Abstract
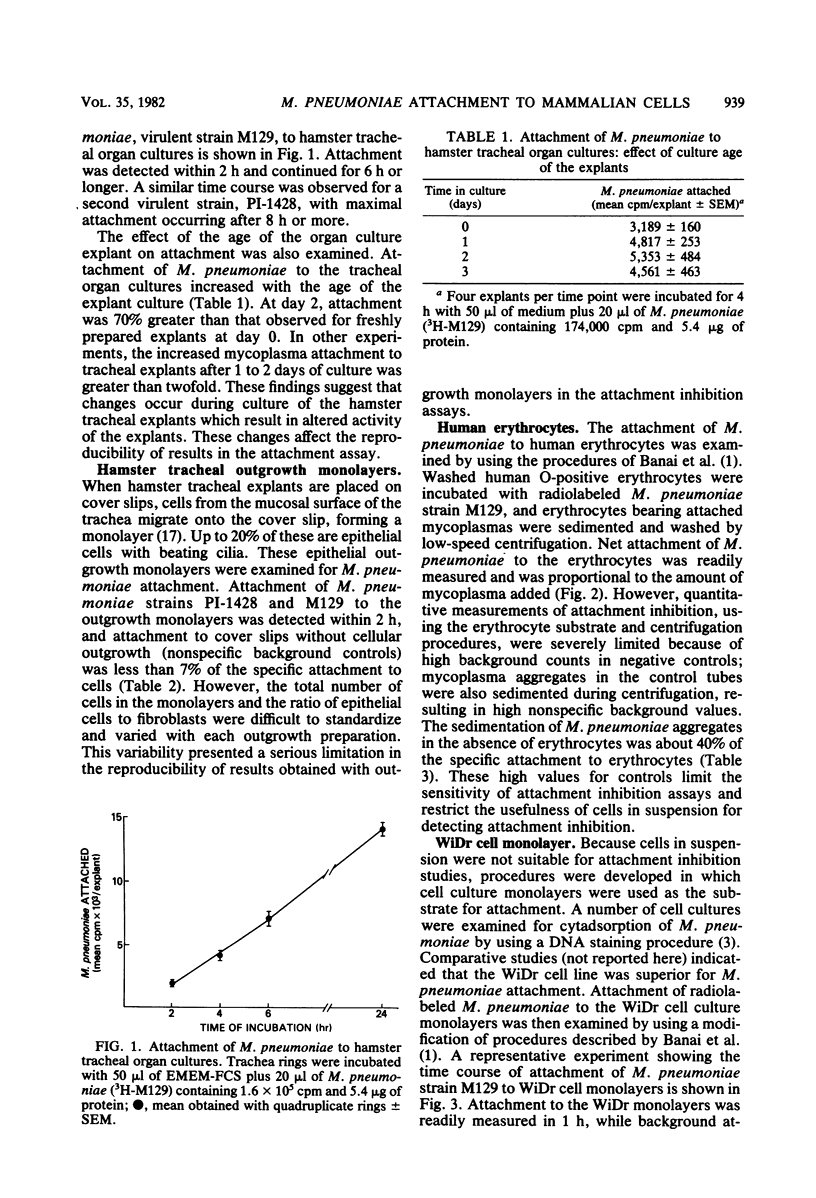
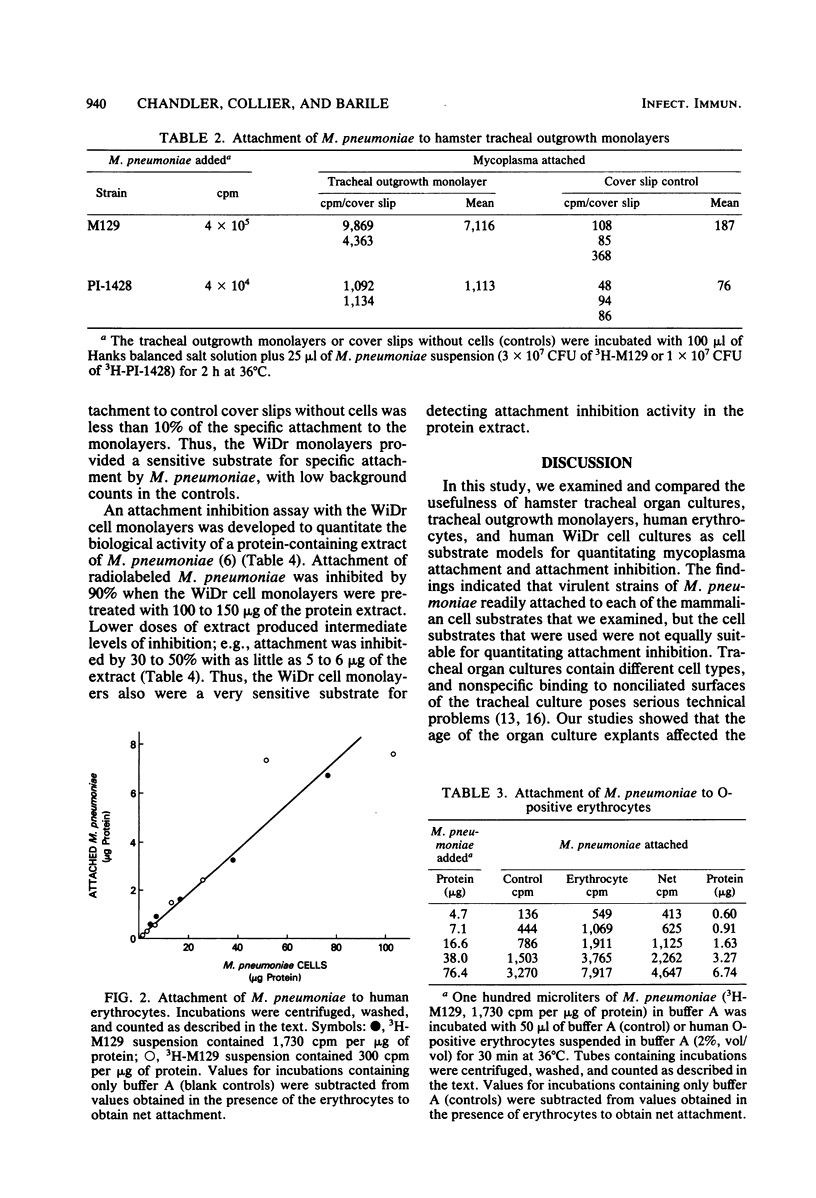
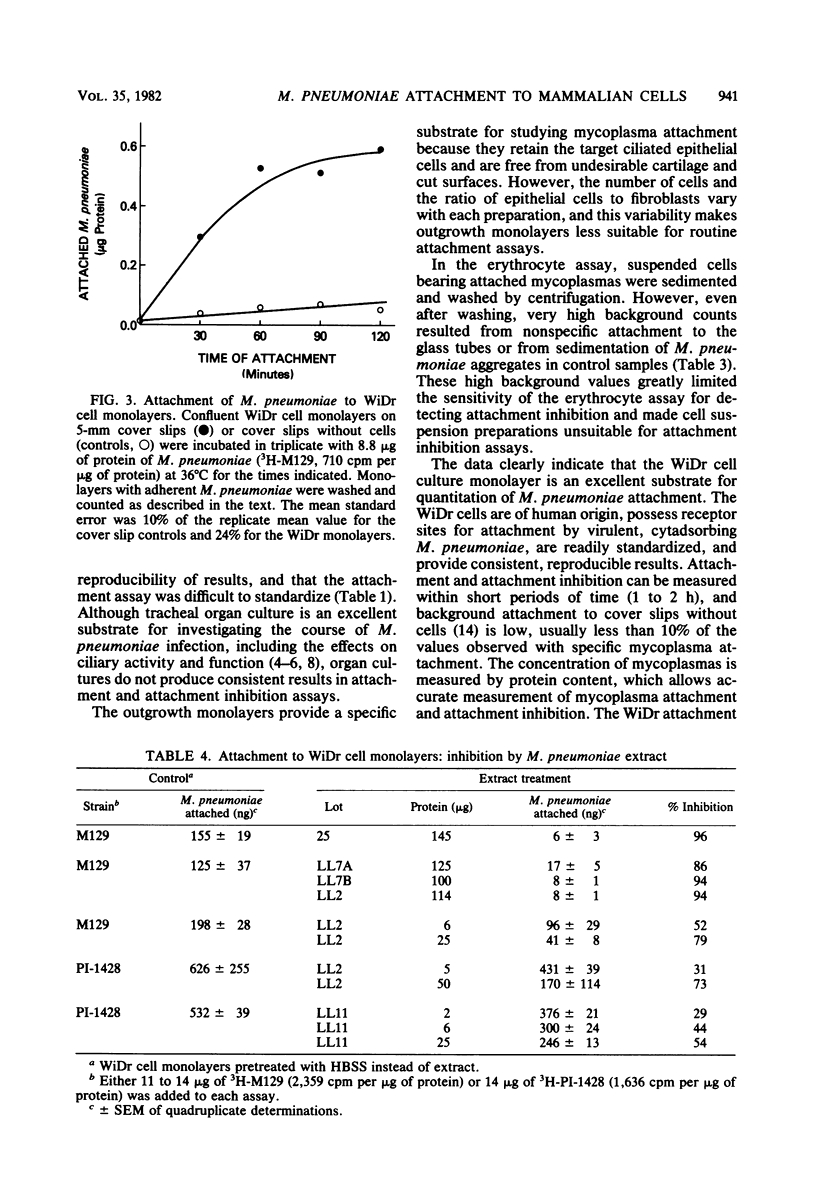
Virulent strains of Mycoplasma pneumoniae, PI-1428 and M129, were radiolabeled wtih [3H]palmitic acid or [3H]thymidine and examined for attachment to hamster tracheal organ cultures, tracheal outgrowth monolayers, human O-positive erythrocytes, and human WiDr carcinoma cell cultures. Although attachment to each cell substrate was readily detected, the WiDr cell culture monolayers provided the most satisfactory substrate for quantitating mycoplasma attachment. Serious technical limitations were encountered with each of the other substrates that we examined; these limitations interfered with reproducibility or sensitivity and rendered tracheal organ cultures and erythrocyte suspensions unsuitable for routine attachment and attachment inhibition assays. Moreover, the WiDr cell monolayer was the most sensitive substrate for determining attachment inhibition activity in protein-containing extracts prepared from M. pneumoniae. The significance of these findings is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banai M., Kahane I., Razin S., Bredt W. Adherence of Mycoplasma gallisepticum to human erythrocytes. Infect Immun. 1978 Aug;21(2):365–372. doi: 10.1128/iai.21.2.365-372.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile M. F., Chandler D. K., Yoshida H., Grabowski M. W., Harasawa R., Ahmed O. A. Hamster challenge potency assay for evaluation of Mycoplasma pneumoniae vaccines. Isr J Med Sci. 1981 Jul;17(7):682–686. [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson J. L., Collier A. M., Clyde W. A., Jr Ciliary membrane alterations occurring in experimental Mycoplasma pneumoniae infection. Science. 1979 Oct 19;206(4416):349–351. doi: 10.1126/science.113877. [DOI] [PubMed] [Google Scholar]
- Carson J. L., Collier A. M., Hu S. C. Ultrastructural observations on cellular and subcellular aspects of experimental Mycoplasma pneumoniae disease. Infect Immun. 1980 Sep;29(3):1117–1124. doi: 10.1128/iai.29.3.1117-1124.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Barile M. F. Ciliostatic, hemagglutinating, and proteolytic activities in a cell extract of Mycoplasma pneumoniae. Infect Immun. 1980 Sep;29(3):1111–1116. doi: 10.1128/iai.29.3.1111-1116.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr Appearance of Mycoplasma pneumoniae in lungs of experimentally infected hamsters and sputum from patients with natural disease. Am Rev Respir Dis. 1974 Dec;110(6):765–773. doi: 10.1164/arrd.1974.110.6P1.765. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr, Denny F. W. Biologic effects of Mycoplasma pneumoniae and other mycoplasmas from man on hamster tracheal organ culture. Proc Soc Exp Biol Med. 1969 Dec;132(3):1153–1158. doi: 10.3181/00379727-132-34385. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr, Denny F. W. Mycoplasma pneumoniae in hamster tracheal organ culture: immunofluorescent and electron microscopic studies. Proc Soc Exp Biol Med. 1971 Feb;136(2):569–573. doi: 10.3181/00379727-136-35313. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. A., Gabridge M. G. Effect of squamous metaplasia on infection of hamster trachea organ cultures with Mycoplasma pneumoniae. Infect Immun. 1977 Feb;15(2):647–655. doi: 10.1128/iai.15.2.647-655.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner J., Bredt W., Razin S. Adherence of Mycoplasma pneumoniae to glass surfaces. Infect Immun. 1979 Oct;26(1):70–75. doi: 10.1128/iai.26.1.70-75.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy H. M., Kenny G. E., Cooney M. K., Allan I. D. Long-term epidemiology of infections with Mycoplasma pneumoniae. J Infect Dis. 1979 Jun;139(6):681–687. doi: 10.1093/infdis/139.6.681. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Agee C. C., Cameron A. M. Differential distribution of ciliated epithelial cells in the trachea of hamsters: implications for studies of pathogenesis. J Infect Dis. 1977 Jan;135(1):9–19. doi: 10.1093/infdis/135.1.9. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Gunderson H., Schaeffer S. L., Barden-Stahl Y. D. Ciliated respiratory epithelial monolayers: new model for Mycoplasma pneumoniae infection. Infect Immun. 1978 Jul;21(1):333–336. doi: 10.1128/iai.21.1.333-336.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Taylor-Robinson D., Davies H. A., Dourmashkin R. R. Interaction of Mycoplasma pneumoniae with human lung fibroblasts: characterization of the in vitro model. Infect Immun. 1979 Jul;25(1):446–454. doi: 10.1128/iai.25.1.446-454.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Taylor-Robinson D. Interaction of Mycoplasma pneumoniae with human lung fibroblasts: role of receptor sites. Infect Immun. 1979 Jul;25(1):455–459. doi: 10.1128/iai.25.1.455-459.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Noguchi P., Wallace R., Johnson J., Earley E. M., O'Brien S., Ferrone S., Pellegrino M. A., Milstien J., Needy C., Browne W. Characterization of the WIDR: a human colon carcinoma cell line. In Vitro. 1979 Jun;15(6):401–408. doi: 10.1007/BF02618407. [DOI] [PubMed] [Google Scholar]
- Powell D. A., Hu P. C., Wilson M., Collier A. M., Baseman J. B. Attachment of Mycoplasma pneumoniae to respiratory epithelium. Infect Immun. 1976 Mar;13(3):959–966. doi: 10.1128/iai.13.3.959-966.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


