagglutination titer, lymphocytosis-promoting activity, adenylate cyclase activity, and presence of agglutinogens by agglutination. In all cases the D−H− colony types showed reduced activities or amounts of antigen compared with their D+H+ parents. Freely diffusible antigens were markedly different between the two phenotypes as noted by double diffusion of antisera added to plates on which colonies of the variants were growing. Antigens solubilized from the two colony types by Triton X-100 were also markedly different as judged by radial immunodiffusion with antifimbrial hemagglutinin, antilymphocytosis-promoting factor, and anti-353/Z adsorbed with autoclaved 353/Z. In addition, autoradiographs of 125I-surface-labeled whole cells separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed unique banding patterns for each colony type. Since all organisms, regardless of colony type, were grown on Bordet-Gengou agar, the differences reported could not be due to medium composition. Differences between phenotypes were also independent of passage number on Bordet-Gengou agar. By analogy to previous studies, the D−H− organisms appear to fulfill the criteria for phase III or phase IV in the system of Leslie and Gardner (P. H. Leslie and A. D. Gardner, J. Hyg. 31:423-434, 1931) or phase III in the system of Kasuga et al. (T. Kasuga, Y. Nakase, K. Ukishima, and K. Takatsu, Kitasato Arch. Exp. Med. 26:121-134, 1954).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aprile M. A. Areexamination of phase IV Bordetella pertussis. Can J Microbiol. 1972 Dec;18(12):1793–1801. doi: 10.1139/m72-281. [DOI] [PubMed] [Google Scholar]
- Arai H., Munoz J. J. Crystallization of pertussigen from Bordetella pertussis. Infect Immun. 1981 Jan;31(1):495–499. doi: 10.1128/iai.31.1.495-499.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai H., Munoz J. J. Fimbrial hemagglutinin in stationary and shake cultures of Bordetella pertussis. Infect Immun. 1979 Aug;25(2):764–767. doi: 10.1128/iai.25.2.764-767.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai H., Munoz J. J. Purification and crystallization of fimbrial hemagglutinin from Bordetella pertussis. Infect Immun. 1979 Jul;25(1):460–462. doi: 10.1128/iai.25.1.460-462.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronne-Shanbury C. J. The importance of agglutinin production in mice in the determination of the definitive serotype of Bordetella pertussis. J Hyg (Lond) 1976 Apr;76(2):257–264. doi: 10.1017/s0022172400055157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow R. J., Wray C. A simple applicator for use in bacteriocin typing. Med Lab Technol. 1975 Jan;32(1):27–30. [PubMed] [Google Scholar]
- ELDERING G., HORNBECK C., BAKER J. Serological study of Bordetella pertussis and related species. J Bacteriol. 1957 Aug;74(2):133–136. doi: 10.1128/jb.74.2.133-136.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- GREAVES R. I. Preservation of living cells by freeze-drying. Ann N Y Acad Sci. 1960 Apr 13;85:723–728. doi: 10.1111/j.1749-6632.1960.tb49992.x. [DOI] [PubMed] [Google Scholar]
- Gow J. A., Parton R., Wardlaw A. C. Radiolabelling of Bordetella pertussis envelope proteins by the 125 I-lactoperoxidase method. Microbios. 1976;15(61-62):209–219. [PubMed] [Google Scholar]
- Hewlett E. L., Urban M. A., Manclark C. R., Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt L. B. Pitfalls in the preparation of monotypic agglutinating antisera for Bordetella pertussis. J Med Microbiol. 1968 Nov;1(2):169–180. doi: 10.1099/00222615-1-2-169. [DOI] [PubMed] [Google Scholar]
- KASUGA T., NAKASE Y., UKISHIMA K., TAKATSU K. Studies on Haemophilis pertussis. III. Some properties of each phase of H. pertussis. Kitasato Arch Exp Med. 1954 Sep;27(3):37–47. [PubMed] [Google Scholar]
- KASUGA T., NAKASE Y., UKISHIMA K., TAKATSU K. Studies on Haemophilus pertussis. I. Antigen structure of H. pertussis and its phases. Kitasato Arch Exp Med. 1953 Nov;26(2-3):121–133. [PubMed] [Google Scholar]
- LACEY B. W. Antigenic modulation of Bordetella pertussis. J Hyg (Lond) 1960 Mar;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Cole R. L. Mouse-protecting and histamine-sensitizing activities of pertussigen and fimbrial hemagglutinin from Bordetella pertussis. Infect Immun. 1981 Apr;32(1):243–250. doi: 10.1128/iai.32.1.243-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Y., Takatsu K., Kasuga T. Antigenic structure and phase variation in Bordetella pertussis. Jpn J Microbiol. 1969 Sep;13(3):283–291. doi: 10.1111/j.1348-0421.1969.tb00468.x. [DOI] [PubMed] [Google Scholar]
- Parker C. Role of the genetics and physiology of Bordetella pertussis in the production of vaccine and the study of host-parasite relationships in pertussis. Adv Appl Microbiol. 1976;20:27–42. doi: 10.1016/s0065-2164(08)70107-2. [DOI] [PubMed] [Google Scholar]
- Parton R., Wardlaw A. C. Cell-envelope proteins of Bordetella pertussis. J Med Microbiol. 1975 Feb;8(1):47–57. doi: 10.1099/00222615-8-1-47. [DOI] [PubMed] [Google Scholar]
- Penn C. W., Veale D. R., Smith H. Selection from gonococci grown in vitro of a colony type with some virulence properties of organisms adapted in vivo. J Gen Microbiol. 1977 May;100(1):147–158. doi: 10.1099/00221287-100-1-147. [DOI] [PubMed] [Google Scholar]
- ROWATT E. The growth of Bordetella pertussis: a review. J Gen Microbiol. 1957 Oct;17(2):297–326. doi: 10.1099/00221287-17-2-297. [DOI] [PubMed] [Google Scholar]
- Ross R. F., Munoz J. Antigens of Bordetella pertussis V. Separation of Agglutinogen 1 and Mouse-Protective Antigen. Infect Immun. 1971 Feb;3(2):243–248. doi: 10.1128/iai.3.2.243-248.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw A. C., Parton R., Hooker M. J. Loss of protective antigen, histamine-sensitising factor and envelope polypeptides in cultural variants of Bordetella pertussis. J Med Microbiol. 1976 Feb;9(1):89–100. doi: 10.1099/00222615-9-1-89. [DOI] [PubMed] [Google Scholar]







