Abstract
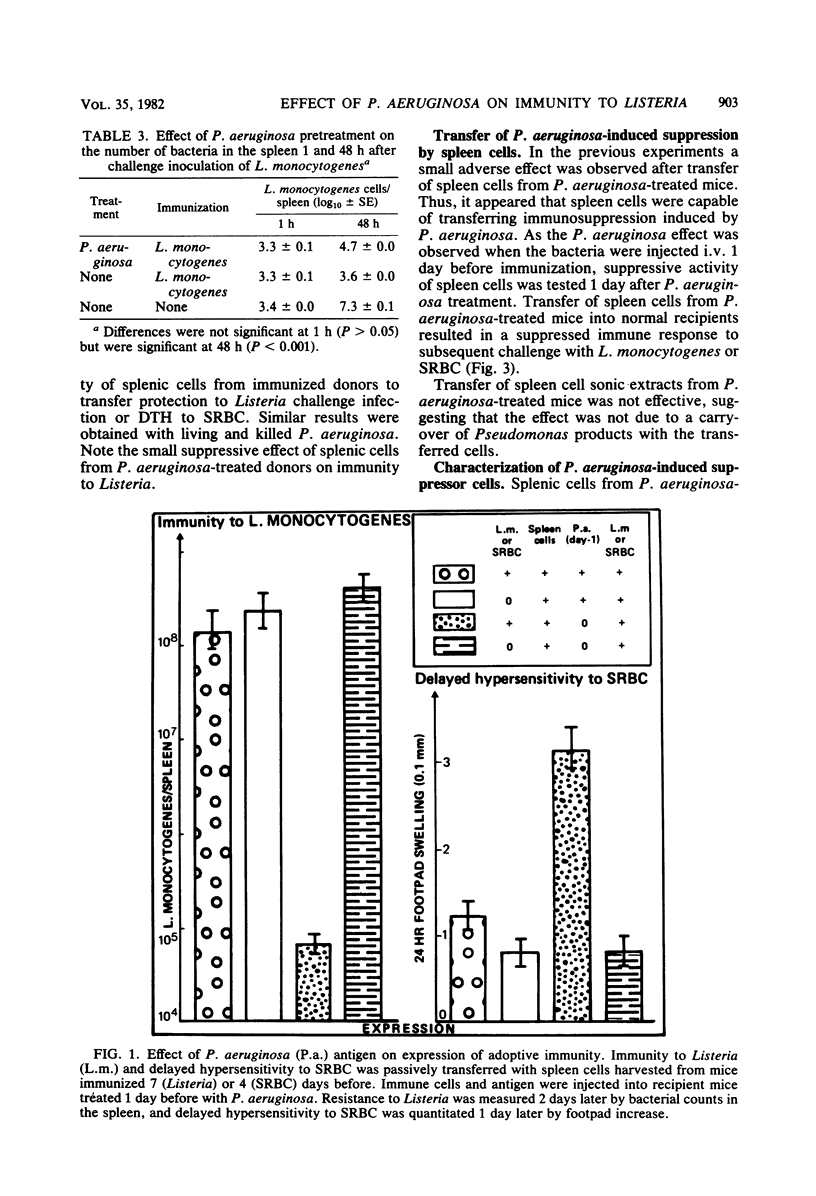
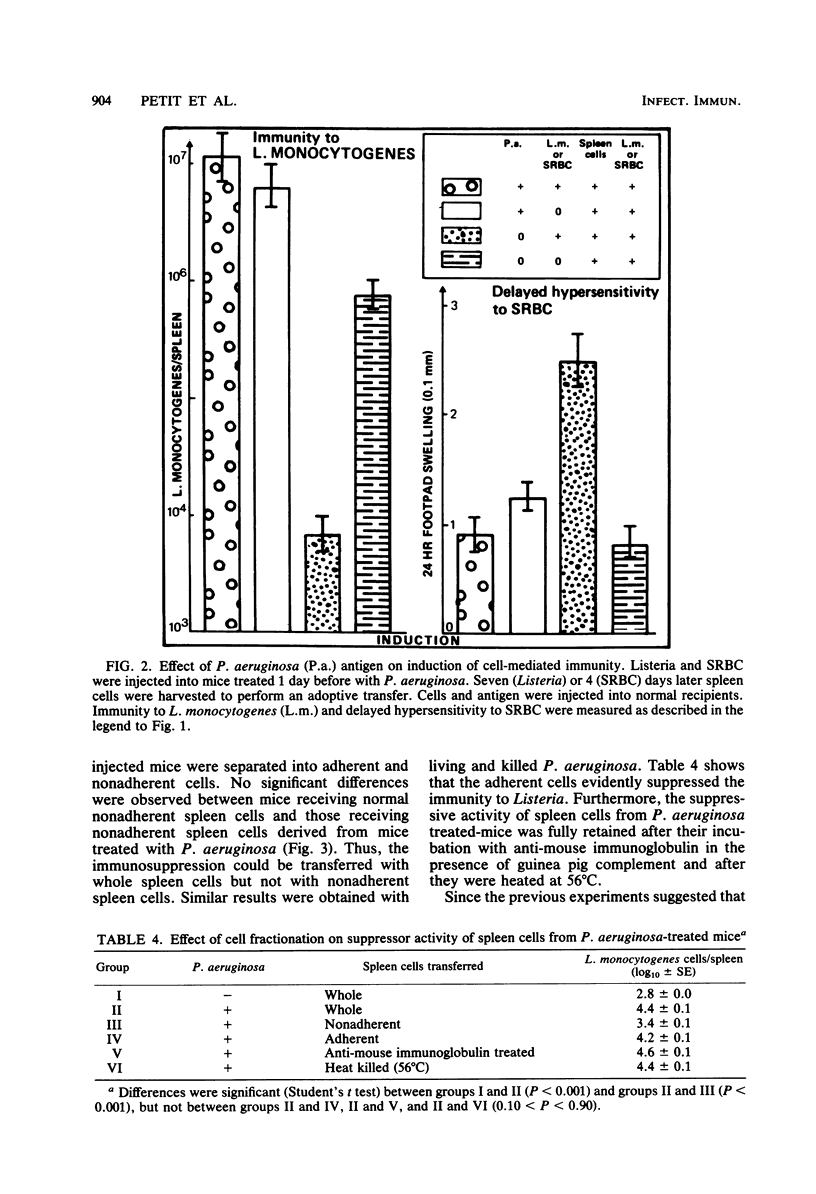
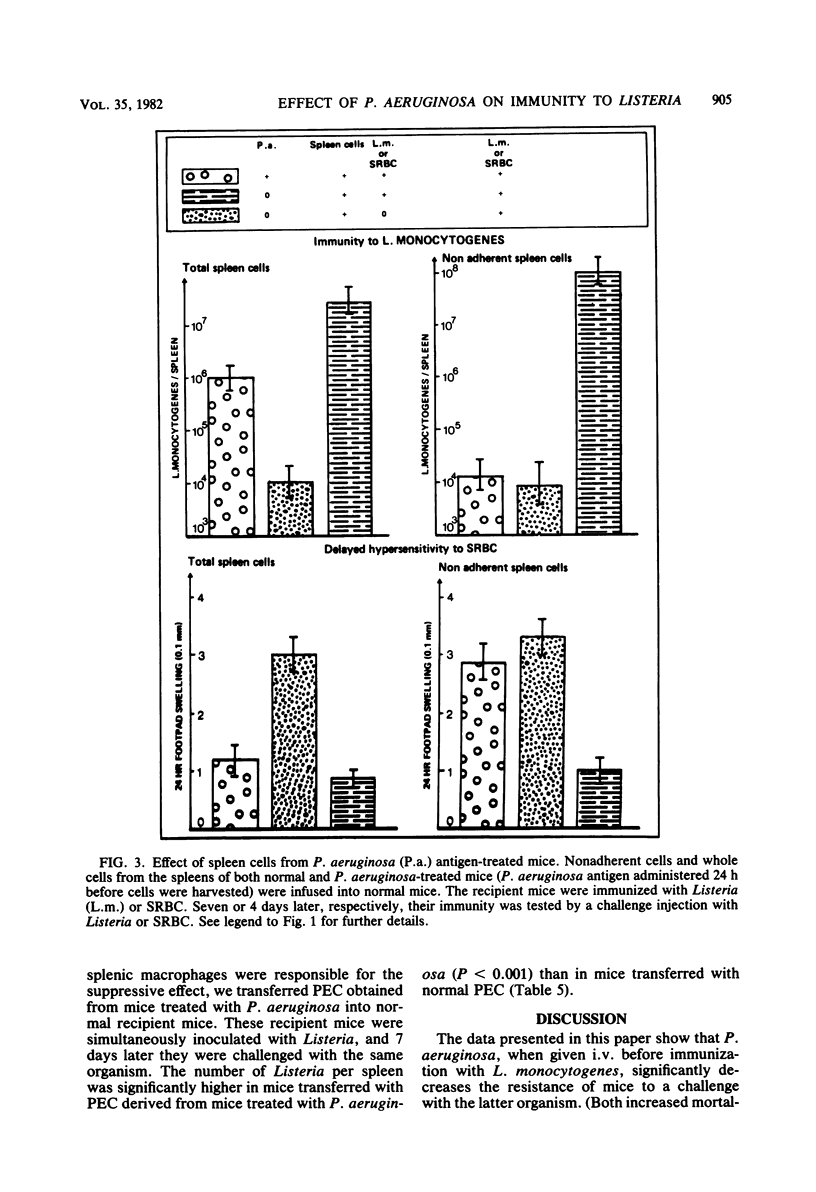
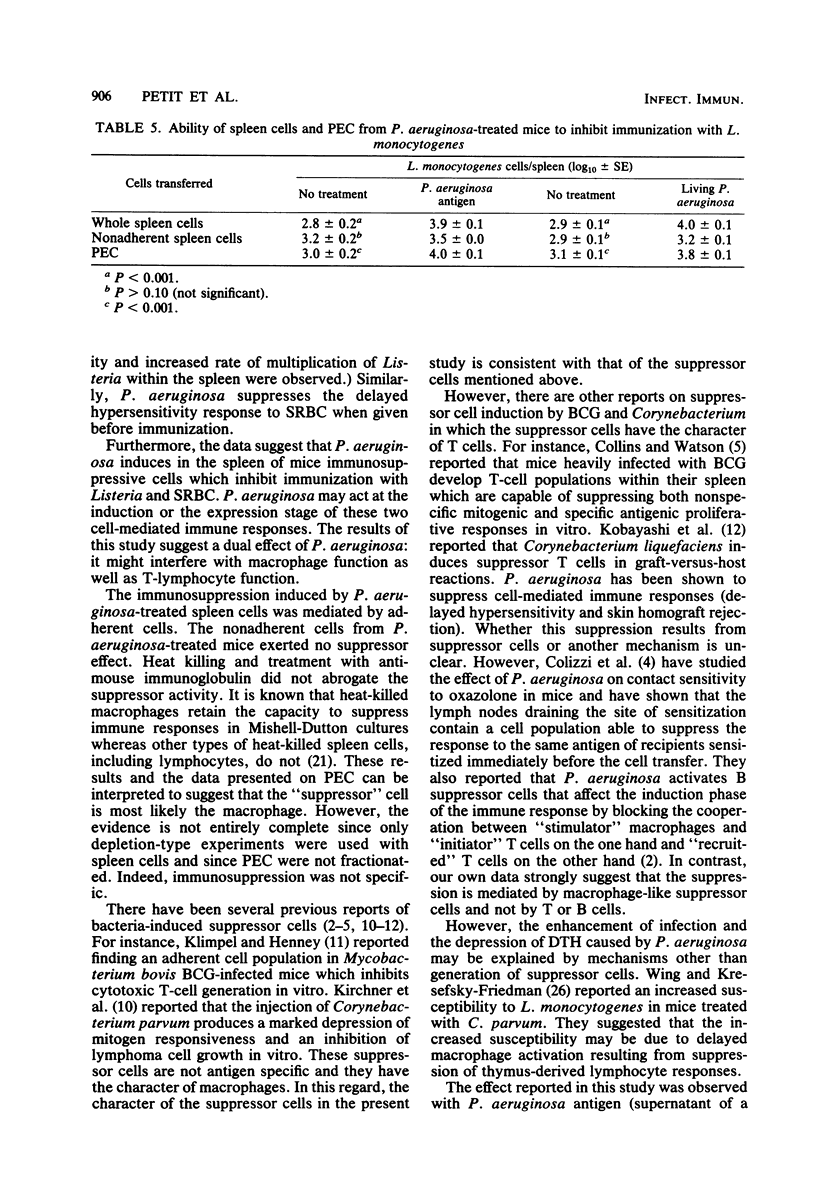
Pseudomonas aeruginosa was studied for its effects on T-cell-mediated responses in mice, as exemplified by anti-Listeria immunity and delayed-type hypersensitivity to sheep erythrocytes. Immunity to Listeria monocytogenes was measured by quantitation of bacteria in spleens and mortality; delayed hypersensitivity to sheep erythrocytes was tested by the footpad reaction. Three different preparations of P. aeruginosa were used: the supernatant of a heat-killed culture, living bacteria, and heat-killed organisms. Similar results were obtained with the three preparations. Administration of P. aeruginosa 24 h before Listeria infection reduced the resistance to the secondary challenge, as measured by increased bacterial multiplication in the spleen and rate of mortality. Cell transfer experiments showed that pretreatment of normal recipient mice with P. aeruginosa prevented them from being adoptively immunized against a Listeria challenge infection with spleen cells from immune donors. They also showed that treatment of donors with P. aeruginosa before immunization affected the capacity of their spleen cells to protect normal recipients against Listeria. Furthermore, spleen and peritoneal exudate cells obtained from mice given P. aeruginosa were capable of preventing immunization of normal recipients against Listeria. Similar results were obtained when the delayed hypersensitivity response to sheep erythrocytes was studied. The suppressive activity of P. aeruginosa-treated spleen cells was lost by removing adherent cells. Conversely, the adherent, heat-killed, anti-immunoglobulin-treated spleen cells exerted a suppressor effect. It thus appears that P. aeruginosa injection changes macrophage and T-lymphocyte activities and results in the development of adherent, macrophage-like suppressor cells in the spleen and peritoneal cavity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIOZZI G., BENACERRAF B., HALPERN B. N. The effect of Salm. typhi and its endotoxin on the phagocytic activity of the reticuloendothelial system in mice. Br J Exp Pathol. 1955 Jun;36(3):226–235. [PMC free article] [PubMed] [Google Scholar]
- Colizzi V., Campa M., Garzelli C., Falcone G. Pseudomonas aeruginosa infection depresses contact sensitivity to oxazolone by enhancing suppressor cell activity. Med Microbiol Immunol. 1979 Aug;167(3):181–188. doi: 10.1007/BF02121184. [DOI] [PubMed] [Google Scholar]
- Colizzi V., Garzelli C., Campa M., Falcone G. Depression of contact sensitivity by enhancement of suppressor cell activity in Pseudomonas aeruginosa-injected mice. Infect Immun. 1978 Aug;21(2):354–359. doi: 10.1128/iai.21.2.354-359.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floersheim G. L., Hopff W. H., Gasser M., Bucher K. Impairment of cell-mediated immune responses by Pseudomonas aeruginosa. Clin Exp Immunol. 1971 Aug;9(2):241–247. [PMC free article] [PubMed] [Google Scholar]
- Freeman R. R. T cell function during fatal and self-limiting malarial infections of mice. Cell Immunol. 1978 Dec;41(2):373–379. doi: 10.1016/0008-8749(78)90234-4. [DOI] [PubMed] [Google Scholar]
- Galleli A., Le Garrec Y., Chedid L. Increased resistance and depressed delayed-type hypersensitivity to Listeria monocytogenes induced by pretreatment with lipopolysaccharide. Infect Immun. 1981 Jan;31(1):88–94. doi: 10.1128/iai.31.1.88-94.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill H. K., Liew F. Y. Regulation of delayed-type hypersensitivity IV. Antigen-specific suppressor cells for delayed-type hypersensitivity induced by lipopolysaccharide and sheep erythrocytes in mice. Eur J Immunol. 1979 Feb;9(2):101–106. doi: 10.1002/eji.1830090202. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Holden H. T., Herberman Splenic suppressor macrophages induced in mice by injection of Corynebacterium parvum. J Immunol. 1975 Nov;115(5):1212–1216. [PubMed] [Google Scholar]
- Klimpel G. R., Henney C. S. BCG-induced suppressor cells. I. Demonstration of a macrophage-like suppressor cell that inhibits cytotoxic T cell generation in vitro. J Immunol. 1978 Feb;120(2):563–569. [PubMed] [Google Scholar]
- Kobayashi M., Kawashima K., Yamada K. Corynebacterium liquefaciens-induced suppressor T cells in graft-versus-host reaction. Transplantation. 1980;29(1):43–46. doi: 10.1097/00007890-198001000-00009. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E., Pardon P. Effects of bacterial lipopolysaccharide on the induction and expression of cell-mediated immunity. I. Depression of the afferent arc. J Immunol. 1975 Jan;114(1 Pt 2):442–446. [PubMed] [Google Scholar]
- Marchal G., Milon G., Hurtrel B., Lagrance P. H. Titration and circulation of cells mediating delayed type hypersensitivity in normal and cyclophosphamide treated mice during response to sheep red blood cells. Immunology. 1978 Dec;35(6):981–987. [PMC free article] [PubMed] [Google Scholar]
- Miller T. E., Mackaness G. B., Lagrange P. H. Immunopotentiation with BCG. II. Modulation of the response to sheep red blood cells. J Natl Cancer Inst. 1973 Nov;51(5):1669–1676. doi: 10.1093/jnci/51.5.1669. [DOI] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. Suppressive effect of bacterial endotoxin on the expression of cell-mediated anti-Listeria immunity. Infect Immun. 1979 Jun;24(3):667–672. doi: 10.1128/iai.24.3.667-672.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. C. Resistance to listeriosis in mice that are deficient in the fifth component of complement. Infect Immun. 1980 Jan;27(1):61–67. doi: 10.1128/iai.27.1.61-67.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. C., Sicard D., Bayo S., Daguet G. L. Effect of active and passive immunization on the development of experimental Pseudomonas aeruginosa pyelonephritis in mice. Can J Microbiol. 1981 Jan;27(1):93–97. doi: 10.1139/m81-015. [DOI] [PubMed] [Google Scholar]
- Petit J. C., Unanue E. R. Effects of bacterial products on lymphocytes and macrophages: their possible role in natural resistance to listeria infetion in mice. J Immunol. 1974 Sep;113(3):984–992. [PubMed] [Google Scholar]
- Ptak W., Gershon R. K. Immunosuppression effected by macrophage surfaces. J Immunol. 1975 Nov;115(5):1346–1350. [PubMed] [Google Scholar]
- Schrier D. J., Allen E. M., Moore V. L. BCG-induced macrophage suppression in mice: suppression of specific and nonspecific antibody-medicated and cellular immunologic responses. Cell Immunol. 1980 Dec;56(2):347–356. doi: 10.1016/0008-8749(80)90110-0. [DOI] [PubMed] [Google Scholar]
- Schwab J. H. Suppression of the immune response by microorganisms. Bacteriol Rev. 1975 Jun;39(2):121–143. doi: 10.1128/br.39.2.121-143.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone H. H., Given K. S., Martin J. D., Jr Delayed rejection of skin homografts in Pseudomonas sepsis. Surg Gynecol Obstet. 1967 May;124(5):1067–1070. [PubMed] [Google Scholar]
- Wing E. J., Kresefsky-Friedman D. Y. Decreased resistance to Listeria monocytogenes in mice injected with killed corynebacterium parvum: association with suppression of cell-mediated immunity. J Infect Dis. 1980 Feb;141(2):203–211. doi: 10.1093/infdis/141.2.203. [DOI] [PubMed] [Google Scholar]



