Abstract
Campylobacter jejuni is a major cause of food-borne disease in industrialized countries. Carbohydrate utilization by C. jejuni is severely restricted, and knowledge about which substrates fuel C. jejuni infection and growth is limited. Some amino acids have been shown to serve as carbon sources both in vitro and in vivo. In the present study we investigated the contribution of serine and proline catabolism to the in vitro and in vivo growth of C. jejuni 81-176. We confirmed that the serine transporter SdaC and the serine ammonia-lyase SdaA are required for serine utilization, and demonstrated that a predicted proline permease PutP and a bifunctional proline/delta-1-pyrroline-5-carboxylate dehydrogenase PutA are required for proline utilization by C. jejuni 81-176. C. jejuni 81-176 mutants unable to utilize serine were shown to be severely defective for colonization of the intestine and systemic tissues in a mouse model of infection. In contrast, C. jejuni 81-176 mutants unable to utilize proline were only defective for intestinal colonization. These results further emphasize the importance of amino acid utilization in C. jejuni colonization of various tissues.
Introduction
Although knowledge of bacterial metabolism during growth in vitro is substantial, much less is known about the metabolism of bacterial pathogens during infection [1], [2]. The understanding of the in vivo metabolism of pathogens has gained importance in recent years as it may facilitate the development of novel antimicrobial drugs [3], [4]. Campylobacter jejuni, a gram-negative, microaerophilic and chemoorganotrophic bacterium, is a major cause of food-borne diarrhea worldwide [5]. C. jejuni efficiently colonizes the intestinal tract of birds, especially poultry, without any adverse effects [6]–[8]. In contrast, infections of humans can lead to severe diarrheal illness [9], [10] and in rare cases to an acute polyneuropathy, the Guillain-Barré Syndrome, as sequelae to the foodborne disease [11]. Relatively little is known about the molecular mechanisms of C. jejuni pathogenesis [12], [13]. Previous studies suggest that adherence to as well as invasion into intestinal epithelial cells and the production of cytolethal distending toxin (CDT) contribute to the pathogenesis of C. jejuni [14]–[18]. Furthermore, motility and chemotaxis [19]–[22], a N-gylcosylation system as well as surface structures like lipooligosaccharide, capsule and the O-glycosylated flagella have been shown to participate in the experimental infection of animals [20], [23]–[30]. Some studies have also begun to define the metabolic requirements for C. jejuni growth in vitro [31], [32]. Unlike other enteropathogenic bacteria, C. jejuni cannot utilize glucose as a growth substrate since it lacks the glycolytic enzyme phosphofructokinase of the Embden-Meyerhof-Parnas (EMP) pathway [33]–[35]. Furthermore, C. jejuni does not encode a complete pentose phosphate (PPP) or Entner-Doudoroff (ED) pathway [33], [34]. C. jejuni has been considered asaccharolytic, but recent studies demonstrated that a subset of C. jejuni strains encode a gene cluster required for the utilization of fucose as an energy source [36], [37]. Instead of glucose and other carbohydrates C. jejuni can utilize several glucogenic amino acids as efficient growth substrates [38]–[42], although there are intriguing strain differences in the ability to utilize specific amino acids [43]. In addition to glucogenic amino acids, the TCA cycle intermediates fumarate, malate, succinate and α-ketoglutarate [44], as well as pyruvate [45], [46] and lactate [35], [47] can serve as growth-promoting substrates for various C. jejuni strains in vitro.
The metabolic requirements for C. jejuni colonization in different animal model systems are being defined, although only few metabolic traits have so far been demonstrated to be important for infection. As for many other pathogens, zinc and iron acquisition plays an important role in C. jejuni colonization and mutations in zinc, ferrous as well as ferric iron uptake systems reduced the ability of C. jejuni to colonize the chicken intestine [48]–[50]. Its ability to carry out oxygen-independent respiration mediated by a gluconate dehydrogenase and nitrate, nitrite, fumarate as well as dimethylsulfoxide reductases are also required for efficient colonization [51]–[54]. While it is well documented that amino acids represent major energy and carbon sources for the in vitro growth of C. jejuni, only a few studies have examined the contribution of amino acid catabolism to bacterial colonization. For example, serine and aspartate catabolism were shown to be important for C. jejuni colonization of the avian gut [38], [45] and mutation of a putative branched amino acid transporter resulted in an attenuated colonization phenotype in chicken [20], [55]. The γ-glutamyltranspeptidase (GGT) dependent utilization of glutamine and glutathione by C. jejuni 81-176 supports the colonization of the murine intestine [51]. In addition, asparagine catabolism enhances the tissue-specific colonization process of C. jejuni 81-176 in a murine infection model [43]. Here we show that the ability to utilize the glucogenic amino acids serine and proline is also important for the colonization of specific tissues by C. jejuni.
Results
Serine Catabolism is a Variable Metabolic Trait in Campylobacter
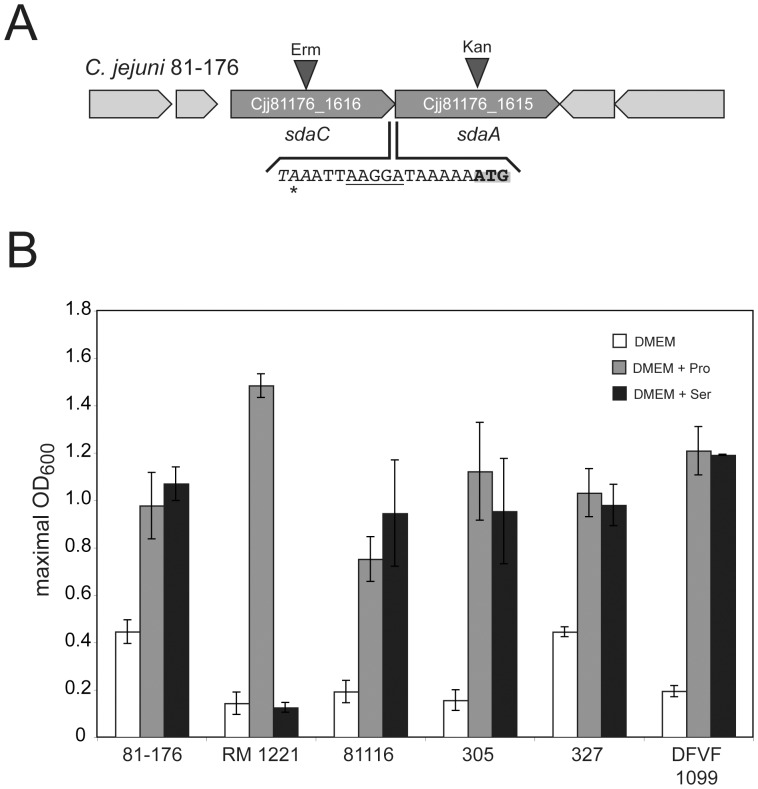
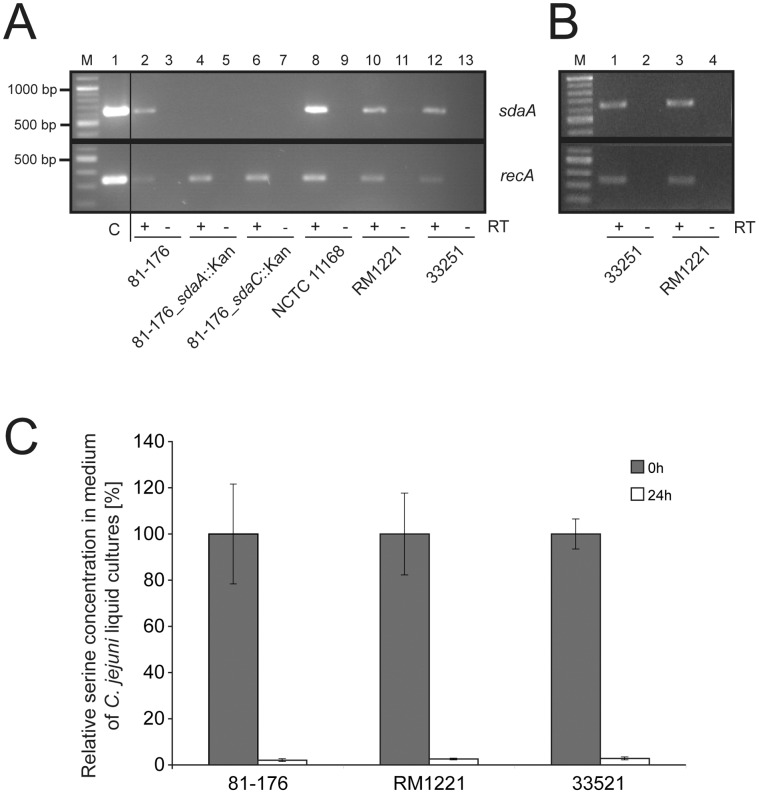
It was previously shown, that serine serves as an efficient nutrient for the in vitro growth of various C. jejuni isolates [39], [42], [45]. Serine utilization in C. jejuni is mediated by the L-serine dehydratase SdaA and the serine transporter SdaC [45] as described for other bacteria. Our examination of published [33], [34], [51], [56]–[64] and unpublished C. jejuni genome sequences revealed a high conservation of the genes encoding for SdaA and SdaC in C jejuni (Figure 1A, Figures S1 and S2, Tables S1 and S2). Strikingly, we recently identified C. jejuni 33251 as the only strain of 13 isolates tested that was unable to utilize serine [43]. This was surprising considering the observed conservation of sdaA and sdaC among C. jejuni isolates. To understand the inability of C. jejuni 33251 to utilize serine, we sequenced its sdaCA locus. We identified only few nucleotide differences in the sdaA and sdaC genes that do not result in the inactivation of respective open reading frames of this isolate. In addition, we found a small insertion leading to 3 additional amino acids in the coding sequence of SdaA (Figures S1, S2, S3 and S4). We confirmed that mutations of sdaA and/or sdaC are not necessarily responsible for the abolished serine catabolism in certain C. jejuni strains by analyzing the sequenced C. jejuni isolates RM1221, 81116, 305, 327 and DFVF1099. While these five strains encode for nearly identical sdaA and sdaC gene products (Figures S3 and S4), C. jejuni RM1221 was, like C. jejuni 33251, unable to grow with serine as sole energy source, whereas the C. jejuni 81116, 305, 327 and DFVF1099 catabolized serine efficiently (Figure 1B). We hypothesized that reduced expression of the sdaCA operon could be responsible for the inability of C. jejuni 33251 and RM1221 to grow with serine. To examine this, mRNA from the strains C. jejuni 81-176, NCTC 11168, 33251 and RM1221 was isolated and reverse transcriptase-PCR analysis of sdaA was used to elucidate if differences in the expression between the wild-type strains occur. Interestingly, the serine utilizing and non-utilizing C. jejuni strains showed similar sdaA expression as C. jejuni 81-176 and NCTC 11168 when cultivated in nutrient rich BHI medium (Figure 2A) or in DMEM (Figure 2B). To clarify if differences in the uptake of serine by the different C. jejuni isolates was causing the defective growth phenotypes of C. jejuni RM1221 and 33251, we analyzed the decrease of serine concentration in the growth medium with GC-MS analysis. For all three tested C. jejuni isolates efficient serine uptake was observed, as the amount of serine in the culture medium vanished to a similar extent over the time period of 24 hours (Figure 2C). This suggested that other strain-specific characteristics are accountable for the inability of C. jejuni 33251 and RM1221 to catabolize serine. We suspected that perhaps dissimilarities in the L-serine dehydratase activity of individual C. jejuni isolates could be responsible for their variable growth with serine. Using the enzymatic assay previously described for the measurement of the SdaA activity in C. jejuni NCTC 11168 [45], we detected in the cell extracts of C. jejuni 33251 and RM1221 a small but significant reduction of L-serine dehydratase activity in comparison to C. jejuni 81-176 (Table S5). Taken together our data suggest that neither differences in the sdaCA gene expression nor serine uptake are responsible for the inability of C. jejuni 33251 and RM1221 to grow this serine, but rather a reduced serine deyhdratase activity or other yet not identified factors.
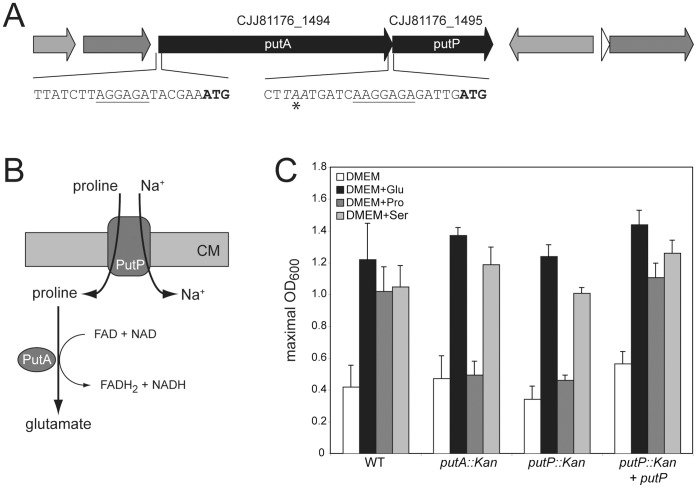
Figure 1. Serine utilization locus in C. jejuni.
(A) The serine utilization locus of C. jejuni consists of the sdaCA operon, which is conserved in all sequenced C. jejuni strains. The genes sdaC and sdaA encode for a serine uptake transporter and a L-serine dehydratase, respectively. The intergenic sequence is shown with the sdaA start codon (ATG), the sdaA shine-dalgarno squence (underlined) and the sdaC stop codon (*). Mutants of sdaA and sdaC were constructed by insertion of kanamycin and erythromycin resistance cassettes in respective genes as indicated. (B) Comparison of different C. jejuni wild-type isolates to utilize serine as growth substrate. The shown optical densities are the mean values ±SD that were maximally reached by indicated wild-type C. jejuni strains RM1221, 81116, 305, 327 and DFVF1099 during a growth period of 48 hours in DMEM supplemented with 20 mM of the indicated amino acids.
Figure 2. Comparison of sdaA expression and serine uptake in different C. jejuni isolates.
The expression of the sdaCA operon in various wild-type C. jejuni strains as well as the sdaA and sdaC mutants of C. jejuni 81-176 cultivated in BHI medium (A) or DMEM (B) was examined by reverse transcriptase (RT)-PCR analysis using sdaA specific primer (upper panel). RT-PCRs with recA specific primers are shown in the lower panel for comparison. To verify the absence of chromosomal DNA contamination in the RNA preparations, PCRs were performed using RNA samples without RT treatment (- RT) as templates. PCRs with respective sdaA and recA primers and chromosomal DNA of C. jejuni 81-176 served as positive controls (A, lane 1, upper and lower panel). (C) The capability of C. jejuni isolates 81-176, RM1221 and 33251 to take up serine from the medium was measured by monitoring the relative changes of serine concentration in the culture supernatant over a cultivation period of 24 hours by GC-MS analysis. The bacteria were grown in Hanks Balance Salt Solution (HBSS) with 1% casein hydrolysate as amino acid sources and supplemented with vitamins and iron. The relative serine concentration at the beginning of the experiment was set as 100%. The mean values ±SD are the results of two independent experiments measured in triplicates.
Serine Catabolism is Required for Efficient C. jejuni 81-176 Mouse Colonization
It has previously been demonstrated that inactivation of the sdaA gene in C. jejuni NCTC 11168 resulted in reduced persistence in the intestine of infected chicken [45]. Here we examined whether the serine catabolism of C. jejuni 81-176 provides an advantage for its colonization of different tissues in a murine infection model [30]. As predicted from studies in C. jejuni strain NCTC 11168 [45], inactivation of the sdaA or sdaC genes in C. jejuni 81-176 abolished its ability to utilize serine as a carbon source (Figure S5). Both mutants exhibited comparable growth to the wild type strain when cultivated in DMEM supplemented with other amino acids. Furthermore, the phenotype of the sdaA mutant was reversed by the reintroduction of a copy of the wild-type sdaA gene.
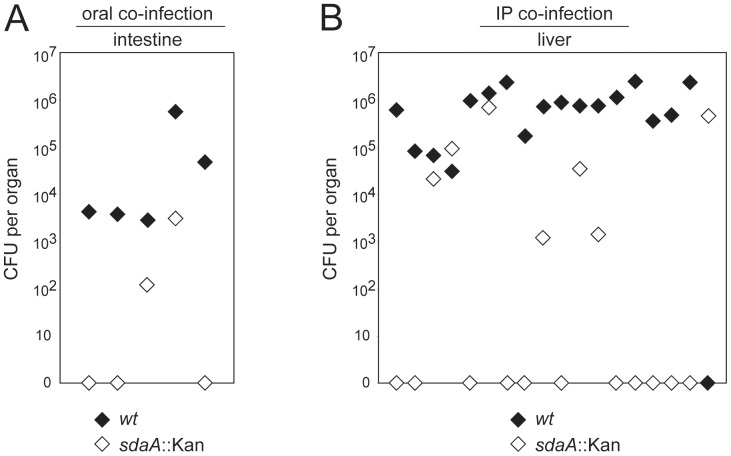
To investigate how serine catabolism affects C. jejuni 81-176 colonization and persistence in the murine infection model, we inoculated myd88 _/_ C57BL/6 mice orally or intraperitoneally with equal numbers of wild-type C. jejuni 81-176 and its isogenic sdaA mutant. Five weeks after infection animals were sacrificed and the colony forming units (CFUs) of C. jejuni in intestine and liver were determined (Figure 3). High CFUs numbers of the wild-type C. jejuni strain could be recovered from the intestine of orally infected mice. In contrast, the sdaA mutant had a reduced capacity to persist in the murine intestine (Figure 3A, Figure S6A). In addition, the sdaA mutant could not be recovered from the liver of most animals (11 out of 18) or was recovered in significantly reduced numbers in comparison to the wild-type strain (Figure 3B, Figure S6A). Taken together, the wild-type strain was able to outcompete the sdaA mutant in 16 of the 18 intraperitoneally infected animals. These results indicate that in contrast to the ggt and ansB mutants, which exhibited a tissue-specific colonization defect, the C. jejuni sdaA mutant strain was defective for persistence in all tissues tested. It also stresses the importance of serine catabolism for C. jejuni 81-176 in vivo since the growth of the sdaA mutant was less dramatically affected when co-cultivated with the wild-type strain in vitro (Figure S7).
Figure 3. Role of serine catabolism in C. jejuni 81-176 mouse colonization.
Shown is the colonization efficiency of C. jejuni 81-176 wild-type (black diamonds) and its isogenic sdaA mutant strain (white diamonds) in a murine model of infection. The colonization of the intestine (A) and liver (B) was evaluated 5 weeks after oral or intraperitoneal co-infection of myd88−/− nramp−/− mice, respectively. Each pair of black and white diamonds represents the CFU numbers of the C. jejuni 81-176 wild-type strain and its sdaA mutant recovered from the indicated organs of an individual animal. The combined results of one (A) and two (B) independent infection experiments are shown. The Mann–Whitney U test ( = Wilcoxon rank-sum test) was used to calculate the P values for the statistical differences between the CFU numbers of the different strains: P<0.05 (A) and P<0.001 (B).
The Role of Peb1A on the Persistence of C. jejuni in Different Murine Tissues
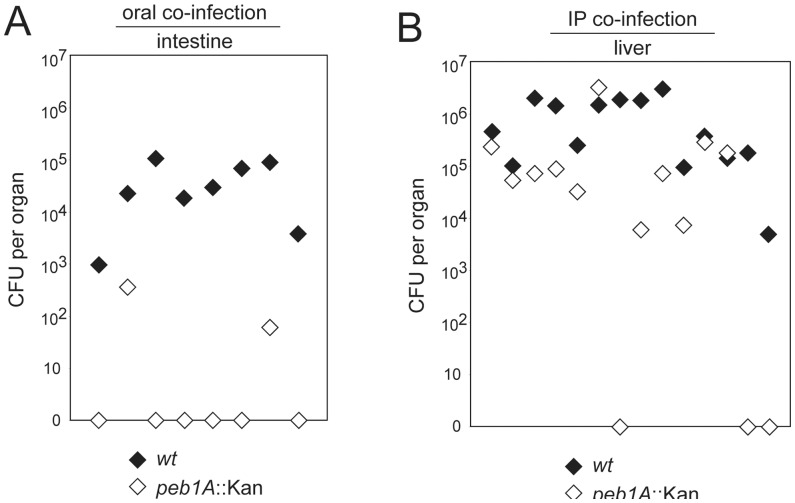
The pronounced and broad colonization defect of the sdaA mutant suggests a particularly important role for serine catabolism in the ability of C. jejuni to persist both in the intestine and systemically. This is in contrast to the more moderate and tissue specific defects observed in C. jejuni mutants unable to utilize other amino acids such as glutamine or asparagine [43], [51]. To gain more insight into the relative contribution of amino acids in C. jejuni colonization, we examined the ability of a C. jejuni peb1A mutant strain to colonize different tissues. Peb1A is a periplasmic aspartate-glutamate binding protein [40], [65] that is required for the growth with aspartate and glutamate by C. jejuni [40], [43]. Certain C. jejuni isolates like C. jejuni 81-176 secrete an asparaginase and γ-glutamyltranspeptidase enabling the efficient deamination of asparagine and glutamine and subsequently the additional generation of aspartate and glutamate in the periplasm [43]. Thus a C. jejuni 81-176 peb1A mutant is simultaneously defective in its ability to utilize all four amino acids resulting in slightly reduced growth efficiency when grown in rich media in vitro as compared to the wild-type strain during co-cultivation experiments (Figure S7). Equal numbers of wild-type C. jejuni 81-176 and the isogenic peb1A mutant were administrated orally or intraperitoneally into myd88−/− C57BL/6 mice. A significant difference in the bacterial load of the intestine with wild-type strain and its peb1A mutant was observed 5 weeks after oral infection and in all cases the wild-type strain was able to outcompete the mutant (Figure 4A, Figure S6B). Moreover, the peb1A mutant could not be recovered in 6 of the 8 infected animals. This observation is in good agreement with previous results, describing the colonization defect of the C. jejuni 81-176 peb1A mutant in BALB/c mice [66]. Upon intraperitoneal co-infection the wild-type strain was recovered from the liver in higher numbers than the mutant strain in 9 out of 14 infected mice, and the peb1A mutant of C. jejuni 81-176 was not recovered from the liver of three infected animals at all (Figure 4B, Figure S6B). These experiments clearly demonstrated the importance of Peb1A for the persistence of C. jejuni 81-176 in the liver.
Figure 4. Peb1A-mediated amino acid uptake in C. jejuni 81-176 is important for its persistence in the murine intestine and liver.
Mice were orally (A) or intraperitoneally (B) co-infected with the C. jejuni 81-176 wild-type strain (black diamonds) and the peb1A mutant (white diamonds). The bacterial load in the intestine and liver of the infected animals was examined 5 weeks after infection. The pairs of black and white diamonds indicate the CFU numbers of C. jejuni 81-176 and its peb1A mutant that could be recovered from the indicated organs of one animal. The combined results of two independent infection experiments are shown. The Mann–Whitney U test was used to calculate the P values for the statistical differences between the CFU numbers of the different strains: P<0.001 (A) and P<0.05 (B).
Proline Metabolism in C. jejuni 81-176
Several studies have documented that C. jejuni can utilize proline for in vitro growth [38], [39], [42], [43]. However, the specific genes involved in the catabolism of proline have not been experimentally investigated. All available C. jejuni genome sequences [33], [34], [51], [56]–[62], [64] show the presence of a homologue of PutP, a proline-sodium symporter, and a homologue of PutA that catalyzes the conversion of proline to glutamate (Figure 5A and 5B). PutA represents a bifunctional enzyme combining the enzymatic activities of a proline dehydrogenase and a 1-pyrroline-5-dehydrogenase. Because reducing equivalents in the form of FADH and NADH are generated by the PutA-mediated oxidation of proline to glutamate, the catabolism of proline not only provides energy but also affects the intracellular redox level of C. jejuni [67]. The arrangement of putA (CJJ81176_1495) and putP (CJJ81176_1494) in the genome of C. jejuni 81-176 suggests that the two genes are organized in an operon structure (Figure 5A), which is in contrast to the organization of the putA and putP orthologues in enterobacteria (Figure S8). PutA is well conserved in the epsilon-bacteria and exhibits high homology to PutA orthologues in the unrelated species Bacteroides spp. and Corynebacterium spp. (Table S3). Likewise, PutP is highly conserved in C. jejuni and exhibits significant homology to the PutP orthologues found in Bacillus spp., Enterobacter spp. and Pseudomonas spp. (Table S4). We therefore investigated the potential role of the putA and putP homologues in the ability of C. jejuni 81-176 to utilize proline. We found that insertional inactivation of putA or putP completely abolished the ability of C. jejuni to utilize proline as a carbon source, whereas no defects in the utilization of glutamate or serine as growth substrates were detected (Figure 5C). These results indicated that PutA and PutP are required for the catabolism of proline in C. jejuni. Interestingly, an abolished putP-mediated proline catabolism does not affect the mutant in growth competition experiments with the wild-type strain when co-cultivated in vitro using nutrient rich BHI medium (Figure S7).
Figure 5. Proline metabolism in C. jejuni 81-176.
(A) The gene locus of C. jejuni 81-176 encoding the proline permease PutP and the bifunctional proline dehydrogenase PutA is shown. The start codons (bold) and Shine-Dalgarno-sequences (underlined) of putA and putP as well as the intergenic region are indicated below the gene region. (B) Schematic model of the proline uptake system in C. jejuni. After proline is being co-imported with sodium ions by the permease PutP into C. jejuni, the cytoplasmic, bifunctional proline dehydrogenase PutA converts proline into glutamate. (C) The ability of C. jejuni 81-176 wild-type strain, its isogenic putA and putP mutants as well as the complemented putP mutant strains to utilize proline as a growth substrate. Values are the mean ± SD of at least three determinations of maximal reached optical density by C. jejuni 81-176 and its mutant derivates in liquid culture. The growth of C. jejuni in DMEM supplemented with 20 mM glutamate, proline or serine occurred over 24 hours at 37°C in 10% CO2.
Role of Proline Catabolism in C. jejuni Mouse Colonization
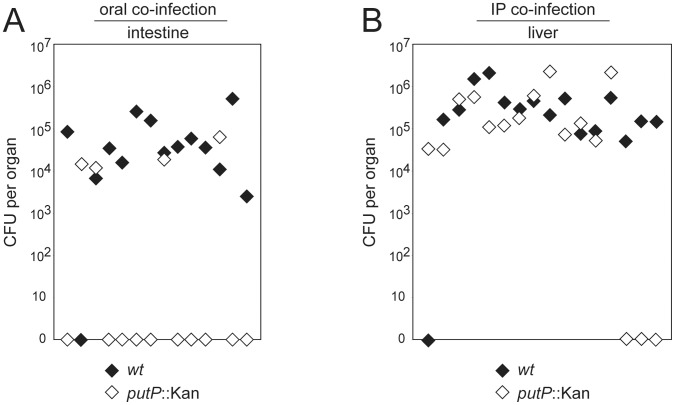
Since only limited information is available on the contribution of proline catabolism to bacterial pathogen colonization, we examined the role of proline utilization in C. jejuni 81-176 mouse colonization. We infected myd88−/− mice orally or intraperitoneally with equal numbers of wild-type C. jejuni 81-176 and the isogenic putP mutant strain and examined bacterial loads in different tissues. We found significantly lower numbers of the C. jejuni putP mutant in the intestine of infected mice 5 weeks after the oral co-administration of the two bacterial strains (Figure 6A, Figure S6C). These results indicate that proline metabolism is important for efficient C. jejuni colonization of and persistence in the mouse intestine of infected animals. In contrast, we found no difference between the bacterial loads of wild-type strain and the putP mutant in the livers of infected animals 5 weeks after intraperitoneal administration (Figure 6B, Figure S6C). These results indicate that proline catabolism, like glutamine catabolism, is dispensable for the colonization of systemic tissues by C. jejuni. Therefore, the utilization of proline also confers a tissue specific advantage for C. jejuni colonization.
Figure 6. Tissue-specific impact of proline catabolism on C. jejuni 81-176 mouse colonization.
Shown is the colonization efficiency of C. jejuni 81-176 wild-type (black diamonds) and its isogenic putP mutant strain (white diamonds) in a murine model of infection. The colonization of the intestine (A) and liver (B) was evaluated 5 weeks after oral (A) or intraperitoneal (B) co-infection of myd88 −/−, nramp −/− mice, respectively. Each pair of black and white diamonds represents the CFU numbers of C. jejuni 81-176 wild-type strain and its putP mutant recovered from the indicated organs of an individual animal. The combined results of four (A) and three (B) independent infection experiments are shown. The Mann–Whitney U test was used to calculate the P values for the statistical differences between the CFU numbers of the different strains: P<0.001 (A) and P ≥ 0.05 (B).
Capability of C. jejuni Mutants Defective in Amino Acid Catabolism to Cope with Oxidative and Osmotic Stress
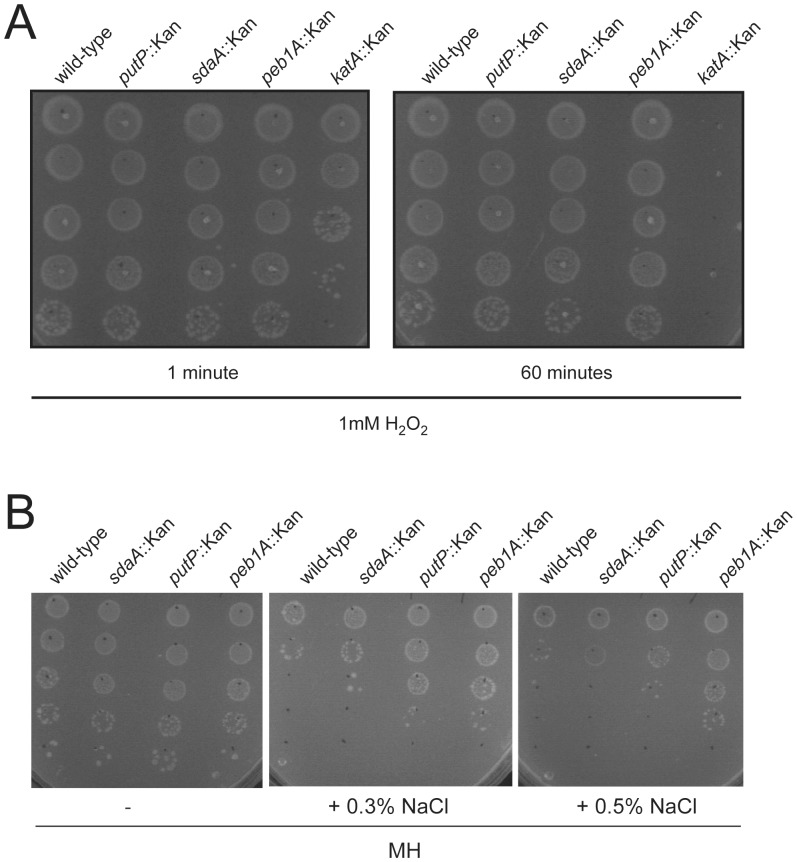
It has been recently shown that mutations in the amino acid transporters paqP and paqQ have altered tolerance to osmotic and oxidative stress [68]. Therefore we tested the ability of the C. jejuni 81-176 wild-type as well as its peb1A, putP, sdaA and katA mutants to cope with oxidative stress by exposing them to hydrogen peroxide (H2O2). Whereas the viability of the katA mutant was dramatically affected by the H2O2 treatment similar to previous descriptions [69], we found no difference between the peb1A, putP and sdaA mutants and wild-type C. jejuni 81-176 in their plating efficiency after exposure to oxidative stress (Figure 7A). To examine whether mutations in peb1A, putP and sdaA affect the ability of C. jejuni to cope with osmotic stress, we examined the plating efficiency of the different mutants in media containing varying concentration of sodium chloride. None of the tested mutants showed an increased sensitivity to osmotic stress (Figure 7B). The putP and peb1A mutants were even more resistant to 0.5% NaCl than the wild-type strain. We do not yet know the cause of this unexpected phenotype.
Figure 7. Effect of oxidative and osmotic stress on the viability of C. jejuni 81-176 mutants with defective amino acid catabolism.
(A) Shown is the capability of C. jejuni 81-176 wild-type and the sdaA, putP and peb1A mutants to survive oxidative stress caused by treatment with H2O2. Serial dilutions of the bacterial suspensions after the different treatments were spotted on Brucella broth agar plates after the indicated incubation times. (B) The resistance of C. jejuni 81-176 wild-type and its isogenic sdaA, putP and peb1A mutants to variable osmotic stress conditions is presented. Liquid cultures were adjusted to an OD600 of 0.1 and serial dilutions were spotted on Mueller Hinton agar plates supplemented with 0%, and 0.5% NaCl.
Taking together these results indicate that the colonization defect observed in these mutant strains is due to their defective acquisition of growth substrates rather than their elevated sensitivity to oxidative and osmotic stresses.
Discussion
Although C. jejuni is a fastidious bacterium in vitro and exhibits significant metabolic restrictions in comparison to other enteropathogenic bacteria [35], [70], this pathogen requires a remarkably low infection dose to successfully colonize different hosts [71], [72]. Therefore C. jejuni must have the capability to acquire and utilize nutrients efficiently to circumvent the competition by the host microbiota. Unlike other enteropathogenic bacteria C. jejuni is unable to use glucose as growth substrate [35] and only a subset of C. jejuni isolates harbor a fucP gene cluster that facilitates the catabolism of fucose [36], [37]. Instead of carbohydrates, the glucogenic amino acids aspartate, asparagine, glutamate, glutamine, proline and serine represent important nutrients fueling the in vitro growth of C. jejuni [38]–[40], [42], [43], [45], [55], [68].
So far, only a few studies have examined the impact of amino acid catabolism on the capacity of C. jejuni to thrive in different hosts [38], [55], [68] and to persist in different tissues [43], [51]. Velayudhan and Kelly (2004) have previously shown that a sdaA mutant of C. jejuni NCTC 11168 is defective in utilizing serine and exhibits a significant colonization defect in chickens. We were interested to examine whether serine catabolism provides C. jejuni with an advantage to colonize animals other than chickens. We found that a C. jejuni sdaA mutant is required for colonization in a mouse model of infection indicating that serine utilization may be required for the colonization of many animal species. Other metabolic traits exhibit similar benefits for the infection process of C. jejuni in various hosts. For example, C. jejuni mutants incapable to use glutamine and glutathione as growth substrates, not only showed a colonization defect in mice [51] but also in chicken [73]. Furthermore, the AspA-mediated aspartate utilization is important for the colonization of C. jejuni in murine [74] and avian [38] hosts. Besides amino acid catabolism, the FeoB-mediated uptake of ferrous iron provides a general benefit for C. jejuni during the colonization of the avian and porcine gut [49]. Interestingly, not all physiological properties of C. jejuni exhibit a host-independent affect on its colonization proficiency but show a host-specific defect instead: the ability of some C. jejuni strains to metabolize fucose is required for efficient colonization of pigs but not chickens [37] and a C. jejuni mutant defective in gluconate respiration shows a diminished colonization efficiency in chickens but not in mice [52]. Future studies will be required to clarify how specific metabolic traits of C. jejuni shape its persistence in specific hosts.
Several in vitro studies have shown that C. jejuni utilizes proline as a growth substrate [38], [39], [42]. It was suggested that proline represents a less favoured growth substrate in comparison to glutamate or serine for Campylobacter [42]. Nevertheless, proline catabolism seems to be a highly conserved phenotypic trait in C. jejuni [43] and C. coli isolates (Wensel and Hofreuter, unpublished data). We have shown here that proline catabolism is required for efficient colonization of the murine intestine. Strikingly, the inability of the putP mutant to utilize proline for growth did not affect its persistence in the murine liver. This tissue-specific colonization phenotype resembles the behaviour of the previously characterized ggt mutant of C. jejuni 81-176, which was also defective for the colonization of the murine gut but could colonize the liver of the infected mice to the same extent as the wild-type strain [43], [51]. The importance of proline catabolism in host colonization has also been shown in Helicobacter spp. [75]–[77], Rhizobium meliloti [78] and Staphylococcus aureus [79]. The PutA mediated oxidation of proline to glutamate generates the reducing equivalents FADH and NADH. Subsequently, the electrons are transferred to the respiration chain and partially to molecular oxygen generating H2O2 [76]. Future experiments have to clarify to what extent the observed colonization defect of the C. jejuni 81-176 putP mutant is the result of a reduced energy catabolism or an imbalanced intracellular redox environment.
The protective role of amino acid metabolism in counteracting osmotic and oxidative stress is well documented in enteric bacteria [80]. In particular, the amino acids glutamate and proline serve as osmoprotective substances in various bacteria [81], [82]. Though the effect of glutamine metabolism on the ability of C. jejuni to withstand osmotic and oxidative stress was recently described [68], the peb1A, putP nor sdaA mutants of C. jejuni 81-176 did not show reduced sensitivity to these types of stresses. These observations indicate that the reduced colonization efficiency of the here-described C. jejuni 81-176 mutants is most likely due to their inability to utilize the specific amino acids and not to other secondary effects.
The present study has extended our previous observations indicating that the catabolism of certain amino acids confers tissue-specific advantages for C. jejuni colonization: Serine supports the growth of C. jejuni 81-176 in the murine intestine and liver, indicating that the uptake of neither lactate nor of pyruvate could compensate the restricted catabolism of the sdaA mutant at either infection site. The catabolism of proline and glutamine provides an advantage for the persistence of C. jejuni 81-176 in the intestine but not in the liver of infected mice. As glutamate is generated by the catabolism of glutamine as well as proline, our studies suggest that glutamate is a crucial growth substrate for the colonization of the murine intestine by C. jejuni 81-176. These results also imply that glutamate is not available in sufficient amounts at the intestinal niche that C. jejuni occupies, since glutamate would be expected to overcome the catabolic defects of the C. jejuni 81-176 ggt and putA mutants as shown in vitro. We have demonstrated previously that asparagine utilization by C. jejuni 81-176 is especially beneficial for the persistence in the liver but not the intestine [43]. The importance of asparagine catabolism for the liver colonization by C. jejuni 81-176 is further supported by our here-presented observations that the peb1A mutant, defective in the utilization of asparagine, aspartate, glutamate and glutamine, exhibits a similar colonization defect in the murine liver as an ansB mutant of C. jejuni 81-176, solely incapable to utilize asparagine [43]. Interestingly, the tissue specific necessity for certain amino acids has also been shown in S. aureus [83] and recently in Francisella novicida, [84].
There is remarkable variability in the metabolic profiles of C. jejuni isolates [36], [43], [44], [85] and serine catabolism is one such non-conserved metabolic trait in C. jejuni. Though C. jejuni ATCC 33251 and RM1221 are not able to utilize serine under the in vitro growth condition tested, we did not detect any obvious changes in the coding sequences of either sdaA or sdaC that could account for this phenotype. Furthermore, both strains express the sdaCA operon and are able to take up serine from the culture medium like C. jejuni 81-176. As C. jejuni ATCC 33251 and RM1221 showed only slightly reduced SdaA activities in comparison to the strain C. jejuni 81-176, future experiments have to clarify if these differences are responsible for the abolished serine catabolism. More likely, defects in other, yet unidentified genes could be required for serine utilization and may explain the inability of certain C. jejuni strains to utilize this amino acid.
Though it has been demonstrated for C. jejuni NCTC 11168 that serine catabolism is important for the colonization of chickens [45], C. jejuni RM1221 with its inability to utilize serine was originally isolated from chicken [86]. Considering the metabolic diversity of C. jejuni isolates, further studies will be required to elucidate any metabolic characteristics C. jejuni RM1221 may harbor that could compensate its inability to grow with serine. In this context, C. jejuni RM1221 showed the best growth with proline in comparison to all other wild-type strains tested (Figure 1B). Though C. jejuni is non-glycolytic [35], certain isolates like C. jejuni NCTC 11168 and RM1221 encode for genes involved in fucose catabolism [37]. Differences in the utilization of fucose or certain other yet not identified carbohydrates perhaps allow C. jejuni RM1221 to overcome its restricted amino acid catabolism during the colonization process. Also future studies have to examine if the efficient catabolism of citric acid cycle intermediates like fumarate or succinate [38], [44] could compensate the abolished serine catabolism in RM1221 in vivo.
We are just beginning to understand the specific nutrients upon which pathogenic bacteria rely during host colonization [1], [2]. It is becoming increasingly clear that there are significant differences between the metabolic requirements in vitro and in vivo. Various studies have shown a clear correlation between certain metabolic traits and the ability of C. jejuni to colonize different animals. So understanding the metabolism of C. jejuni during the infection process may facilitate the development of anti-microbial drugs that specifically target essential metabolic pathways in C. jejuni.
Materials and Methods
Bacterial Strains, Media and Culture Conditions
C. jejuni was routinely cultivated on Brucella broth agar plates and tryptic soy agar (TSA) or Columbia agar plates containing 5% (vol/vol) sheep blood at 37°C in 10% CO2 atmosphere. To select for C. jejuni mutants carrying antibiotic-resistant determinants, either kanamycin, chloramphenicol or erythromycin were added to Brucella broth (BB) agar plates with a final concentration of 50 mg/l, 7.5 mg/l or 10 mg/l, respectively. For growth experiments in 4 ml liquid cultures, C. jejuni was inoculated with a starting OD600 of approximately 0.1 in Brain Heart Infusion (BHI; BD 237500) medium or in defined Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen 11965), supplemented with Fe(II)-ascrobate (Sigma; AO207) containing 20 mM of the indicated amino acids (Sigma). The liquid cultures were incubated in a rotating wheel (50 rpm) at 10% CO2 and 37°C. Bacterial growth in liquid cultures was monitored with a spectrophotometer (Spectronic 20, Genesys) by measuring the optical density of the cultures at 600 nm (OD600). All C. jejuni strains were stored at –80°C in BHI broth containing 40% glycerol.
Escherichia coli DH5α was cultured at 37°C on Luria-Bertani (LB) medium agar plates supplemented when necessary with the antibiotics kanamycin (50 mg/l), chloramphenicol (30 mg/l) or erythromycin (100 mg/l).
Natural Transformation of C. jejuni
Natural transformation was used to genetically manipulate C. jejuni 81-176 similar as described previously (18). Briefly, C. jejuni 81-176 was resuspended in 1 ml BHI medium with an OD600 of 0.1. Approximately 1 µg of DNA was added and the bacterial suspension was incubated overnight (37°C, 10% CO2). The next day bacteria were spread onto Brucella Broth plates supplemented with the appropriate antibiotics.
Construction of Recombinant Plasmids for Generating C. jejuni Mutants
To generate an isogenic sdaA (CJJ81176_1615) mutant of C. jejuni 81-176 the 1.3 kb sdaA gene with 500 bp of the upstream and downstream regions was PCR amplified with the primers sdaA_fwd and sdaA_rev (see Table S6 for primer information) and cloned into the BamHI/PstI-restriction sites of pBluescriptIIKS. The aphA3-cassette was released from pILL600 (see Table S7 for plasmids used in this study) by SmaI digestion and introduced into a unique NheI site within the cloned sdaA gene by blunt end ligation. The resulting plasmid pSB3622 was introduced into C. jejuni 81-176 by natural transformation as described above and C. jejuni transformants were selected with Brucella broth plates containing kanamycin (50 mg/l). To complement the C. jejuni 81-176 sdaA mutant strain, the sdaA gene of C. jejuni 81-176 was PCR amplified with its Shine-Dalgarno sequence by using the primers OW2 and OW3 and cloned into the NcoI/SpeI sites of pSB3021 [30]. The resulting plasmid pOW4 was verified by sequencing and transformed into the sdaA mutant of C. jejuni 81-176. Transformants were selected on Brucella broth agar plates supplemented with chloramphenicol (7.5 mg/l). A sdaC mutant of C. jejuni 81-176 was generated by introducing an erythromycin resistance marker into the gene CJJ81176_1616: a 2.9 kb sdaA-sdaC fragment was PCR amplified with the primers DHO285 and DHO284 and cloned into the XbaI/XhoI-restriction sites of pBluescriptIISK resulting into the plasmid pSK1. An erythromycin cassette was amplified by PCR with the oligonucleotides DHO349 and DHO350 using pDHO36 as template. The PCR fragment was inserted into a unique EcoRI site of sdaC generating pOW34. This plasmid was transformed into C. jejuni 81-176. Transformants with an interrupted sdaC gene grew on Brucella Broth plates supplemented with erythromycin (10 mg/l).
To construct the putA (CJJ81176_1495) insertion mutant of C. jejuni 81-176 a gene fragment of about 3.0 kb was PCR amplified from the chromosome of C. jejuni 81-176 using the oligonucletides DHO231_putA-5′ and DHO232_putA-3′. The PCR product was cloned as XbaI/XhoI-fragment into the compatible digested vector pBluescriptIIKS generating pSB3030. The plasmid was electroporated into the dam- E. coli strain GM2199 (Table S7) and a singular ClaI restriction site of the putA gence was used to insert an aphA3 kanamycin resistence cassette, which was isolated as ClaI-fragment from pILL600. The resulting plasmid pSB3031 was introduced into C. jejuni 81-176 by natural transformation. Transformants with an inactivated putA gene were selected on Brucella broth plates supplemented with kanamycin (50 mg/l). A putP mutant of C. jejuni 81-176 was constructed by PCR amplification of the putP gene (CJJ81176_1494) with the primer DHO233_putP-5′ and DHO234_putP-3′ and cloning of the XbaI/XhoI-digested PCR fragment into pBluescriptIIKS. A unique HindIII restriction site within the putP gene was used to insert the aphA3 resistance marker that was PCR amplified from pILL600 with the primers DHO2 and DHO52 resulting in plasmid pSB3032. The putP mutant was obtained by transformation of pSB3032 into C. jejuni 81-176 and selection of transformants on Brucella broth agar plates supplemented with kanamycin (50 mg/l). Complementation of the putP mutant was done by PCR amplification of the putP gene of C. jejuni 81-176 with the primes DHO286_putP-SD and DHO287_putP-3′, followed by cloning the NcoI−/SpeI-digested PCR fragment into the complementation vector pSB3021 resulting in the plasmid pSB3033. Natural transformation of C. jejuni 81-176 with plasmid pSB3033 generated the complemented putP mutant. A katA insertion mutant was constructed by in vitro transposon mutagenesis as described before [87] using a modified Tn552 transposon with an outward oriented cat and aphA3 cassette. The generation of the isogenic peb1A mutant has been described before [74].
Gene Expression Analysis by Reverse Transcriptase PCR Analysis
For the extraction of total RNA from C. jejuni aliquots of bacterial liquid cultures were taken after 18 hours of incubation and added to two volumes of RNAprotect bacteria reagent (Qiagen). The mixture was mixed immediately and incubated for 10 minutes at room temperature. After centrifugation (10 min, 7000 rpm) the supernatant was removed and the pellet was used for RNA extraction by RNeasy Mini kit (Qiagen) following the manufacturer’s instructions. RNA was eluted in 40 µL nuclease free water and remaining DNA was digested by DNase I (NEB) following the manufacturer’s instructions. The quantity and purity of RNA were measured spectrophotometrically using a NanoDrop 1000 (Thermo Scientific). For cDNA synthesis 40 ng RNA were added to a total volume of 12 µL nuclease free water containing 1 µL dNTPs and 1 µL random hexameres (Qiagen). The SuperScript II Kit (Fermentas) was used for the reverse transcription according to the manufacturer’s instructions. Equal amounts of the synthesized cDNA was used as templates for the partial PCR amplification of the sdaA gene with the primers DHO352 and OW10 generating a 628 bp PCR product. As reference the recA gene was amplified with the primers SR15 and SR16. The PCR was carried out in a total volume of 50 µL containing 2 µL cDNA, 5 µL TaqBuffer, 2 µL dNTPs, 1.5 µL MgSO4, 0.75 µL Primers each, 0.5 µL Taq polymerase (NEB), and 38.5 µL water with 30 cycles of 95°C for 15 s (denature), 58°C for 60 s (annealing), and 72°C for 45 s (extension). As a control to exclude amplification of DNA contamination an aliquot of RNA without RT treatment was used instead of cDNA as PCR template.
Measurement of Serine Dehydratase Activity in Cell Extracts
C. jejuni was grown in liquid cultures (200 ml BHI) for 18 hours at 37°C under microaerophilic conditions using an Anaerocult C pack (Merck). Cells were harvested by centrifugation (20 min, 7000 rpm, 4°C), washed with cold 0,1 M triethanolamine buffer (pH 7,5), and resuspended in 500 µl of the same buffer. Lysis was followed by sonification with the Branson Sonifier 450 (5 min, 30% of duty). The cell extracts were clarified from cell debris by centrifugation (30 min, 14000 rpm, 4°C) and the supernatants were used for the enzymatic assay the same day. The amount of total protein was determined using the Pierce® BCA Protein Assay Kit (ThermoScientific). The serine dehydratase activity of the cell lysates was measured as described previously [88]: Briefly, the assay was carried out in a total volume of 1 mL 50 mM HEBS buffer (pH 8,4) containing 150 µM NADH (Roth), 2,7 U LDH (Sigma), and cell-free extract. The reaction was started by adding 50 mM L-serine (Sigma Aldrich) and the decrease of NADH was monitored photometrically at 360 nm.
Serine Uptake by C. jejuni
For the comparison of serine uptake from the growth medium by different C. jejuni isolates, cultures of C. jejuni 81-176, C. jejuni RM1221 and C. jejuni 33251 were grown in 50 ml Hank`s Balanced Salt Solution supplemented with Fe2+ in the form of Iron(II)-ascorbate (Sigma, A0207), MEM Vitamin Solution (Invitrogen, 11120) and 1% Casamino acids (Roth, AE41.1) as a carbon source. All cultivations were done at 37°C and 150 r.p.m. on a rotary shaker under oxygen reduced atmosphere using Anaerocult C-Packs (Merck, 116275). The uptake of serine in C. jejuni was studied by analyzing the changes in the amount of serine in the culture supernatants. Samples of 2 ml of supernatant were taken at the particular time points. The bacterial cells were removed by centrifugation (17000 g, 5 min, 4°C) and an aliquot of the filtrated supernatant was used for GC-MS analysis. This aliquot (100 µl) of the supernatant was diluted in 500 µl water, containing 4 µg of ribitol as an internal standard. The samples were mixed, dried under vacuum at room temperature and stored at −20°C. Derivatization of the samples was done by using 40 µl pyridine, containing methoxyamine hydrochloride (20 mg ml-1) and 60 µl N-Methyl-N-trimethylsilyltrifluoro-acetamide (MSTFA). The GC-MS analysis was done on a Thermo GC Ultra coupled to a DSQII mass spectrometer equipped with an AS3000 autosampler (ThermoScientific, Dreieich, Germany) as previously described [89] with the following exceptions: Helium flow was set to 1.1 ml min-1 and the temperature was increased to final 325°C. Solvent delay time was 5.80 min. Data analysis was performed with Metabolite Detector (version 2.07; [90]) as described [89] and quantification was done by using one unique fragment ion for each metabolite. For statistical analysis, data were first normalized dividing the peak area of every detected compound in each sample by the peak area of the respective internal standard ribitol. Before further data evaluations derivates belonging to one substance were summarized. Afterwards the mean and the standard deviation of 5 biological samples with 2 technical replicates each were calculated.
Osmotic Stress Assay
The capability of C. jejuni to tolerate osmotic stress was assayed by its growth on Mueller Hinton broth plates supplemented with various concentration of NaCl. C. jejuni cells were harvested from blood pates, resuspended in BHI medium and cultured for several hours in an atmosphere of 10% CO2 at 37°C. The bacterial suspension was adjusted to an OD600 of 0.1 and serially diluted. From each dilution 10 µl were spotted on Mueller Hinton (Difco #225220) agar plates supplemented with 0% and 0.5% NaCl. The plates were incubated in 10% CO2 at 37°C.
Oxidative Stress Assay
The capability of C. jejuni to cope with oxidative stress was evaluated by exposure to 1 mM hydrogen peroxide (H2O2; Applichem A4269.1000) in Brucella broth. A bacterial suspension of an OD600 of 0.1 was incubated for various times in the H2O2-containing media. Afterwards bacteria were serially diluted and 10 µl of each dilution were spotted on Brucella Broth agar plates. The plates were incubated in 10% CO2 at 37°C.
Co-cultivation Experiments
To compare the growth characteristics of C. jejuni 81-176 and its isogenic mutants in vitro, 10 ml of BHI medium were inoculated in a flask with an equal amount of wild-type and mutant strain to a final OD600 of 0.1. The flask was incubated in a jar at 37°C and 150 r.p.m. on a rotary shaker under oxygen-reduced atmosphere using Anaerocult C-Packs (Merck, 116275). After 20 hours, aliquots of the culture were taken and plated after serial dilution on Brucella broth agar plates and Brucella broth agar plates containing kanamycin to enumerate the wild-type and mutant strain. To determine if C. jejuni 81-176 or its mutants have growth advantage under in vitro cultivation condition, the competitive index was calculated: CI = (mutantoutput/wild-typeoutput)/(mutantinoculum/wild-typeinoculum). Input represents the CFUs of the inoculum and output the recovered CFUs after 20 hours.
Mice Infection Experiments
For animal infection experiments myd88 −/−, nramp1 −/−, male age-matched (6 to 8 weeks old) C57BL/6 mice were used as previously described (72). Briefly, C. jejuni 81-176 wild-type strain and its isogenic mutants were grown in BHI medium to an OD600 of about 0.6 and then applied in equal amounts of 109 or 107 CFUs for oral or intraperitoneal (i.p.) co-infection of mice, respectively. The colonization levels of the different C. jejuni strains were monitored by enumerating the number of CFUs in the feces of inoculated animals. At the end of the experiment mice were sacrificed, and the intestine and liver were aseptically removed and homogenized in HBSS. The bacterial loads in their organs of infected animals were enumerated by plating on Brucella broth agar plates containing Campylobacter-selective supplements (Oxoid SR0167E) as well as on Campylobacter-selective plates containing the antibiotic kanamycin (50 mg/l) to differentiate between C. jejuni wild-type and mutant strains. Statistical analysis of the co-infection experiments were carried out with the non-parametric, two-sided Mann–Whitney U test ( = Wilcoxon rank-sum test) using a Web-based algorithm (http://elegans.som.vcu.edu/~leon/stats/utest.html). The Mann–Whitney U test was used to examine if the observed differences in the numbers of recovered CFUs of the wild-type strain and its isogenic mutants from co-infected mice were significant. Therefore the recovered CFUs of the wild-type strain and the CFUs of the mutant strains were grouped in two datasets and directly analyzed with the web-based algorithm. The competitive index was determined for each infected mice with the equation (mutantoutput/wild-typeoutput)/(mutantinoculum/wild-typeinoculum). Output numbers equal the bacterial loads of wild-type and mutant strains recovered from the organs. The CFUs of the inoculums were determined through plating of serial dilutions of the bacterial suspensions that had been adjusted to an equal number of wild-type and mutant bacteria. All animal work was approved by the Yale University Institutional Animal Care and Use Committee (IACUC) and all animals were maintained as well as animal experiments were conducted according to the guidelines of the IACUC regulations.
Supporting Information
Nucleotide sequence comparison of the L-serine dehydratase sdaA genes in indifferent C. jejuni isolates. The nucleotide sequences of sdaA open reading frames from different C. jejuni strains were compared using ClustalW (www.ebi.ac.uk/Tools/msa/clustalW2). The sources for the DNA sequences are as follows: CG8486 (Cj8486_1666c; NZ_AASY01000001.2), ATCC 33251 (this study), CF93-6 (CJJCF936_1718; NZ_AANJ01000002.1), 84-25 (CJJ8425_1708; NZ_AANT02000001.1), NCTC 11168 (Cj1624c; NC_002163.1), IA3902 (CJSA_1536; CP001876.1), DFVF1099 (CSQ_0902; ADHK01000020.1; this study), 305 (CSS_1725; ADHL01000259.1; this study), RM1221 (CJE1796; NC_003912.7), S3 (CJS3_1705; CP001960.1), 260.94 (CJJ26094_1675; NZ_AANK01000006.1), ICDCCJ07001 (ICDCCJ07001_1539; NC_014802.1), HB93-13 (CJJHB9313_1615; NZ_AANQ01000001.1), 81-176 (CJJ81176_1615; AASL01000001.1), 81116 (C8J_1526; NC_009839.1), M1 (CJM1_1565; CP001900.1), 327 (CSU_0676; ADHM01000033.1; this study), 1336 (C1336_000330073; NZ_ADGL01000024.1), CG8421 (Cj8421_1678; NZ_ABGQ01000002.1), 414 (C414_000010127; NZ_ADGM01000001.1).
(DOC)
Nucleotide sequence comparison of the serine transporter sdaC genes in different C. jejuni isolates. The published sequences of the sdaC genes from several C. jejuni isolates were compared using ClustalW (www.ebi.ac.uk/Tools/msa/clustalW2). The sources for the nucleotide sequences were obtained from GenBank (NCBI) and have following accession numbers: 260.94 (CJJ26094_1676; NZ_AANK01000006), ICDCCJ07001 (ICDCCJ07001_1540; NC_014802), 81116 (C8J_1527; NC_009839), M1 (CJM1_1566; CP001900), 327(CSU_0678; ADHM01000033.1), DFVF1099 (CSQ_0900; ADHK01000020), 305 (CSS_1724; ADHL01000259.1), IA3902 (CJSA_1537; CP001876), NCTC11168 (Cj1625c; NC_002163), CG8486 (Cj8486_1667c; NZ_AASY01000001), 84-25 (CJJ8425_1709; NZ_AANT02000001), CF93-6 (CJJCF936_1719; NZ_AANJ01000002), CG8421 (Cj8421_1679; NZ_ABGQ01000002), ATCC 33251 (this study), RM1221 (CJE1797; NC_003912), S3 (CJS3_1706; CP001960), HB93-13 (CJJHB9313_1616; NZ_AANQ01000001), 81-176 (CJJ81176_1616; NC_008787), 1336 (C1336_000330074; NZ_ADGL01000024), 414 (C414_000010126; NZ_ADGM01000001).
(DOC)
Amino acid sequence comparison of SdaA proteins from different C. jejuni strains. ClustaW (www.ebi.ac.uk/Tools/msa/clustalW2) was used for the alignment of the SdaA amino acid sequences from various C. jejuni isolates. The accession numbers were as follows: CF93-6 (CJJCF936_1718; ZP_01067690), 84-25 (CJJ8425_1708; ZP_01099834), NCTC 11168 (Cj1624c; YP_002344993), IA3902 (CJSA_1536; ADC29171), DFVF1099 (CSQ_0902, this study), 305 (CSS_1725; this study), CG8486 (Cj8486_1666c; ZP_01809456), RM1221 (CJE1796; YP_179767), S3 (CJS3_1705; ADT73404), 260.94 (CJJ26094_1675; NZ_AANK01000006.1), 81-176 (CJJ81176_1615; YP_001001267), 1336 (C1336_000330073; ZP_06374482), HB93-13 (CJJHB9313_1615; ZP_01070837), M1 (CJM1_1565; ADN91750), 81116 (C8J_1526; YP_001483100), ICDCCJ07001 (ICDCCJ07001_1539; YP_004067035), 327 (CSU_0676; this study), CG8421 (Cj8421_1678; ZP_03222408), ATCC 33251 (this study), 414 (C414_000010127; ZP_06371291).
(DOC)
Comparison of SdaC serine transporter protein sequences in various C. jejuni isolates. ClustaW (www.ebi.ac.uk/Tools/msa/clustalW2) was used for the alignment of the SdaC amino acid sequences from listed C. jejuni isolates. The protein sequence accession numbers for the SdaC serine transporters of the different C. jejuni strains were as follows: RM1221 (CJE1797; YP_179768), S3 (CJS3_1706, ADT73405), CF93-6 (CJJCF936_1719; ZP_01067605), 84-25 (CJJ8425_1709; ZP_01099564); CG8486 (Cj8486_1667c; ZP_01809457), NCTC 11168 (Cj1625c; YP_002344994), IA3902 (CJSA_1537; ADC29172), DFVF1099 (CSQ_0900; EFV06958), 305 (CSS_1724; EFV08300); CG8421 (Cj8421_1679; ZP_03222409); ATCC 33251 (this study); 260.94 (CJJ26094_1676; ZP_01070445), HB93-13 (CJJHB9313_1616; ZP_01071370); 81-176 (CJJ81176_1616; ZP_02271921), 81116 (C8J_1527; YP_001483101), ICDCCJ07001 (ICDCCJ07001_1540; YP_004067036), M1 (CJM1_1566; ADN91751); 327 (CSU_0678; EFV10631), 1336 (C1336_000330074; ZP_06374483), 414 (C414_000010126; ZP_06371290).
(DOC)
Growth of C. jejuni 81-176 and its isogenic sdaA and sdaC mutants. Growth characteristics of the C. jejuni 81-176 wild-type strain, its isogenic sdaC and sdaA mutants as well as a complemented sdaA mutant in DMEM and DMEM supplemented with 20 mM serine, glutamate or proline. The maximal optical densities (OD600) of liquid cultures from indicated C. jejuni strains over a time period of 24 hours are presented.
(TIF)
Competitive index of mice co-infection with C. jejuni 81-176 and its sdaA , peb1A or putP mutants. The competitive index, CI = (mutantoutput/wild-typeoutput)/(mutantinoculum/wild-typeinoculum), was calculated for each mouse infected with C. jejuni 81-176 wild-type strain and its indicated mutant. The output numbers representing the CFUs of wild-type and mutant strains recovered from the intestine or the liver of each animal are plotted in Figures 3, 4 and 6. Each mouse was infected with approximately the same number of wild-type and mutant strain as determined by the CFU counting of the inoculum.
(TIF)
In vitro growth competition experiments of C. jejuni 81-176 and its mutants. Shown are the co-cultivation experiments of C. jejuni 81-176 with its peb1A, putP and sdaA mutants, respectively. Equal amounts of wild-type and a mutant strain were incubated in nutrient rich BHI medium over night and the CFUs of each strain were determined after 20 hours. Each symbol represents the calculated competitive index for one co-cultivation experiment.
(TIF)
Comparison of the putA / putP gene cluster in Campylobacter and Enterobacteria . The schematic comparison of the putAputP gene locus and its flanking regions of several Campylobacter and Enterobacter strains was derived from the comparative genome database xBASE2 (Chaudhuri RR et al. (2008) Nucleic Acids Res., D543-6) in particular CampyDB (www.xbase.ac.uk/campydb) and ColiDB (www.xbase.ac.uk/colibase). The putA and putP genes are marked in light and dark grey, respectively. The genes of the flaking regions are represented as white arrows.
(TIF)
Proteobacteria with homologues to the L-serine dehydratase SdaA of C. jejuni 81-176. The table shows the homology between the SdaA protein of C. jejuni 81-176 and the SdaA proteins in other proteobacteria with their given accession numbers. The percent of amino acids identical and similar (conserved amino acid exchanges) between the serine ammonia-lyase of C. jejuni 81-176 and the other SdaA proteins were determined by BLASTP analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The order of the table reflects the score values calculated by the BLASTP algorithm. C. jejuni isolates are marked in red, other Campylobacter species in orange and Helicobacter species in yellow. Only a subset of C. jejuni isolates are listed, but all sequenced C. jejuni strains encode for SdaA homologues that are 100% or 99% identical to the SdaA protein of C. jejuni 81-176.
(DOC)
Proteobacteria with homologues to the serine transporter SdaC of C. jejuni 81-176. The table shows the homology between the SdaC protein of C. jejuni 81-176 and the SdaC proteins in other proteobacteria. The order represents the grade of homology according to the score calculated by the BlastP algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi). C. jejuni isolates are marked in red other Campylobacter species in orange and Helicobacter species in yellow. Sequenced C. jejuni strains that are not represented in the table encode for SdaC homologues that are at least 99% identical to the SdaC of C. jejuni 81-176.
(DOC)
Proteobacteria with homologues to the proline dehydrogenase/delta 1-pyrroline-5-carboxylate dehydrogenase PutA of C. jejuni 81-176. The table shows the homology between the PutA protein of C. jejuni 81-176 and the PutA proteins in other proteobacteria with their given accession numbers. The percent of amino acids identical and similar (conserved amino acid exchanges) between C. jejuni 81-176 PutA and the PutA proteins of other presented proteobacteria were determined by BLASTP analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The order of the table reflects the score values calculated by the BLASTP algorithm. C. jejuni isolates are marked in red, other Campylobacter species in orange and Helicobacter species in yellow. Only a subset of C. jejuni isolates are listed, but all sequenced C. jejuni strains encode for SdaA homologues that are at least 99% identical to the SdaA protein of C. jejuni 81-176.
(DOC)
Proteobacteria with homologues to the proline transporter PutP of C. jejuni 81-176. The table illustrates the homology between the PutP protein of C. jejuni 81-176 and the PutP proteins in other proteobacteria. The accession numbers of the PutP proteins from indicated bacteria are listed. The order represents the grade of homology according to the score calculated by the BlastP algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi). C. jejuni isolates are marked in red, Campyobacter species besides C. jejuni are highlighted in orange and Helicobacter species in yellow. Not all C. jejuni isolates are listed, but every sequenced C. jejuni strain encodes for a PutP homologue that is at least 98% identical to the PutP protein of C. jejuni 81-176.
(DOC)
L-serine dehydratase activity in cell extracts of C. jejuni isolates. The mean serine dehydratase activities with standard deviations are shown for measurements repeated four (a) and three (b) times in triplicates. The statistical significance in the differences of SdaA activity between C. jejuni 81-176 and C. jejuni 33251 or RM1221 was calculated by Student t test: * P<0.01; ** P<0.05.
(DOC)
Primers used in this study.
(DOC)
Strains and plasmids used in this study.
(DOC)
Acknowledgments
We thank Patricia Guerry (Enteric Disease Department, Naval Medical Research Center, Silver Spring, MD), Thomas Alter (Institute of Food Hygiene, Free University Berlin), Steffen Backert (School of Biomolecular and Biomedical Science, University College Dublin), and Susanne Knøchel (Department of Food Science, Copenhagen University) for providing C. jejuni strains. The E. coli strain GM2199 was kindly given us by Rainer Haas (Max von Pettenkofer-Institute, Ludwig-Maximilians-University, Munich). We thank María Lara-Tejero for providing the myd88 −/− mice, Heather Carleton-Romer for animal care and Shalaka Samant as well as Sonja Kunstmann for experimental support. The authors would like to thank the members of the laboratories for critical review of the manuscript.
Funding Statement
This work was supported by a grant from the Ellison Medical Foundation to JEG and by the Deutsche Forschungsgemeinschaft with the grant DFG HO 4553/1-1 and the grant HO 4553/1-2 as part of the priority program 1316 ‘Host Adapted Metabolism of Bacterial Pathogens’ to DH. The Hannover Biomedical Research School (HBRS) and the Center for Infection Biology (ZIB) supported JM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brown SA, Palmer KL, Whiteley M (2008) Revisiting the host as a growth medium. Nat Rev Microbiol 6: 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rohmer L, Hocquet D, Miller SI (2011) Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Microbiol 19: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buysse JM (2001) The role of genomics in antibacterial target discovery. Curr Med Chem 8: 1713–1726. [DOI] [PubMed] [Google Scholar]
- 4. Plaimas K, Eils R, Konig R (2010) Identifying essential genes in bacterial metabolic networks with machine learning methods. BMC Syst Biol 4: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allos BM (2001) Campylobacter jejuni Infections: update on emerging issues and trends. Clin Infect Dis 32: 1201–1206. [DOI] [PubMed] [Google Scholar]
- 6. de Zoete MR, van Putten JP, Wagenaar JA (2007) Vaccination of chickens against Campylobacter. Vaccine 25: 5548–5557. [DOI] [PubMed] [Google Scholar]
- 7. Lee MD, Newell DG (2006) Campylobacter in poultry: filling an ecological niche. Avian Dis 50: 1–9. [DOI] [PubMed] [Google Scholar]
- 8. Newell DG, Fearnley C (2003) Sources of Campylobacter colonization in broiler chickens. Appl Environ Microbiol 69: 4343–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butzler JP (2004) Campylobacter, from obscurity to celebrity. Clin Microbiol Infect 10: 868–876. [DOI] [PubMed] [Google Scholar]
- 10. Blaser MJ (1997) Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect Dis 176 Suppl 2 S103–105. [DOI] [PubMed] [Google Scholar]
- 11. Yuki N (2001) Infectious origins of, and molecular mimicry in, Guillain-Barre and Fisher syndromes. Lancet Infect Dis 1: 29–37. [DOI] [PubMed] [Google Scholar]
- 12. Young KT, Davis LM, Dirita VJ (2007) Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol 5: 665–679. [DOI] [PubMed] [Google Scholar]
- 13. van Putten JP, van Alphen LB, Wosten MM, de Zoete MR (2009) Molecular mechanisms of campylobacter infection. Curr Top Microbiol Immunol 337: 197–229. [DOI] [PubMed] [Google Scholar]
- 14. Fox JG, Rogers AB, Whary MT, Ge Z, Taylor NS, et al. (2004) Gastroenteritis in NF-kappaB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect Immun 72: 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hickey TE, McVeigh AL, Scott DA, Michielutti RE, Bixby A, et al. (2000) Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect Immun 68: 6535–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krause-Gruszczynska M, Rohde M, Hartig R, Genth H, Schmidt G, et al. (2007) Role of the small Rho GTPases Rac1 and Cdc42 in host cell invasion of Campylobacter jejuni. Cell Microbiol 9: 2431–2444. [DOI] [PubMed] [Google Scholar]
- 17. van Alphen LB, Bleumink-Pluym NM, Rochat KD, van Balkom BW, Wosten MM, et al. (2008) Active migration into the subcellular space precedes Campylobacter jejuni invasion of epithelial cells. Cell Microbiol 10: 53–66. [DOI] [PubMed] [Google Scholar]
- 18. Watson RO, Galan JE (2008) Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog 4: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, et al. (2011) Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One 6: e20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hendrixson DR, DiRita VJ (2004) Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol Microbiol 52: 471–484. [DOI] [PubMed] [Google Scholar]
- 21. Takata T, Fujimoto S, Amako K (1992) Isolation of nonchemotactic mutants of Campylobacter jejuni and their colonization of the mouse intestinal tract. Infect Immun 60: 3596–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao R, Burr DH, Guerry P (1997) CheY-mediated modulation of Campylobacter jejuni virulence. Mol Microbiol 23: 1021–1031. [DOI] [PubMed] [Google Scholar]
- 23. Bachtiar BM, Coloe PJ, Fry BN (2007) Knockout mutagenesis of the kpsE gene of Campylobacter jejuni 81116 and its involvement in bacterium-host interactions. FEMS Immunol Med Microbiol 49: 149–154. [DOI] [PubMed] [Google Scholar]
- 24. Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, et al. (2001) A phase-variable capsule is involved in virulence of Campylobacter jejuni 81–176. Mol Microbiol 40: 769–777. [DOI] [PubMed] [Google Scholar]
- 25. Chang C, Miller JF (2006) Campylobacter jejuni colonization of mice with limited enteric flora. Infect Immun 74: 5261–5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones MA, Marston KL, Woodall CA, Maskell DJ, Linton D, et al. (2004) Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infect Immun 72: 3769–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karlyshev AV, Everest P, Linton D, Cawthraw S, Newell DG, et al. (2004) The Campylobacter jejuni general glycosylation system is important for attachment to human epithelial cells and in the colonization of chicks. Microbiology 150: 1957–1964. [DOI] [PubMed] [Google Scholar]
- 28. Morooka T, Umeda A, Amako K (1985) Motility as an intestinal colonization factor for Campylobacter jejuni. J Gen Microbiol 131: 1973–1980. [DOI] [PubMed] [Google Scholar]
- 29. Naito M, Frirdich E, Fields JA, Pryjma M, Li J, et al. (2010) Effects of sequential Campylobacter jejuni 81–176 lipooligosaccharide core truncations on biofilm formation, stress survival, and pathogenesis. J Bacteriol 192: 2182–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watson RO, Novik V, Hofreuter D, Lara-Tejero M, Galan JE (2007) A MyD88-deficient mouse model reveals a role for Nramp1 in Campylobacter jejuni infection. Infect Immun 75: 1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Metris A, Reuter M, Gaskin DJ, Baranyi J, van Vliet AH (2011) In vivo and in silico determination of essential genes of Campylobacter jejuni. BMC Genomics 12: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stahl M, Stintzi A (2011) Identification of essential genes in C. jejuni genome highlights hyper-variable plasticity regions. Funct Integr Genomics 11: 241–257. [DOI] [PubMed] [Google Scholar]
- 33. Fouts DE, Mongodin EF, Mandrell RE, Miller WG, Rasko DA, et al. (2005) Major structural differences and novel potential virulence mechanisms from the genomes of multiple campylobacter species. PLoS Biol 3: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, et al. (2000) The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403: 665–668. [DOI] [PubMed] [Google Scholar]
- 35. Velayudhan J, Kelly DJ (2002) Analysis of gluconeogenic and anaplerotic enzymes in Campylobacter jejuni: an essential role for phosphoenolpyruvate carboxykinase. Microbiology 148: 685–694. [DOI] [PubMed] [Google Scholar]
- 36. Muraoka WT, Zhang Q (2011) Phenotypic and genotypic evidence for L-fucose utilization by Campylobacter jejuni. J Bacteriol 193: 1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stahl M, Friis LM, Nothaft H, Liu X, Li J, et al. (2011) L-fucose utilization provides Campylobacter jejuni with a competitive advantage. Proc Natl Acad Sci U S A 108: 7194–7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guccione E, Leon-Kempis Mdel R, Pearson BM, Hitchin E, Mulholland F, et al. (2008) Amino acid-dependent growth of Campylobacter jejuni: key roles for aspartase (AspA) under microaerobic and oxygen-limited conditions and identification of AspB (Cj0762), essential for growth on glutamate. Mol Microbiol 69: 77–93. [DOI] [PubMed] [Google Scholar]
- 39. Leach S, Harvey P, Wali R (1997) Changes with growth rate in the membrane lipid composition of and amino acid utilization by continuous cultures of Campylobacter jejuni. J Appl Microbiol 82: 631–640. [DOI] [PubMed] [Google Scholar]
- 40. Leon-Kempis Mdel R, Guccione E, Mulholland F, Williamson MP, Kelly DJ (2006) The Campylobacter jejuni PEB1a adhesin is an aspartate/glutamate-binding protein of an ABC transporter essential for microaerobic growth on dicarboxylic amino acids. Mol Microbiol 60: 1262–1275. [DOI] [PubMed] [Google Scholar]
- 41. Westfall HN, Rollins DM, Weiss E (1986) Substrate utilization by Campylobacter jejuni and Campylobacter coli. Appl Environ Microbiol 52: 700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wright JA, Grant AJ, Hurd D, Harrison M, Guccione EJ, et al. (2009) Metabolite and transcriptome analysis of Campylobacter jejuni in vitro growth reveals a stationary-phase physiological switch. Microbiology 155: 80–94. [DOI] [PubMed] [Google Scholar]
- 43. Hofreuter D, Novik V, Galan JE (2008) Metabolic diversity in Campylobacter jejuni enhances specific tissue colonization. Cell Host Microbe 4: 425–433. [DOI] [PubMed] [Google Scholar]
- 44. Mohammed KA, Miles RJ, Halablab MA (2004) The pattern and kinetics of substrate metabolism of Campylobacter jejuni and Campylobacter coli. Lett Appl Microbiol 39: 261–266. [DOI] [PubMed] [Google Scholar]
- 45. Velayudhan J, Jones MA, Barrow PA, Kelly DJ (2004) L-serine catabolism via an oxygen-labile L-serine dehydratase is essential for colonization of the avian gut by Campylobacter jejuni. Infect Immun 72: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mendz GL, Ball GE, Meek DJ (1997) Pyruvate metabolism in Campylobacter spp. Biochim Biophys Acta 1334: 291–302. [DOI] [PubMed] [Google Scholar]
- 47. Thomas MT, Shepherd M, Poole RK, van Vliet AH, Kelly DJ, et al. (2011) Two respiratory enzyme systems in Campylobacter jejuni NCTC 11168 contribute to growth on L-lactate. Environ Microbiol 13: 48–61. [DOI] [PubMed] [Google Scholar]
- 48. Davis LM, Kakuda T, DiRita VJ (2009) A Campylobacter jejuni znuA orthologue is essential for growth in low-zinc environments and chick colonization. J Bacteriol 191: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Naikare H, Palyada K, Panciera R, Marlow D, Stintzi A (2006) Major role for FeoB in Campylobacter jejuni ferrous iron acquisition, gut colonization, and intracellular survival. Infect Immun 74: 5433–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu F, Zeng X, Haigh RD, Ketley JM, Lin J (2010) Identification and characterization of a new ferric enterobactin receptor, CfrB, in Campylobacter. J Bacteriol 192: 4425–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hofreuter D, Tsai J, Watson RO, Novik V, Altman B, et al. (2006) Unique features of a highly pathogenic Campylobacter jejuni strain. Infect Immun 74: 4694–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pajaniappan M, Hall JE, Cawthraw SA, Newell DG, Gaynor EC, et al. (2008) A temperature-regulated Campylobacter jejuni gluconate dehydrogenase is involved in respiration-dependent energy conservation and chicken colonization. Mol Microbiol 68: 474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weingarten RA, Grimes JL, Olson JW (2008) Role of Campylobacter jejuni respiratory oxidases and reductases in host colonization. Appl Environ Microbiol 74: 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weingarten RA, Taveirne ME, Olson JW (2009) The dual-functioning fumarate reductase is the sole succinate:quinone reductase in Campylobacter jejuni and is required for full host colonization. J Bacteriol. [DOI] [PMC free article] [PubMed]
- 55. Ribardo DA, Hendrixson DR (2011) Analysis of the LIV system of Campylobacter jejuni reveals alternative roles for LivJ and LivK in commensalism beyond branched-chain amino acid transport. J Bacteriol 193: 6233–6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cooper KK, Cooper MA, Zuccolo A, Law B, Joens LA (2011) Complete genome sequence of Campylobacter jejuni strain S3. J Bacteriol 193: 1491–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Friis C, Wassenaar TM, Javed MA, Snipen L, Lagesen K, et al. (2010) Genomic characterization of Campylobacter jejuni strain M1. PLoS One 5: e12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hepworth PJ, Ashelford KE, Hinds J, Gould KA, Witney AA, et al. (2011) Genomic variations define divergence of water/wildlife-associated Campylobacter jejuni niche specialists from common clonal complexes. Environ Microbiol 13: 1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pearson BM, Gaskin DJ, Segers RP, Wells JM, Nuijten PJ, et al. (2007) The complete genome sequence of Campylobacter jejuni strain 81116 (NCTC11828). J Bacteriol 189: 8402–8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Poly F, Read T, Tribble DR, Baqar S, Lorenzo M, et al. (2007) Genome sequence of a clinical isolate of Campylobacter jejuni from Thailand. Infect Immun 75: 3425–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Poly F, Read TD, Chen YH, Monteiro MA, Serichantalergs O, et al. (2008) Characterization of two Campylobacter jejuni strains for use in volunteer experimental-infection studies. Infect Immun 76: 5655–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Takamiya M, Ozen A, Rasmussen M, Alter T, Gilbert T, et al. (2011) Genome Sequence of Campylobacter jejuni strain 327, a strain isolated from a turkey slaughterhouse. Stand Genomic Sci 4: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takamiya M, Ozen A, Rasmussen M, Alter T, Gilbert T, et al. (2011) Genome sequences of two stress-tolerant Campylobacter jejuni poultry strains, 305 and DFVF1099. J Bacteriol 193: 5546–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang M, He L, Li Q, Sun H, Gu Y, et al. (2010) Genomic characterization of the Guillain-Barre syndrome-associated Campylobacter jejuni ICDCCJ07001 Isolate. PLoS One 5: e15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pei Z, Blaser MJ (1993) PEB1, the major cell-binding factor of Campylobacter jejuni, is a homolog of the binding component in gram-negative nutrient transport systems. J Biol Chem 268: 18717–18725. [PubMed] [Google Scholar]
- 66. Pei Z, Burucoa C, Grignon B, Baqar S, Huang XZ, et al. (1998) Mutation in the peb1A locus of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect Immun 66: 938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Servet C, Ghelis T, Richard L, Zilberstein A, Savoure A (2012) Proline dehydrogenase: a key enzyme in controlling cellular homeostasis. Front Biosci 17: 607–620. [DOI] [PubMed] [Google Scholar]
- 68. Lin AE, Krastel K, Hobb RI, Thompson SA, Cvitkovitch DG, et al. (2009) Atypical roles for Campylobacter jejuni amino acid ATP binding cassette transporter components PaqP and PaqQ in bacterial stress tolerance and pathogen-host cell dynamics. Infect Immun 77: 4912–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Day WA Jr, Sajecki JL, Pitts TM, Joens LA (2000) Role of catalase in Campylobacter jejuni intracellular survival. Infect Immun 68: 6337–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelly DJ (2001) The physiology and metabolism of Campylobacter jejuni and Helicobacter pylori. Symp Ser Soc Appl Microbiol: 16S–24S. [DOI] [PubMed]
- 71. Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ (1988) Experimental Campylobacter jejuni infection in humans. J Infect Dis 157: 472–479. [DOI] [PubMed] [Google Scholar]
- 72. Robinson DA (1981) Infective dose of Campylobacter jejuni in milk. Br Med J (Clin Res Ed) 282: 1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Barnes IH, Bagnall MC, Browning DD, Thompson SA, Manning G, et al. (2007) Gamma-glutamyl transpeptidase has a role in the persistent colonization of the avian gut by Campylobacter jejuni. Microb Pathog 43: 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Novik V, Hofreuter D, Galan JE (2010) Identification of Campylobacter jejuni genes involved in its interaction with epithelial cells. Infect Immun 78: 3540–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kavermann H, Burns BP, Angermuller K, Odenbreit S, Fischer W, et al. (2003) Identification and characterization of Helicobacter pylori genes essential for gastric colonization. J Exp Med 197: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Krishnan N, Doster AR, Duhamel GE, Becker DF (2008) Characterization of a Helicobacter hepaticus putA mutant strain in host colonization and oxidative stress. Infect Immun 76: 3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nakajima K, Inatsu S, Mizote T, Nagata Y, Aoyama K, et al. (2008) Possible involvement of put A gene in Helicobacter pylori colonization in the stomach and motility. Biomed Res 29: 9–18. [DOI] [PubMed] [Google Scholar]
- 78. Jimenez-Zurdo JI, Garcia-Rodriguez FM, Toro N (1997) The Rhizobium meliloti putA gene: its role in the establishment of the symbiotic interaction with alfalfa. Mol Microbiol 23: 85–93. [DOI] [PubMed] [Google Scholar]
- 79. Schwan WR, Coulter SN, Ng EY, Langhorne MH, Ritchie HD, et al. (1998) Identification and characterization of the PutP proline permease that contributes to in vivo survival of Staphylococcus aureus in animal models. Infect Immun 66: 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Empadinhas N, da Costa MS (2008) Osmoadaptation mechanisms in prokaryotes: distribution of compatible solutes. Int Microbiol 11: 151–161. [PubMed] [Google Scholar]
- 81. Wood JM (1988) Proline porters effect the utilization of proline as nutrient or osmoprotectant for bacteria. J Membr Biol 106: 183–202. [DOI] [PubMed] [Google Scholar]
- 82. Booth IR, Higgins CF (1990) Enteric bacteria and osmotic stress: intracellular potassium glutamate as a secondary signal of osmotic stress? FEMS Microbiol Rev 6: 239–246. [DOI] [PubMed] [Google Scholar]
- 83. Coulter SN, Schwan WR, Ng EY, Langhorne MH, Ritchie HD, et al. (1998) Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol Microbiol 30: 393–404. [DOI] [PubMed] [Google Scholar]
- 84. Peng K, Monack DM (2010) Indoleamine 2,3-dioxygenase 1 is a lung-specific innate immune defense mechanism that inhibits growth of Francisella tularensis tryptophan auxotrophs. Infect Immun 78: 2723–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gripp E, Hlahla D, Didelot X, Kops F, Maurischat S, et al. (2011) Closely related Campylobacter jejuni strains from different sources reveal a generalist rather than a specialist lifestyle. BMC Genomics 12: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Miller WG, Bates AH, Horn ST, Brandl MT, Wachtel MR, et al. (2000) Detection on surfaces and in Caco-2 cells of Campylobacter jejuni cells transformed with new gfp, yfp, and cfp marker plasmids. Appl Environ Microbiol 66: 5426–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Colegio OR, Griffin TJ, Grindley ND, Galan JE (2001) In vitro transposition system for efficient generation of random mutants of Campylobacter jejuni. J Bacteriol 183: 2384–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Grabowski R, Buckel W (1991) Purification and properties of an iron-sulfur-containing and pyridoxal-phosphate-independent L-serine dehydratase from Peptostreptococcus asaccharolyticus. Eur J Biochem 199: 89–94. [DOI] [PubMed] [Google Scholar]
- 89. Abu Dawud R, Schreiber K, Schomburg D, Adjaye J (2012) Human embryonic stem cells and embryonal carcinoma cells have overlapping and distinct metabolic signatures. PLoS One 7: e39896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hiller K, Hangebrauk J, Jager C, Spura J, Schreiber K, et al. (2009) MetaboliteDetector: comprehensive analysis tool for targeted and nontargeted GC/MS based metabolome analysis. Anal Chem 81: 3429–3439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nucleotide sequence comparison of the L-serine dehydratase sdaA genes in indifferent C. jejuni isolates. The nucleotide sequences of sdaA open reading frames from different C. jejuni strains were compared using ClustalW (www.ebi.ac.uk/Tools/msa/clustalW2). The sources for the DNA sequences are as follows: CG8486 (Cj8486_1666c; NZ_AASY01000001.2), ATCC 33251 (this study), CF93-6 (CJJCF936_1718; NZ_AANJ01000002.1), 84-25 (CJJ8425_1708; NZ_AANT02000001.1), NCTC 11168 (Cj1624c; NC_002163.1), IA3902 (CJSA_1536; CP001876.1), DFVF1099 (CSQ_0902; ADHK01000020.1; this study), 305 (CSS_1725; ADHL01000259.1; this study), RM1221 (CJE1796; NC_003912.7), S3 (CJS3_1705; CP001960.1), 260.94 (CJJ26094_1675; NZ_AANK01000006.1), ICDCCJ07001 (ICDCCJ07001_1539; NC_014802.1), HB93-13 (CJJHB9313_1615; NZ_AANQ01000001.1), 81-176 (CJJ81176_1615; AASL01000001.1), 81116 (C8J_1526; NC_009839.1), M1 (CJM1_1565; CP001900.1), 327 (CSU_0676; ADHM01000033.1; this study), 1336 (C1336_000330073; NZ_ADGL01000024.1), CG8421 (Cj8421_1678; NZ_ABGQ01000002.1), 414 (C414_000010127; NZ_ADGM01000001.1).
(DOC)
Nucleotide sequence comparison of the serine transporter sdaC genes in different C. jejuni isolates. The published sequences of the sdaC genes from several C. jejuni isolates were compared using ClustalW (www.ebi.ac.uk/Tools/msa/clustalW2). The sources for the nucleotide sequences were obtained from GenBank (NCBI) and have following accession numbers: 260.94 (CJJ26094_1676; NZ_AANK01000006), ICDCCJ07001 (ICDCCJ07001_1540; NC_014802), 81116 (C8J_1527; NC_009839), M1 (CJM1_1566; CP001900), 327(CSU_0678; ADHM01000033.1), DFVF1099 (CSQ_0900; ADHK01000020), 305 (CSS_1724; ADHL01000259.1), IA3902 (CJSA_1537; CP001876), NCTC11168 (Cj1625c; NC_002163), CG8486 (Cj8486_1667c; NZ_AASY01000001), 84-25 (CJJ8425_1709; NZ_AANT02000001), CF93-6 (CJJCF936_1719; NZ_AANJ01000002), CG8421 (Cj8421_1679; NZ_ABGQ01000002), ATCC 33251 (this study), RM1221 (CJE1797; NC_003912), S3 (CJS3_1706; CP001960), HB93-13 (CJJHB9313_1616; NZ_AANQ01000001), 81-176 (CJJ81176_1616; NC_008787), 1336 (C1336_000330074; NZ_ADGL01000024), 414 (C414_000010126; NZ_ADGM01000001).
(DOC)
Amino acid sequence comparison of SdaA proteins from different C. jejuni strains. ClustaW (www.ebi.ac.uk/Tools/msa/clustalW2) was used for the alignment of the SdaA amino acid sequences from various C. jejuni isolates. The accession numbers were as follows: CF93-6 (CJJCF936_1718; ZP_01067690), 84-25 (CJJ8425_1708; ZP_01099834), NCTC 11168 (Cj1624c; YP_002344993), IA3902 (CJSA_1536; ADC29171), DFVF1099 (CSQ_0902, this study), 305 (CSS_1725; this study), CG8486 (Cj8486_1666c; ZP_01809456), RM1221 (CJE1796; YP_179767), S3 (CJS3_1705; ADT73404), 260.94 (CJJ26094_1675; NZ_AANK01000006.1), 81-176 (CJJ81176_1615; YP_001001267), 1336 (C1336_000330073; ZP_06374482), HB93-13 (CJJHB9313_1615; ZP_01070837), M1 (CJM1_1565; ADN91750), 81116 (C8J_1526; YP_001483100), ICDCCJ07001 (ICDCCJ07001_1539; YP_004067035), 327 (CSU_0676; this study), CG8421 (Cj8421_1678; ZP_03222408), ATCC 33251 (this study), 414 (C414_000010127; ZP_06371291).
(DOC)
Comparison of SdaC serine transporter protein sequences in various C. jejuni isolates. ClustaW (www.ebi.ac.uk/Tools/msa/clustalW2) was used for the alignment of the SdaC amino acid sequences from listed C. jejuni isolates. The protein sequence accession numbers for the SdaC serine transporters of the different C. jejuni strains were as follows: RM1221 (CJE1797; YP_179768), S3 (CJS3_1706, ADT73405), CF93-6 (CJJCF936_1719; ZP_01067605), 84-25 (CJJ8425_1709; ZP_01099564); CG8486 (Cj8486_1667c; ZP_01809457), NCTC 11168 (Cj1625c; YP_002344994), IA3902 (CJSA_1537; ADC29172), DFVF1099 (CSQ_0900; EFV06958), 305 (CSS_1724; EFV08300); CG8421 (Cj8421_1679; ZP_03222409); ATCC 33251 (this study); 260.94 (CJJ26094_1676; ZP_01070445), HB93-13 (CJJHB9313_1616; ZP_01071370); 81-176 (CJJ81176_1616; ZP_02271921), 81116 (C8J_1527; YP_001483101), ICDCCJ07001 (ICDCCJ07001_1540; YP_004067036), M1 (CJM1_1566; ADN91751); 327 (CSU_0678; EFV10631), 1336 (C1336_000330074; ZP_06374483), 414 (C414_000010126; ZP_06371290).
(DOC)
Growth of C. jejuni 81-176 and its isogenic sdaA and sdaC mutants. Growth characteristics of the C. jejuni 81-176 wild-type strain, its isogenic sdaC and sdaA mutants as well as a complemented sdaA mutant in DMEM and DMEM supplemented with 20 mM serine, glutamate or proline. The maximal optical densities (OD600) of liquid cultures from indicated C. jejuni strains over a time period of 24 hours are presented.
(TIF)
Competitive index of mice co-infection with C. jejuni 81-176 and its sdaA , peb1A or putP mutants. The competitive index, CI = (mutantoutput/wild-typeoutput)/(mutantinoculum/wild-typeinoculum), was calculated for each mouse infected with C. jejuni 81-176 wild-type strain and its indicated mutant. The output numbers representing the CFUs of wild-type and mutant strains recovered from the intestine or the liver of each animal are plotted in Figures 3, 4 and 6. Each mouse was infected with approximately the same number of wild-type and mutant strain as determined by the CFU counting of the inoculum.
(TIF)
In vitro growth competition experiments of C. jejuni 81-176 and its mutants. Shown are the co-cultivation experiments of C. jejuni 81-176 with its peb1A, putP and sdaA mutants, respectively. Equal amounts of wild-type and a mutant strain were incubated in nutrient rich BHI medium over night and the CFUs of each strain were determined after 20 hours. Each symbol represents the calculated competitive index for one co-cultivation experiment.
(TIF)
Comparison of the putA / putP gene cluster in Campylobacter and Enterobacteria . The schematic comparison of the putAputP gene locus and its flanking regions of several Campylobacter and Enterobacter strains was derived from the comparative genome database xBASE2 (Chaudhuri RR et al. (2008) Nucleic Acids Res., D543-6) in particular CampyDB (www.xbase.ac.uk/campydb) and ColiDB (www.xbase.ac.uk/colibase). The putA and putP genes are marked in light and dark grey, respectively. The genes of the flaking regions are represented as white arrows.
(TIF)
Proteobacteria with homologues to the L-serine dehydratase SdaA of C. jejuni 81-176. The table shows the homology between the SdaA protein of C. jejuni 81-176 and the SdaA proteins in other proteobacteria with their given accession numbers. The percent of amino acids identical and similar (conserved amino acid exchanges) between the serine ammonia-lyase of C. jejuni 81-176 and the other SdaA proteins were determined by BLASTP analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The order of the table reflects the score values calculated by the BLASTP algorithm. C. jejuni isolates are marked in red, other Campylobacter species in orange and Helicobacter species in yellow. Only a subset of C. jejuni isolates are listed, but all sequenced C. jejuni strains encode for SdaA homologues that are 100% or 99% identical to the SdaA protein of C. jejuni 81-176.
(DOC)
Proteobacteria with homologues to the serine transporter SdaC of C. jejuni 81-176. The table shows the homology between the SdaC protein of C. jejuni 81-176 and the SdaC proteins in other proteobacteria. The order represents the grade of homology according to the score calculated by the BlastP algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi). C. jejuni isolates are marked in red other Campylobacter species in orange and Helicobacter species in yellow. Sequenced C. jejuni strains that are not represented in the table encode for SdaC homologues that are at least 99% identical to the SdaC of C. jejuni 81-176.
(DOC)
Proteobacteria with homologues to the proline dehydrogenase/delta 1-pyrroline-5-carboxylate dehydrogenase PutA of C. jejuni 81-176. The table shows the homology between the PutA protein of C. jejuni 81-176 and the PutA proteins in other proteobacteria with their given accession numbers. The percent of amino acids identical and similar (conserved amino acid exchanges) between C. jejuni 81-176 PutA and the PutA proteins of other presented proteobacteria were determined by BLASTP analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The order of the table reflects the score values calculated by the BLASTP algorithm. C. jejuni isolates are marked in red, other Campylobacter species in orange and Helicobacter species in yellow. Only a subset of C. jejuni isolates are listed, but all sequenced C. jejuni strains encode for SdaA homologues that are at least 99% identical to the SdaA protein of C. jejuni 81-176.
(DOC)
Proteobacteria with homologues to the proline transporter PutP of C. jejuni 81-176. The table illustrates the homology between the PutP protein of C. jejuni 81-176 and the PutP proteins in other proteobacteria. The accession numbers of the PutP proteins from indicated bacteria are listed. The order represents the grade of homology according to the score calculated by the BlastP algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi). C. jejuni isolates are marked in red, Campyobacter species besides C. jejuni are highlighted in orange and Helicobacter species in yellow. Not all C. jejuni isolates are listed, but every sequenced C. jejuni strain encodes for a PutP homologue that is at least 98% identical to the PutP protein of C. jejuni 81-176.
(DOC)
L-serine dehydratase activity in cell extracts of C. jejuni isolates. The mean serine dehydratase activities with standard deviations are shown for measurements repeated four (a) and three (b) times in triplicates. The statistical significance in the differences of SdaA activity between C. jejuni 81-176 and C. jejuni 33251 or RM1221 was calculated by Student t test: * P<0.01; ** P<0.05.
(DOC)
Primers used in this study.
(DOC)
Strains and plasmids used in this study.
(DOC)