Abstract
Aim
Hydrogen sulfide (H2S) is a promising cardioprotective agent and a potential modulator of cardiac ion currents. Yet its cardiac effects on humans are poorly understood due to lack of functional cardiomyocytes. This study investigates electrophysiological responses of human pluripotent stem cells (hPSCs) derived cardiomyocytes towards H2S.
Methods and Results
Cardiomyocytes of ventricular, atrial and nodal subtypes differentiated from H9 embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) were electrophysiologically characterized. The effect of NaHS, a donor of H2S, on action potential (AP), outward rectifier potassium currents (I Ks and I Kr), L-type Ca2+ currents (I CaL) and hyperpolarization-activated inward current (I f) were determined by patch-clamp electrophysiology and confocal calcium imaging. In a concentration-dependent manner, NaHS (100 to 300 µM) consistently altered the action potential properties including prolonging action potential duration (APD) and slowing down contracting rates of ventricular-and atrial-like cardiomyocytes derived from both hESCs and hiPSCs. Moreover, inhibitions of slow and rapid I K (I Ks and I Kr), I CaL and I f were found in NaHS treated cardiomyocytes and it could collectively contribute to the remodeling of AP properties.
Conclusions
This is the first demonstration of effects of H2S on cardiac electrophysiology of human ventricular-like, atrial-like and nodal-like cardiomyocytes. It reaffirmed the inhibitory effect of H2S on I CaL and revealed additional novel inhibitory effects on I f, I Ks and I Kr currents in human cardiomyocytes.
Introduction
Safety and efficacy evaluation of pharmaceuticals for cardiac indications are hindered due to a shortage of suitable human in vitro cellular models. Such limitation has in part precipitated in market withdrawal of several cardiac as well as non-cardiac drugs recently [1], [2]. Current available cardiotoxicity screening platform that relying on animal-derived cardiomyocytes or cell lines ectopically expressing ion channels are not ideal due to species difference in the former and inadequate ion channel interactions in the latter [3]. Human pluripotent stem cell (hPSC) including human embryonic stem cells (hESC) and human induced pluripotent stem cells (hiPSCs) are capable of cardiomyogenesis [4], [5]. hESC- and hiPSC-derived cardiomyocytes (hESC-CMs and hiPSC-CMs) present an unprecedented window for an expedited evaluation of chemical entities for known cardiac toxicity or hitherto unknown cardiac implications. In contrast to hESCs, hiPSCs are capable of giving rise to a renewable source of cardiomyocytes from individual patients. These patient-derived cardiomyocytes offer an immensely valuable resource in evaluating individual-specific responses towards pharmaceutical agents especially anti-arrhythmics that have a narrow therapeutic index that are often compounded by idiosyncratic patient response.
Hydrogen sulfide (H2S) is a recently identified physiological gaseous molecule associated with cardiovascular benefits such as vasodilation and cardiac protection [6]. It is known to affect multiple ion channels that could have implication in cardiac arrhythmia [7]. However, its influence on electrical remodeling of human cardiomyocytes is yet to be understood and its effect on individual ventricular, atrial and nodal cardiomyocyte subtypes has not been explored.
In this study, we demonstrated that H2S altered the action potential (AP) properties of hESC- and hiPSC-derived ventricular (V)-, atrial (A)-like CMs. H2S not only blocked L-type Ca2+ current (I CaL), but also showed inhibitory effects on the slow and rapid outward rectifier K+ currents (I Ks and I Kr).
Methods
Generation of Human Embryonic Stem Cell-derived Cardiomyocytes
H9 hESCs (WiCell, Madison, USA) were maintained on mouse embryonic fibroblasts feeder (Millipore, USA) in hESC medium (80% Knockout Dulbecco’s Modified Eagle Medium or DMEM, 20% Serum replacement, 1% non-essential amino acid, 1 mM L-glutamine, 0.1 mM beta-mercaptoethanol and 4 ng/ml bFGF). Unless specified, all culture reagents were from Invitrogen. Embryoid bodies (EBs) were generated after mechanical dissection of hESCs before being maintained in suspension culture in a cardiomyogenic medium which contains DMEM High Glucose 485 ml, L-GLutamine 5 ml, NEAA 5 ml, Selenium Transferrin 5 ml (Sigma) and 2-mercaptoethanol 3.5 ul supplemented with 5 µM SB 203580 (Sigma), a specific p38-MAPK inhibitor in low adherent 6-well plates (Corning, USA) [8]. Subsequently, contracting EB aggregates (emerged from Day 15 onwards) were plated on 0.1% gelatin coated dishes and maintained in DMEM containing 2% FBS. On day 21, the contracting outgrowth of cardiomyocytes were mechanically dissected out and dissociated in Collagenase B (Roche) [9] into small cell clusters (containing 10–30 cells) and continually cultured in DMEM supplemented with 2% FBS for 2 weeks before examination.
Characterization of Human Pluripotent Stem Cell-derived Cardiomyocytes
Human iPSC-derived cardiomyocytes (hiPSC-CMs) were acquired from Cell Dynamic International (CDI, Wisconsin, USA) and they have been well characterized [10]. All cardiomyocytes used in this study were 5 weeks post cardiac differentiation. For structural characterization, cardiomyocytes were seeded on glass coverslips and immunocytochemically stained for sarcomeric cardiac markers using antibodies against cardiac sarcomeric α-actinin (clone EA-35. Sigma), β-myosin heavy chain or β-MHC (Alexis Biochemicals, FL, USA) followed by Alexa Fluo® 488 goat anti-rabbit IgG (Invitrogen, CA, USA); and cardiac Titin (1∶10) (Sigma-Alrich, MO, USA) followed by Alexa Fluo® 555 donkey anti-rabbit IgG. For electrophysiological characterization, H9 hESC-CMs and hiPSC-CMs were identified with spontaneous action potential (AP) recorded by Patch-Clamp technology later described. Ventricular (V)-, atrial (A) - and nodal (N)-like subtypes of cardiomyocytes were determined by their characteristic AP properties [11].
Evaluation of the Effect of H2S on hESC- and hiPSC-CMs
Freshly prepared NaHS stock solution (1000 mM) was added to cardiomyocytes using a micro-perfusion system. For the concentration curve, NaHS was sequentially added to the cells from low to high concentrations ranging from 100 to 1500 µM.
Patch-clamp Electrophysiology
Cardiomyocytes seeded on 3.5 cm diameter petri dishes were transferred to a recording chamber mounted on the stage of an inverted microscope (TE2000-S, Nikon, Tokyo, Japan). Whole-cell AP and ion currents were recorded with a Patch-Clamp amplifier (Axon 200B, Axon Instruments, Foster City, CA, USA). Patch pipettes were fabricated with a Sutter P-97 horizontal puller (Sutter Instrument, Novato, CA) and had a resistance of 2–4 MΩ when filled with the internal solution. In experiments, 70–90% series resistance was compensated. Currents and voltage protocol generation, data acquisition and analysis, were performed using Clampex and Clampfit software (version 10.0, Axon Instruments). Except Ca2+ current measurement, all experiments were performed at 37°C.
Measurement of action potential
V-, A- and N-like CMs were identified by action potential (AP) patterns recorded by whole cell patch-clamp configuration in normal Tyrode’s solution [(in mM): NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 1, glucose 10, HEPES10, pH 7.4 with NaOH] in current-clamp mode. From H9 hESC-CMs, the AP homogeneity of the subpopulation within each CM cluster (10 to 30 CMs) was determined by testing all “patchable” cardiomyocytes (over 70%) within the cluster.
Measurement of outward K+ current
In the voltage-clamp mode, the outward K+ current was measured on the same V-like cardiomyocytes after AP recording. Immediately after the AP measurement, Ca2+ and Na+ currents (I Ca and I Na) were blocked with 0.5 mM CdCl2 and 20 µmol/L TTX in normal Tyrode’s solution, respectively. The outward K+ currents were elicited by a 500-ms depolarization from holding potential of −80-mV to voltages ranging from −40 to +70 mV in 10-mV steps. Pipette solution for AP and I K (in mM): KCl 130, MgCl2 1, MgATP 3, EGTA 10, and HEPES 10, pH 7.2 with KOH. Modified extracellular solution for the AP and outward K+ currents (in mM): normal Tyrode’s solution plus 0.5 mM CdCl2 and 20 µmol/L TTX to block I Ca and I Na, respectively.
Measurement of L-type Ca2+ currents
The H9 hESC-CM clusters contain homogeneous V-like cardiomyocytes (after consistent identification of 5 V-like cardiomyocytes in that cluster) were subsequently used for measuring of L-type Ca2+ currents (I CaL). The I CaL was evoked by a 400-ms pulse to +60 mV from the holding potential of −40 mV in 10 mV increment. Pipette solution for I CaL was prepared (in mM): CsCl 120, MgCl2 3, MgATP 5, EGTA 10, HEPES 5, pH 7.2 with CsOH. A modified pipette solution for AP and I CaL was prepared (in mM): KCl 130, MgCl2 1, MgATP 3, EGTA 10, and HEPES 10, pH 7.2 with KOH. Extracellular solution for calcium current (I CaL) (in mM): TEA-Cl 140, CsCl 5.4, 4-AP 1, MgCl2 1.2, CaCl2 1.8, HEPES 5, glucose 10, pH 7.4 with CsOH. The recording was performed at room temperature.
Measurement of hyperpolarization-activated inward current (If)
If current was recorded in a single hiPSC-CM. The external solution contained (in mM) NaCl 132, KCl 4, MgCl2 1.2, CaCl2 1.8, glucose 5, and HEPES, 10 (pH 7.4 with NaOH). The pipette solution contained (in mM) KCl 150, K2ATP 1, MgCl2 5, and HEPES 3 (pH 7.2 with KOH). Cells were hyperpolarized from a holding potential of −40 mV to test potentials of −50 mV to −110 mV for 3000 ms to elicit currents, which were completely blocked by CsCl (2 mM).
Measurement of Confocal Ca2+ Transients
Calcium imaging using confocal fluorescent microscope was conducted in combination with AP recordings. Contracting clusters of homogenous H9 hESC-derived V-CMs on 3.5-cm glass bottomed dishes were identified and labeled after a consistent recording of over 5 CMs showing homogenous AP patterns of V-CM or N-CM. After 2 hours recovery in culture medium, CMs were loaded with 6 µg/mL Fluo-4 AM (Molecular Probes) for 15 min at 37°C and changed to normal Tyrode solution. Ca2+ transients were recorded by a LSM-710 laser scanning confocal microscope (Carl Zeiss, Inc Germany) with a × 40 oil immersion objective, numeric aperture = 1.3. Fluo-4 was excited at 488 nm using a 25 mW argon laser (with intensity attenuated to 1%). Fluorescence emission was measured at >505 nm. Images were acquired in the line (X-T)-scan mode with 512 pixels per line at a rate of 3 ms per scan. The scan line was oriented along the longitudinal axis of the cell, at pixel intervals of 0.15 µm. The axial resolution was set at 1.5 µm according to the manufacture’s specifications. In some experiments, Ca2+ transients were measured with the confocal microscope operating in the frame (X-Y) imaging mode. Ca2+ images were analyzed using a computer program written in IDL 5.4 software [12].
Statistical Analysis
For ion current density, data were presented as mean±SEM (standard error of the mean) and reflected measurements of multiple cells. For the rest, data were expressed as mean±SD. Data were analyzed by 2-tailed Student t tests. P values below 0.05 were considered to indicate a statistically significant difference.
Results
Cardiac Differentiation of H9 hESCs and Subsequent Characterization
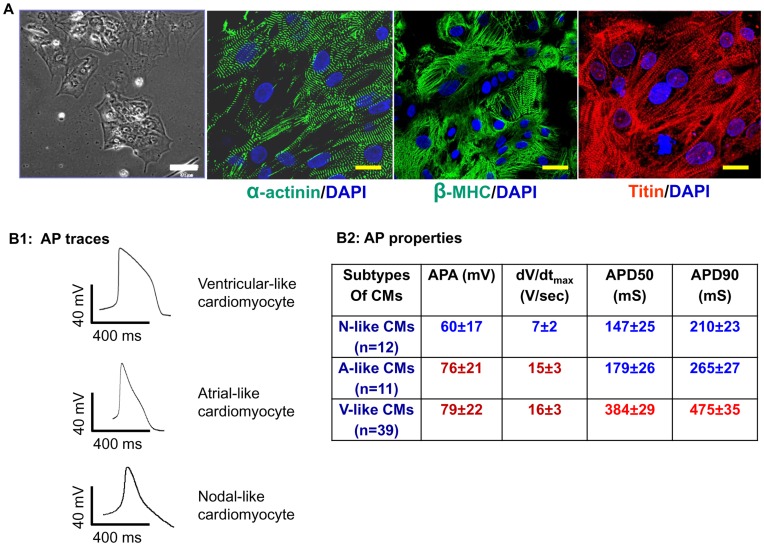
Contracting EBs were obtained from H9 hESCs after 15 days of cardiac differentiation with differentiation efficiency (contracting EBs vs. total EBs) ranging from 29±3% to 50±5%, which is consistent with previous reports [13]. Dissociated hESC-CMs showed sarcomeric protein expression similar to those in previous reports ( Fig. 1A ). [4], [5] Among dissociated hESC-CMs, ventricular (V)-, atrial (A)- and nodal (N)-like subtypes ( Fig. 1B1 ) were identified by corresponding action potential (AP) properties which included action potential amplitude (APA), 50% and 90% of action potential duration (APD50 and APD90), and maximal rate of depolarization or maximal upstroke velocity (dV/dtmax) ( Fig. 1B2 ). However, unlike adult quiescent CMs, A- and V-like hESC-and hiPSC-CMs were capable of spontaneous contractions in culture. The proportion of 3 subtypes of CMs from H9 hESCs was similar to previous reports with V-like CMs constituting the majority subtype (60∼70%) of total cardiomyocyte populations [10], [11].
Figure 1. Generation and characterization of cardiomyocytes differentiated from H9 hESCs.
A: Light microscopy and immunocytochemistry image of H9 hESC-CMs dissociated from contracting EBs. Scale bar: 100 µm. Fluorescent images of H9 hESC-CMs stained with antibodies against α-actinin, β-MHC and Titin showed typical cardiac sarcomeres. Scale bar: 30 µm. B1∼2: Action potential trace of three subtypes of cardiomyocytes derived from H9 hESCs and corresponding specific action potential properties. APA: action potential amplitude. dV/dtmax: maximal rate of depolarization or maximal upstroke velocity. APD50: action potential duration at 50%. APD90: action potential duration at 90%.
Effect of H2S on hPSC-derived Cardiomyocytes
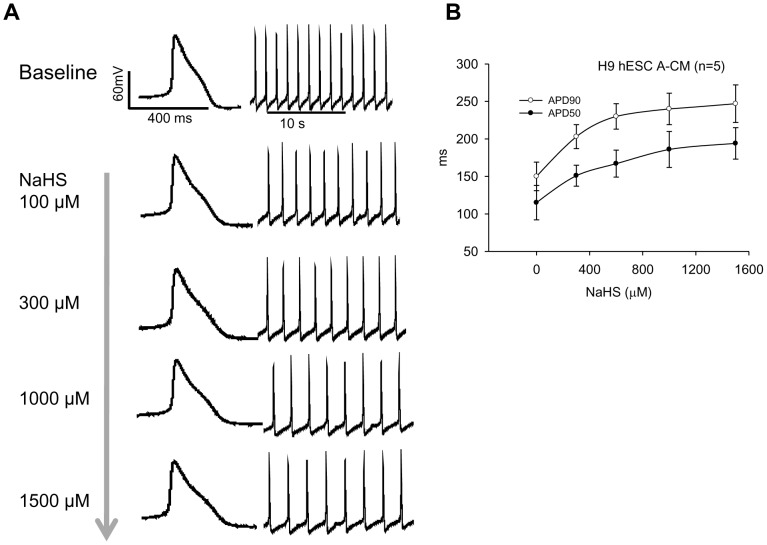
NaHS (a H2S donor) exerted a dose-dependent (from 100 to 1500 µM) effect on both H9 hESC- and hiPSC-CMs. Fig. 2 shows that NaHS dose-dependently prolonged the action potential duration of a H9 hESC-derived atrial-like CMs ( Fig. 2A ) and both APD50 and ADP90 were significantly increased with approximately 50% APD prolongation (IC50) achieved at 300 µM ( Fig. 2B ). As the H2S solution contains a concentration that is approximately 33% of the original concentration of NaHS [14], 300 µM of NaHS is equivalent of ∼100 µmol/L of H2S. Counting on the rapid decay of H2S under in vitro condition, this concentration is close to the top limit of the physiological concentration of H2S which is 50∼90 µM [15], [16]. Accordingly, the subsequent experiments were performed mostly with 100 µM of NaHS and 300 µM of NaHS was used to test the maximal effects.
Figure 2. Dose-dependent effect of H2S on H9 hESC-CMs.
A: A H9 hESC-derived atrial-like cardiomyocyte concentration-dependently responded to an increasing dose (100∼1500 µM) of NaHS presented as prolongation of action potential duration (APD50 and ADP90) and slowing of contraction rates. B: The corresponding IC50 curve.
Action Potential Response of hPSC-CMs towards NaHS
Exposure to NaHS evoked distinct electrical responses from the 3 different subtypes of cardiomyocytes indicated by altered action potential properties. Following NaHS exposure, beating rates were significantly reduced in V- and A-like CMs derived from H9 hESC (p<0.05) and hiPSC (p<0.05) ( Fig. 3A ). No difference was found with N-like CMs (data not shown).
Figure 3. Action potential response of hESC- and hiPSC-CMs towards H2S.
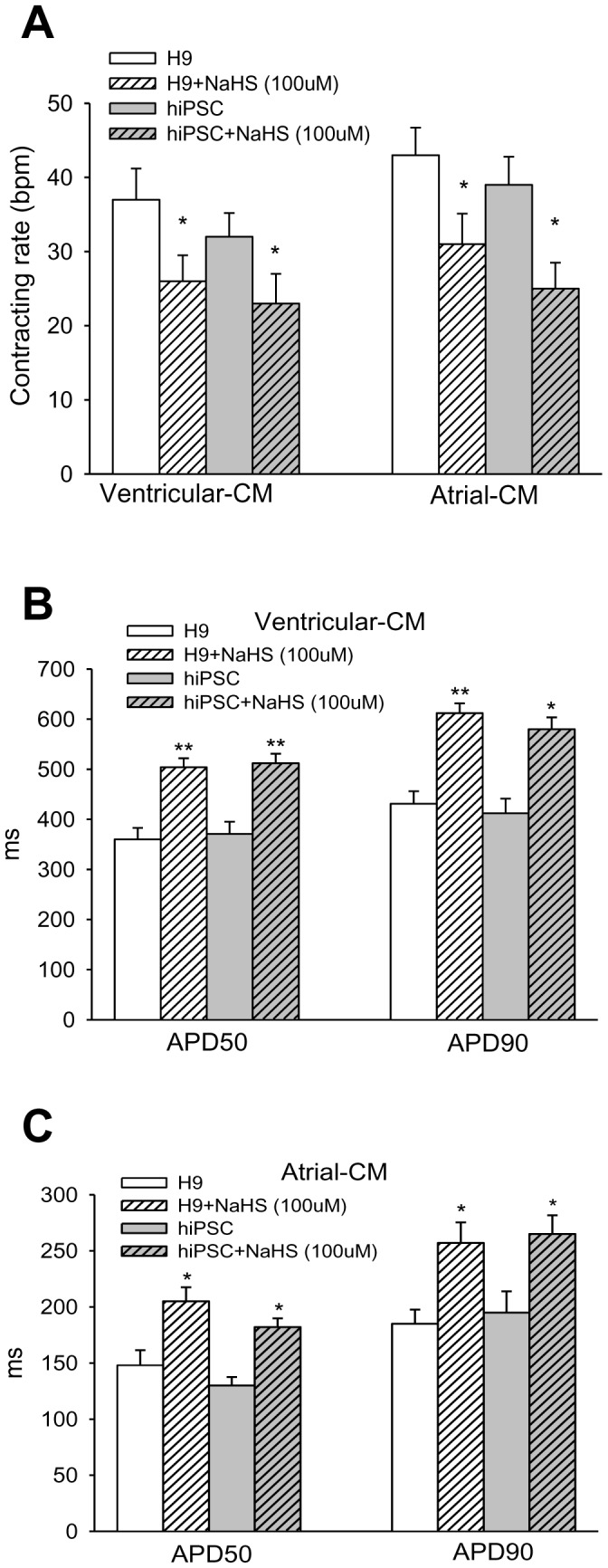
A∼B: Responses of action potential traces of H9 hESC- and hiPSC-derived V- and A-like CMs towards H2S. Changes of the AP properties of different subtypes of cardiomyocytes before and after NaHS (100 µM) exposure were analyzed. C: Beating rates in H9 hESC- and hiPSC-derived V- and A-like CMs. D∼E: Action potential duration (APD) in the V-like CMs (D) and A-like CMs (E). *P<0.05; **p<0.01 (NaHS treated vs. baseline).
APDs in the V- and A-like CMs derived from H9 hESCs and hiPSCs were significantly prolonged following NaHS exposure. There was a significant prolongation of APD in the V-like CMs derived from H9 hESCs (APD50∶504.7±17.8 ms vs. 360.3±23.5 ms, p<0.01; APD90∶612.5±19.5 ms vs. 431.2±25.0 ms, p<0.01) and hiPSCs (APD50∶519.2±15.9 ms vs. 374.7±24.6 ms, p<0.01; APD90∶593.4±17.9 ms vs. 418.2±23.6 ms, p<0.01) following exposure to NaHS ( Fig. 3B ). Similar APD prolongation was detected in A-like CMs derived from H9 hESC (APD50∶205.6±12.6 ms vs. 148.1±13.4 ms, p<0.05; APD90∶275.6±18.3 ms vs. 185.8±12.6 ms, p<0.05) and hiPSC (APD50∶182.4±6.7 ms vs. 127.8±5.5 ms, p<0.01; APD90∶266.3±7.9 ms vs. 195.6±11.5 ms, p<0.01) respectively ( Fig. 3C ). However, no statistically significant changes in APD were observed in the N-like CMs derived from H9 hESCs and hiPSCs (data not shown). In addition, no significant changes in action potential amplitude (APA), maximal upstroke velocity (dV/dtmax) and maximum diastolic potential (MDP) were found following NaHS treatment (data not shown).
Ion Currents Response of hESC- and hiPSC-derived CM towards H2S
To investigate the mechanism behind the effect of H2S on prolongation of APD and slowing down of the contraction rates, effects of H2S in altering ion currents in H9 hESC- and hiPSC- derived V-like CMs were investigated.
Exposure to NaHS Significantly Reduced Outward Potassium Currents (I K) in H9 hESC- and hiPSC-derived CMs
Outward rectifier potassium currents (I K) including slow and rapid I K (I Ks and I Kr) contribute to the 2nd and 3rd phase of AP. With a modified protocol that facilitates the combined measurement of potassium currents with action potential, I K was recorded in V- and A- like CMs identified by AP measurement. Fig. 4 showed representative data recorded with V-like CMs.
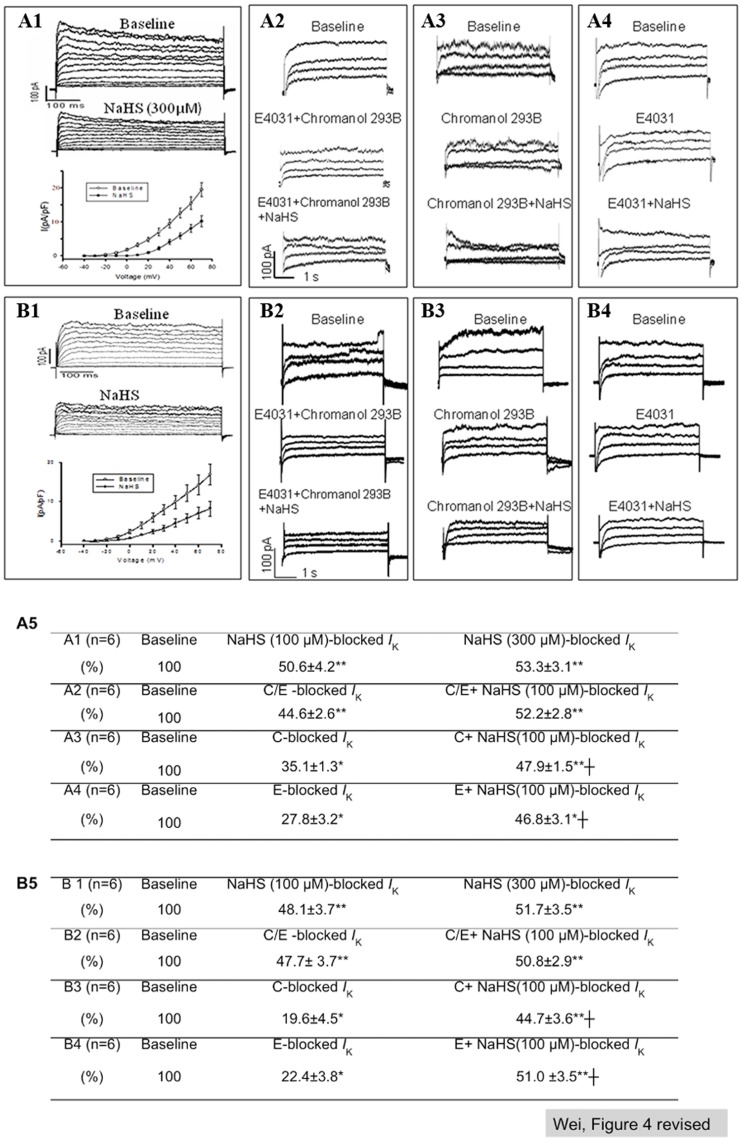
Figure 4. IK response of hESC- and hiPSC-CMs towards H2S.
A, B: IK recorded on V-CMs derived from H9 hESCs (A) and hiPSCs (B) and A1, B1: IK recorded before and after NaHS (300 µM) treatment. A2, B2: IK recorded at baseline, with pre-treatment with a mixture of 5 µM E-4031 and 5 µM Chromanol 293B, and with subsequently treatment with NaHS (100 µM). A3, B3: IK recorded at baseline, with pre-treatment with 5 µM Chromanol 293B, and with subsequently treatment with NaHS (100 µM). A4, B4: IK recorded at baseline, with pre-treatment with 5 µM E-4031, and with subsequently treatment with NaHS (100 µM). A5, B5: Quantification of IK response of hESC-CMs (A5) and hiPSC-CMs (B5) towards Chromanol 293B, E-4031 and NaHS observed in A1∼A4 and B1∼B4, respectively. Relative quantity (% of baseline IK) of Chromanol 293B-, E-4031- and NaHS-sensitive IK were calculated by subtracting post-treatment IK from baseline. *P<0.05; **p<0.01 (NaHS or IK blocker(s) treated vs. baseline). ┼p<0.05 (NaHS treated vs. IK blocker treated). C/E: Chromanol 293B+E-4031; C: Chromanol 293B; E: E-4031. Number of repeats (n) = 6.
It was found that exposure to NaHS (100 to 300 µM) significantly reduced total I K in both hESC-CMs ( Fig. 4A ) and hiPSC-CMs ( Fig. 4B ). The current-voltage (I−V) relationships demonstrated a suppression of NaHS (300 µM) on I K density (pA/pF) ( Fig. 4A1 and Fig. 4B1 ). However, such effect of NaHS on I K was found to be abolished after pretreatment of cardiomyocytes with a mixture of I K blockers containing 5 µM E-4031 (blocks I Kr) and Chromanol 293B (blocks I Ks) ( Fig. 4A2 and Fig. 4B2 ). Separately, the effects of NaHS on I Ks and I Kr were independently validated. Fig. 4A3 and Fig. 4B3 show that Chromanol 293B (a I Ks blocker) alone suppressed I K in both hESC-derived and iPSC-derived V-like CMs respctively. Addition of NaHS to Chromanol 293B treated cells led to further attenuation of I K to a level close to that of NaHS treatment alone, suggesting that NaHS could additionally inhibit I Kr. Similarly, Fig. 4A4 and Fig. 4B4 show that E-4031 (a I Kr blocker) suppressed I K in both hESC-derived and hiPSC-derived V-like CMs respectively. Additional NaHS treatment resulted in a further reduction in I K to a level close to NaHS treatment alone, suggesting that NaHS could additionally inhibit I Ks. The effects of NaHS on I Ks and I Kr in hESC-CMs and hiPSC-CMs were quantified and summarized in Fig. 4A5 and Fig. 4B5 respectively. Compared to the baseline, it was noted that NaHS registered a ∼50% suppression of I K in hESC-CM (A1) and hiPSC-CM (B1). Such effect of NaHS was equivalent to the inhibitory effects on I K (44.6±2.6 and 47.7±3.7) achieved by combined Chromanol 293B and E-4031 which blocked both I Ks and I Kr in hESC-CMs (A2) and hiPSC-CMs (B2). After subtracting the effects of NaHS from the effects of both I K blockers, additional treatment with NaHS did not show a significant further inhibition on I K (A2 and B2), suggesting that the effect of NaHS overlapped with those of Chromanol 293B and E-4031 blockers. Moreover, it was noted that NaHS exerted additive inhibitory effect on total I K in the respective presence of inhibitory effects of Chromanol 293B on I Ks (A3 and B3) and E-4031 on I Kr (A4 and B4), suggesting that NaHS suppressed both I Ks and I Kr in both hESC-CMs and hiPSC-CMs. However, the effects of NaHS on A-like CMs were inconclusive due to insufficient number of cells (n <4 for each subgroup) tested. No effects of NaHS on N-like CMs were observed (data not shown).
Exposure to NaHS Significantly Inhibited L-type Ca2+ Current in H9 hESC-CMs
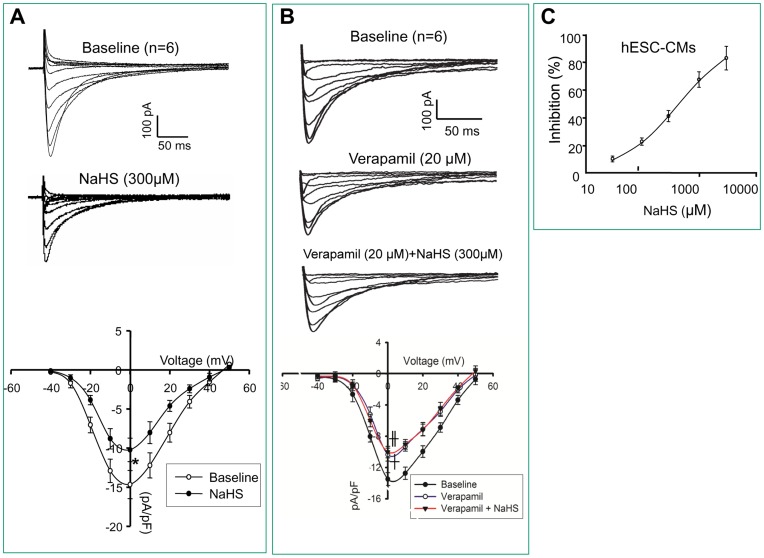
Ca2+ signaling plays a crucial role in cardiac excitation-contraction (EC) coupling. L-type Ca2+ channel is responsible for Ca2+ influx triggered by the electrical signal during cardiac contraction. I CaL contributes to the 2nd phase of action potential and is more prominent in ventricular cardiomyocytes. Cardiomyocytes exposed to NaHS showed a decreased I CaL density. Fig. 5A shows that the I CaL density was significantly decreased in H9 hESC-derived V-like CMs exposed to 300 µM NaHS from −15±2.9 pA/pF to −10±3.2 pA/pF at 0 mV (p<0.05). The specificity of I CaL was confirmed by 20 µM verapamil, a blocker of I CaL which expectedly reduced the current substantially (p<0.05), while the addition of 300 µM NaHS in the presence of 20 µM verapamil failed to see a further reduction of I CaL, suggesting that the effect of NaHS overlapped with verapamil ( Fig. 5B ). It thus further confirmed the effect of NaHS on I CaL. Furthermore, the I-V relationships following NaHS treatment suggested a concentration-response curve of I CaL inhibition by NaHS ( Fig. 5C ).
Figure 5. Ca2+ current response of hESC-CMs towards H2S.
Representative Ca2+ currents were recorded on V-CMs derived from H9 hESCs. A: Traces of Ca2+ currents recorded before and after NaHS (300 µM) treatment. I/V curve of Ca2+ density was plotted. B: Ca2+ currents recorded at baseline, after verapamil (20 µM) treatment; and with addition of NaHS (300 µM) treatment I/V curve of Ca2+ density was plotted. C: The IC50 of the effect of NaHS on Ca2+ currents. *P<0.05 (NaHS treated vs. baseline). ┼p<0.05 (verapamil treated vs. baseline). ╫p<0.05 (verapamil+NaHS treated vs. baseline). Number of repeats (n) = 6.
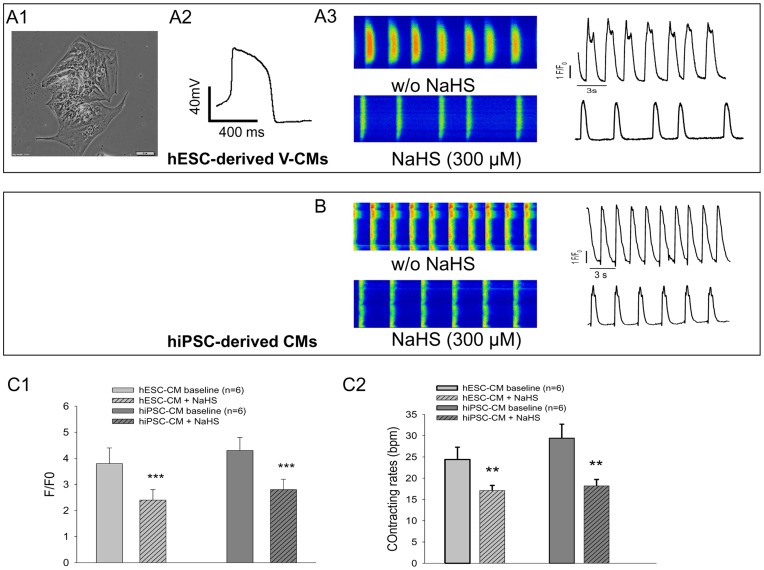
Moreover, confocal scanning of Fluo 4-loaded H9 hESC-derived V-like CMs ( Fig. 6A1, 6A2 ) and hiPSC-CMs (with unidentified subtype) showed that exposure to NaHS (300 µM) decreased firing frequency ( Fig. 6A3 and Fig. 6B ). Expectedly, quantitative data showed reduced amplitude of calcium transients (p<0.001) ( Fig. 6C1 ) and decreased contraction rates (p<0.01) ( Fig. 6C2 ) after NaHS treatment.
Figure 6. Spontaneous Ca2+ transients response of hESC- and hiPSC-CMs towards H2S.
A1∼3: Light microscopy images of a cluster of H9 hESC-derived V-CMs (A1), their AP traces (A2) and the Fluo-4 loaded confocal line-scan calcium images of rhythmic spontaneous Ca2+ transients recorded before and after NaHS (300 µM) treatment (A3). B: Fluo-4 loaded confocal line-scan calcium images of rhythmic spontaneous Ca2+ transients recorded in hiPSC-CMs before and after NaHS (100 µM) treatment. C1: The time course of the ratio of fluorescence to background fluorescence (F/F0) of hESC-CM and hiPSC-CM prior to and post NaHS treatment. C2: the average contracting rates of hESC-CM and hiPSC-CM prior to and post NaHS treatment. **p<0.01, ***p<0.001 (NaHS treated vs. baseline). Number of repeats (n) = 6.
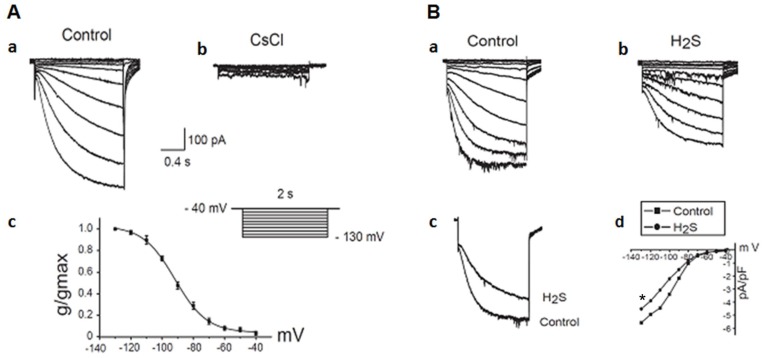
Exposure to NaHS Significantly Inhibited I f Current in hiPSC-derived CMs
Interestingly, I f current was detected in hiPSC-derived V-like CMs which was significantly reduced by addition of specific channel blocker, CsCl ( Fig. 7A ). Similarly, exposure to NaHS (100 µM) significantly decreased I f current in those CMs (p<0.05) ( Fig. 7Bd ).
Figure 7. Effect of H2S on the hyperpolarization-activated inward current (If) in hiPSC-CMs.
A: Voltage- and time-dependence of the If. Original If current recorded in a hiPSC-CM shows the voltage- and time- dependence (voltage clamp protocol in inset). Superimposed traces of a hiPSC-CM recorded before (Aa) and after (Ab) addition of external Cs+ (2 mM). (Ac) The activation curve calculated by fitting a Boltzman distribution to normalized current over a voltage range of −40 mV to −130 mV. Half-maximal activation and slope factor were −90 mV and −10.2 mV, respectively. Number of repeats (n) = 4. B: Original If traces of a hiPSC-CM before (Ba) and after (Bb) the exposure of H2S (100 µM). (Bc) Superimposed traces before and after exposure of H2S (100 µM). (Bd) Current–voltage relationship of If in the absence and presence of H2S (100 µM). **p<0.05 (NaHS treated vs. baseline). Number of repeats (n) = 4.
Discussion
Hydrogen sulfide (H2S) as a recently identified gaseous signalling transmitter has been known to interact with a wide range of ion channels to mediate important physiological responses. These include protecting against myocardial ischemic reperfusion injury and other cardiac protective effects through modulation of ATP-sensitive potassium current (I kATP) [17] and voltage-gated L-type calcium current (I CaL) [18]. Despite its beneficial effects, H2S may have implication in cardiac arrhythmias as it interacts with multiple ion channels involved in membrane action potential [7].
Outward rectifier potassium currents (I K) including slow and rapid I K (I Ks and I Kr) contribute to the 2nd and 3rd phase of AP, and both play an important role in regulating the repolarization of cardiomyocytes. Drugs affecting I Ks and I Kr have been associated with cardiac arrhythmia [1], [2]. Both I Ks and I Kr are the predominant I K in adult human CMs [19] and their presence has been confirmed in hiPSC-CMs [10], [20], [21]. To our knowledge, the findings of inhibitory effect on delayed rectifier I k of human CMs is the first report of such effect by H2S. Our data showed that H2S at physiological concentration could suppress both I Ks and I Kr currents. In addition, H2S was found to mediate I CaL channel inhibition in V-like CMs derived from H9 hESCs. This is consistent with previous report that H2S inhibited I CaL in rat V-like cardiomyocytes [22].
In human CMs, the outwardly rectifying potassium current I Ks and I Kr contribute to phase 2 and 3 repolarization of AP in both V- and A-CMs [23]. In V-CMs, the longer phase 2 of AP of membrane depolarization is contributed mainly by I CaL. In the present study, the suppressed I Ks and I Kr by H2S contributed to prolonged repolarization phase in V-like and A-like CMs with appreciable perturbation of APD. In V-like CMs, however, altered APD could be the consequence of a balanced inhibitory effect of H2S on delayed rectifier I K and on I CaL. Although suppressing I CaL is likely to shorten the APD, inhibiting outwardly rectifying potassium current in phase 2 and phase 3 by H2S may override such effect, resulting in an overall APD prolongation as observed in our study. In contrast to V- and A-like CMs, N-like CMs derived from H9 hESC and hiPSCs did not show significant difference in APD in response to H2S.
In contrast to adult CMs of which only nodal CMs are capable of spontaneous contraction, all CMs derived from hiPSCs are capable of spontaneous contraction [10] indicating their fetal-like phenotype in culture. The identification of I f current in hiPSC-derived V-like CMs in this study further supports the immature status of those hiPSC-CMs. Nevertheless, inhibition of I f may explain the inhibitory effect of H2S on the contraction rates of hiPSC-CMs observed. However, since sulfhydration of KATP by H2S has been attributed to its channel opening effect [24], [25], it will be interesting to study if S-sulfhydration by H2S had any effect on I K, I CaL and I f channel activities in the future. Furthermore, endogenous H2S mediated by cystathionine-β-synthase on the observed outcomes were not studied, though it has been shown to have effect similar to I KATP channel opening effect of exogenously supplied NaHS in rat myocytes [26].
In conclusion, we present the first report of inhibition of both slow and rapid delayed rectifier potassium channels and hyperpolarization-activated inward current in human cardiomyocytes by H2S. Such effect, in combination with its inhibition of I CaL, could contribute to prolonged APD and slowed contracting rates in V-and A-like CMs that may have undesired implications in its vasodilation function in cardiac hemodynamics.
Funding Statement
This study is supported by National Research Foundation, Singapore grant, NRF2008-CRP001-68. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Roy M, Dumaine R, Brown AM (1996) HERG, a primary human ventricular target of the nonsedating antihistamine terfenadine. Circulation 94: 817–823. [DOI] [PubMed] [Google Scholar]
- 2. Cavero I, Mestre M, Guillon JM, Crumb W (2000) Drugs that prolong QT interval as an unwanted effect: assessing their likelihood of inducing hazardous cardiac dysrhythmias. Expert Opin Pharmacother 1: 947–973. [DOI] [PubMed] [Google Scholar]
- 3. Braam SR, Mummery CL (2010) Human stem cell models for predictive cardiac safety pharmacology. Stem Cell Res 4: 155–156. [DOI] [PubMed] [Google Scholar]
- 4. Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, et al. (2001) Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 108: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, et al. (2009) Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res 104: e30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Szabo C (2007) Hydrogen sulphide and its therapeutic potential. Nature reviews Drug discovery 6: 917–935. [DOI] [PubMed] [Google Scholar]
- 7. Tang G, Wu L, Wang R (2010) Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol 37: 753–763. [DOI] [PubMed] [Google Scholar]
- 8. Graichen R, Xu X, Braam SR, Balakrishnan T, Norfiza S, et al. (2008) Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation 76: 357–370. [DOI] [PubMed] [Google Scholar]
- 9. Maltsev VA, Wobus AM, Rohwedel J, Bader M, Hescheler J (1994) Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ Res 75: 233–244. [DOI] [PubMed] [Google Scholar]
- 10. Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, et al. (2011) High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol 301: H2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei H, Tan G, Manasi, Qiu S, Kong G, et al. (2012) One-step derivation of cardiomyocytes and mesenchymal stem cells from human pluripotent stem cells. Stem Cell Res 9: 87–100. [DOI] [PubMed] [Google Scholar]
- 12. Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, et al. (1999) Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J 76: 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu XQ, Graichen R, Soo SY, Balakrishnan T, Rahmat SN, et al. (2008) Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation 76: 958–970. [DOI] [PubMed] [Google Scholar]
- 14. Reiffenstein RJ, Hulbert WC, Roth SH (1992) Toxicology of hydrogen sulfide. Annual review of pharmacology and toxicology 32: 109–134. [DOI] [PubMed] [Google Scholar]
- 15. Wu R, Yao WZ, Chen YH, Geng B, Tang CS (2008) [Plasma level of endogenous hydrogen sulfide in patients with acute asthma]. Beijing Da Xue Xue Bao 40: 505–508. [PubMed] [Google Scholar]
- 16. Chen YH, Yao WZ, Geng B, Ding YL, Lu M, et al. (2005) Endogenous hydrogen sulfide in patients with COPD. Chest 128: 3205–3211. [DOI] [PubMed] [Google Scholar]
- 17. Cheng Y, Ndisang JF, Tang G, Cao K, Wang R (2004) Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–2323. [DOI] [PubMed] [Google Scholar]
- 18. Lavu M, Bhushan S, Lefer DJ (2011) Hydrogen sulfide-mediated cardioprotection: mechanisms and therapeutic potential. Clin Sci (Lond) 120: 219–229. [DOI] [PubMed] [Google Scholar]
- 19. Li GR, Feng J, Yue L, Carrier M, Nattel S (1996) Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res 78: 689–696. [DOI] [PubMed] [Google Scholar]
- 20. Moretti A, Bellin M, Welling A, Jung CB, Lam JT, et al. (2010) Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med 363: 1397–1409. [DOI] [PubMed] [Google Scholar]
- 21. Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, et al. (2011) Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471: 225–229. [DOI] [PubMed] [Google Scholar]
- 22. Sun YG, Cao YX, Wang WW, Ma SF, Yao T, et al. (2008) Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res 79: 632–641. [DOI] [PubMed] [Google Scholar]
- 23. Roden DM, George AL Jr (1996) The cardiac ion channels: relevance to management of arrhythmias. Annu Rev Med 47: 135–148. [DOI] [PubMed] [Google Scholar]
- 24. Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, et al. (2009) H2S signals through protein S-sulfhydration. Sci Signal 2: ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, et al. (2011) Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhong GZ, Li YB, Liu XL, Guo LS, Chen ML, et al. (2010) Hydrogen sulfide opens the KATP channel on rat atrial and ventricular myocytes. Cardiology 115: 120–126. [DOI] [PubMed] [Google Scholar]