Abstract
Measurements of the quantum efficiencies of photosynthetic electron transport through photosystem II (φPSII) and CO2 assimilation (φCO2) were made simultaneously on leaves of maize (Zea mays) crops in the United Kingdom during the early growing season, when chilling conditions were experienced. The activities of a range of enzymes involved with scavenging active O2 species and the levels of key antioxidants were also measured. When leaves were exposed to low temperatures during development, the ratio of φPSII/φCO2 was elevated, indicating the operation of an alternative sink to CO2 for photosynthetic reducing equivalents. The activities of ascorbate peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase, glutathione reductase, and superoxide dismutase and the levels of ascorbate and α-tocopherol were also elevated during chilling periods. This supports the hypothesis that the relative flux of photosynthetic reducing equivalents to O2 via the Mehler reaction is higher when leaves develop under chilling conditions. Lipoxygenase activity and lipid peroxidation were also increased during low temperatures, suggesting that lipoxygenase-mediated peroxidation of membrane lipids contributes to the oxidative damage occurring in chill-stressed leaves.
Exposure of maize (Zea mays) crops to low temperatures during the early growing season in temperate regions results in depressions in photosynthetic productivity and canopy development (Miedema, 1982; Stirling et al., 1991; Baker and Nie, 1994). Chill-induced decreases in CO2 assimilation in maize leaves are associated with inhibition of photosynthesis involving both increased dissipation of excitation energy in the PSII antennae and photodamage to PSII reaction centers (Ortiz-Lopez et al., 1990; Andrews et al., 1995; Fryer et al., 1995; Haldimann et al., 1996), decreases in the activities of Benson-Calvin cycle enzymes (Kingston-Smith et al., 1997), and poor development of the photosynthetic apparatus (Nie and Baker, 1991; Nie et al., 1992, 1995).
Under such environmental stress conditions, which reduce the capacity to assimilate C, it has been suggested that photosynthetic electron flux to O2 will increase, resulting in the increased production of superoxide, H2O2, and hydroxyl radicals (Asada, 1996). These active O2 species are extremely damaging to lipids, proteins, and pigments unless they are rapidly scavenged within the chloroplasts by a group of enzymes consisting of SOD, GTR, DHAR, MDHAR, and APX (Asada, 1996). There is some evidence, although not extensive, that increased levels of these scavenging enzymes may play a role in limiting the degree of photodamage experienced by maize at chilling temperatures (Jahnke et al., 1991; Massacci et al., 1995; Hodges et al., 1997).
In maize leaves at normal growth temperatures, the relationship between photosynthetic electron transport and CO2 assimilation is highly conserved over a wide range of light intensities and CO2 concentrations and also during the induction of photosynthesis in dark-adapted leaves (Genty et al., 1989). Examination of the quantitative relationship between electron transport and CO2 assimilation in maize leaves in air indicated that the majority of the reductants generated by electron transport are consumed by CO2 assimilation and that other sinks, such as N metabolism, O2 reduction via photorespiration, and the Mehler reaction, are minimal (Edwards and Baker, 1993). However, when maize leaves are grown at low temperatures the ratio of electron transport to CO2 assimilation increases (Fryer et al., 1995; Massacci et al., 1995). This indicates that there is an increased allocation of reductants to sinks other than CO2 assimilation.
A similar increase in the ratio of electron transport to CO2 assimilation was also observed when mangrove (Cheeseman, 1994) and sweet sorghum (Massacci et al., 1996) leaves were drought stressed. Therefore, it can be speculated that additional electron sinks to CO2 assimilation, possibly O2, develop during the imposition of environmental stresses that impose restrictions on photosynthetic C metabolism. Such a metabolic change could be a mechanism for preventing photodamage to the photosynthetic apparatus that operates in conjunction with increased dissipation of excitation in the PSII antennae by nonradiative decay processes.
A primary aim of this study was to determine whether the well-established chill-induced suppression of CO2 assimilation during chilling was associated with changes in the relationship between electron transport and CO2 assimilation in maize leaves during the early growing season. If the relationship between electron transport and CO2 assimilation changed, a secondary aim was to evaluate whether these changes were associated with changes in the activity of active O2-scavenging systems. Simultaneous measurements of the quantum efficiencies of linear electron transport through PSII and of CO2 assimilation were made on leaves of maize crops harvested from a field site in southeast UK during May and June in 1994 and 1995, and the activities of a range of enzymes involved with scavenging of active O2 species and the levels of some key antioxidants in the leaves were determined. Also, the extent of lipid peroxidation occurring in the leaves was monitored, because this is another important source of active O2 species that may be associated with the environmental stress responses of leaves.
MATERIALS AND METHODS
Plant Material and Experimental Site
The experimental plot consisted of a 0.08-ha area of sandy, loamy soil in northeast Essex, UK. Before the seeds were sown, the soil was treated with N:P:K (2:1:1) fertilizer at a rate of 100 Kg ha−1. Maize (Zea mays) cvs LG11 and LG20.80 were sown on May 2, 1994, and May 1, 1995, respectively, in randomized rows 0.33 m apart, at a depth of 6 cm, and at 0.2-m intervals to give a population density of approximately 14 plants m−2, similar to that recommended for growers (Tiley and Warbots, 1975). All measurements were made on the youngest fully expanded leaves of the crop.
Climatologic Measurements
Environmental conditions were monitored by a weather station (Delta-T Devices, Newmarket, UK) that was situated within 10 m of the experimental plot. Measurements of PPFD and air temperature were taken at 1-min intervals, and 30-min means were logged.
Measurements of Chlorophyll Fluorescence and CO2 Assimilation
Leaves were harvested from the field by cutting the base of the leaf under water and were then transferred to an adjacent laboratory and placed in a temperature-controlled leaf chamber, which was described by Stirling et al. (1991). Measurements of chlorophyll fluorescence and CO2 assimilation were made simultaneously at 25°C over a PPFD range of 150 to 1500 μmol m−2 s−1. At any given PPFD the fluorescence yield at steady-state photosynthesis, Fs, and the maximum yield produced by a 0.5-s saturating flash (PPFD of 10,000 μmol m−2 s−1), Fm′, were determined using a fluorimeter (PAM-2000, Heinz Walz, Effeltrich, Germany). The φPSII was determined from the equation: φPSII = (Fm′ − Fs)/Fm′, as originally described by Genty et al. (1989). Rates of CO2 uptake by leaves in the light and respiration rates in the dark were measured at 25°C using an IR gas analyzer (model 225-Mk3, Analytical Development, Hoddeson, UK) as described by Stirling et al. (1991).
The φCO2 was determined by dividing the rate of CO2 assimilation (corrected for respiratory losses) by the rate at which quanta were absorbed, which was determined using a Taylor integrating sphere. For analyses of the relationship between φPSII and φCO2, only data from leaves with rates of CO2 assimilation greater than 2 μmol m−2 s−1 were taken. The dark respiration rates in leaves with lower CO2 assimilation rates were often similar to the photosynthetic rates and could potentially give rise to large errors in the estimation of φCO2 due to the dark rate of respiration not reflecting the respiration rate in the light. When photosynthetic rates are considerably greater than respiration rates, such errors are considerably reduced.
APX, GTR, MDHAR, and SOD Assays
Leaf tissue (approximately 85 mg; 2 × 10−3 m2) was harvested from the field, and leaf area was immediately determined with a video-based leaf area meter (model AM, Delta-T Devices, Cambridge, UK). Leaf tissue samples were then frozen in liquid N2 and stored at −80°C. Cell-free homogenates for antioxidant enzyme assays were prepared essentially by the methods described by Jahnke et al. (1991). Leaf tissue samples were ground to a powder with liquid N2 and homogenized in 3 mL of ice-cold extraction buffer (0.1 m K2PO4, pH 7.0, 0.01 m sodium ascorbate, and 5 mm DTPA) and 30 mg of insoluble polyvinylpolypyrrolidone. The homogenate was centrifuged at 3°C for 20 min at 16,000g. The cell-free supernatant containing the antioxidant enzymes was then desalted by passing through a disposable G-25 Sephadex PD-10 column of 9 mL total volume. The column was pre-equilibrated by running 25 mL of ice-cold equilibration/elution buffer (0.1 m K2PO4, pH 7.0, containing 200 μm DTPA) through it prior to sample application. The cell-free extract was eluted with 3.5 mL of column equilibration/elution buffer, the first 0.5 mL was discarded, and the following 2.5 mL (the green chlorophyll-containing fraction) was collected. Antioxidant enzymes were assayed in order of lability (Hull, 1990; Jahnke et al., 1991). APX was assayed immediately after desalting, followed by MDHAR, GTR, and SOD.
MDHAR was assayed by a method modified from Hossain et al. (1984) and Jahnke et al. (1991). The decrease in A340 due to the oxidation of NADH to NAD+ was monitored over the linear 5-min period of the reaction by the generation of monodehydroascorbate via the inclusion of ascorbate oxidase in the reaction mixture of 1 mL total volume. Extract (50 μL) was mixed with 500 μm ascorbate, 150 μm NADH, and 0.2 unit of ascorbate oxidase from Cucurbita sp.; 1 unit of ascorbate oxidase is defined by the manufacturer as the amount that causes the oxidation of 1 μmol of ascorbate to monodehydroascorbate per minute. The balance to 1 mL was made up by monodehydroascorbate assay buffer (0.08 m K2PO4, pH 7.8, containing 200 μm DTPA). The assay was repeated with twice the volume of extract (100 μL) to check that there was a doubling of the reaction rate. If this did not occur, then the ascorbate oxidase solution had lost activity and had become limiting. The rate of conversion of NADH to NAD+ was determined using an extinction coefficient for NADH at 340 nm of 6.2 mm−1 cm−1.
GTR was assayed following a method modified from that of Schaedle and Bassham (1977), Hossain et al. (1984), and Jahnke et al. (1991) based on the decrease in A340 due to the oxidation of NADPH to NADP+ over 5 min. The total reaction mixture volume was 1 mL and contained 500 μm oxidized glutathione, 100 μL of extract, 150 μm NADPH, and GTR assay medium (0.08 m K2PO4, pH 7.8, and 200 μm DTPA). Correction was made for the non-GTR-dependent oxidation of NADPH by excluding the oxidized glutathione from the reaction mixture.
SOD was assayed by the NBT method modified from that described by Beyer and Fridovich (1987). The assay is dependent on competition for the photogenerated superoxide anion radical between the dye NBT (which is oxidized to a fine purple formazan colloid that absorbs at 560 nm and is stabilized in suspension by the presence of a detergent) and SOD in the sample. The total reaction volume was 1 mL and contained from 30 to 800 μL of sample, to which was added 0.025% Triton X-100 (detergent) and 57 μmol of NBT, the balance being made up of SOD assay buffer (0.05 m K2PO4 containing 200 μm DTPA). The reaction was started by adding 0.01 m Met and 1.13 μm riboflavin (the superoxide anion radical photogeneration system) and placing the reaction tube a preset distance from a 60-W fluorescent tube for 7 min. The development of the purple coloration was then determined by measurement of the A560 in a spectrophotometer blanked with SOD assay buffer. An inhibition curve for A560 was constructed against an increasing volume of sample. One unit of SOD was defined as that being contained in the volume of extract that caused a 50% inhibition of the SOD-inhibitable fraction of the NBT reduction (Beyer and Fridovich, 1987).
All enzyme assays were carried out at both 14 and 24°C using a temperature-controlled cuvette. Temperature was monitored by a calibrated thermocouple in the cuvette solutions. Each assay was the mean from eight leaves. Leaf chlorophyll concentrations were determined by the method of Hipkins and Baker (1986).
DHAR Assay
A separate extraction procedure, based on the method of Jahnke et al. (1991), was used for the preparation of a cell-free extract for the assay of DHAR due to the enzyme's lability. Leaf material (approximately 0.20 g; 2 × 10−3 m2 area) was frozen in liquid N2 and ground with a pestle to a powder in a prechilled mortar. The powder was homogenized in PVP (30 mg) and 3 mL of DHAR extraction medium (0.05 m K2PO4 buffer, pH 6.5, containing 20% [v/v] glycerol, 2 × 10−4 m 4-chlororesorcinol, 2 mm DTPA, 1 mm PMSF, 1 mm benzamidine-HCl, 100 μm 2-mercaptobenzothiazole, 0.014 m 2-mercaptoethanol, 200 μm dehydroascorbate, and 5 mm ε-amino-n-caproic acid). The homogenate was immediately centrifuged at 3°C for 20 min. The supernatant was used for the enzyme assay directly.
LOX Assay
Pro-oxidant enzymic lipid peroxidation (LOX activity) was assayed polarographically by the uptake of O2 (Grossman and Zakut, 1978). Extraction was by the method of Kar and Feierarbend (1984). Leaf tissue (300 mg) was ground in liquid N2 to a powder and then homogenized in 2 mL of extraction buffer (50 mm K2PO4 buffer, pH 7.5, containing 0.5% [v/v] Triton X-100). The extract was then centrifuged at 1000g at 4°C for 15 min. The supernatant (0.5 mL) was added to 2.5 mL of buffered linoleate dispersion in a Clark O2 electrode and O2 uptake was measured for 5 min at 24°C. Enzyme activity was expressed in terms of O2 uptake per milligram of chlorophyll per unit of time. Buffered linoleate consisted of 230 mg of linoleate dispersed with 274 mg of Tween 20 in 25 mL of distilled water. The mixture was stirred and neutralized to pH 7.0 (using 1 m KOH) and then made up to 100 mL by adding 75 mL of 0.2 m K2PO4 buffer, pH 6.5. All experiments were replicated with a minimum of four leaves.
Determination of Lipid Peroxidation
MDA content and other thiobarbituric acid-reacting substances were assayed as indicators of the extent of lipid peroxidation in leaf tissue by the method of Hodgson and Raison (1991). Leaf tissue (300 mg) was ground in 4 mL of N2-degassed 10 mm Na2PO4 buffer, pH 7.4, and centrifuged at 1000g for 5 min. Two-hundred microliters of the supernatant was added to a reaction mixture containing 100 μL of 8.1% (w/v) SDS, 750 μL of 20% (w/v) acetic acid, pH 3.5 (NaOH), 750 μL of 0.8% (w/v) aqueous thiobarbituric acid, and 200 μL of distilled water. An identical reaction mixture in which the 200 μL of supernatant was substituted with an equal volume of buffer was simultaneously set up as an absorbance blank. Both reaction mixtures were then incubated at 98°C for 60 min. After cooling to room temperature, the mixtures were centrifuged for 5 min. MDA concentration was calculated by subtracting the A535 from the A600using a molar extinction coefficient of 1.56 × 105 m−1 cm−1.
Ascorbate Content
Ascorbate was measured by a modification of the method of Omaye et al. (1979), based on the coupling of dehydroascorbate to dinitrophenylhydrazine in the presence of thiourea as a mild reducing reagent, and conversion of the resulting dinitrophenylhydrazone to a red compound by sulfuric acid. Each sample was ground in liquid N2 with a little sand. The resulting powder was then transferred into 1.5 mL of 6% (w/v) trichloracetic acid solution and then centrifuged for approximately 7 min at 10,000g. The supernatant (0.5 mL) was then transferred to two 2-mL tubes and diluted with 1.5 mL of trichloracetic acid. To one tube was added 100 mg of HCl-washed activated charcoal to oxidize any ascorbate to dehydroascorbate. Both tubes were sealed, shaken for 1 min, and centrifuged for 3 min at 10,000g. From each of the two tubes, 0.5 mL of supernatant was pipetted into two additional 2-mL tubes. Dinitrophenylhydrazine reagent (0.25 mL; 2 g of 2,4-dinitrophenylhydrazine and 4 g of thiourea in 100 mL of 25% [v/v] H2SO4) was added to one tube of each pair. All four tubes were then incubated for 3 h in a water bath at 37°C to allow the reaction to run to completion. To all tubes 0.63 mL of H2SO4 was then added dropwise on ice. Reagent (0.25 mL) was added to the blank tubes. The A540 of the sample relative to the blank was measured. Standard curves were constructed using l-ascorbic acid in the range of 0 to 4 mm.
α-Tocopherol Content
Leaves (85 mg fresh weight per leaf) were ground to a fine powder in liquid N2, which was transferred to an ice-cold, 9-mL glass tissue homogenizer containing 2 mL of extraction buffer (2 mm sodium isoascorbate, 5 mm MgCl2, and 0.08 m H2SO4, pH 6.8). The macerate was then homogenized and α-tocopherol was extracted by vigorous shaking for 10 min with 1 mL of hexane. The upper organic layer was separated by centrifugation for 10 min at 16,000g. The pellet was collected and twice reextracted before pooling the upper organic layers and evaporating to dryness under N2. Samples were stored in the dark at −80°C under N2. Immediately prior to chromatography, samples were dissolved in 100 μL of methanol. Separation was by reverse-phase chromatography (System Gold HPLC, Beckman) with a C18 ODS-1 column (length 25 cm, i.d. 4 mm). The mobile phase was 3% dichloromethane in 97% methanol (v/v) delivered at a flow rate of 1 mL/min with a 20-μL injection loop. Detection was at 292 nm. Peak areas were integrated and the column was calibrated by using known concentrations of purified α-tocopherol.
RESULTS
Environmental Conditions
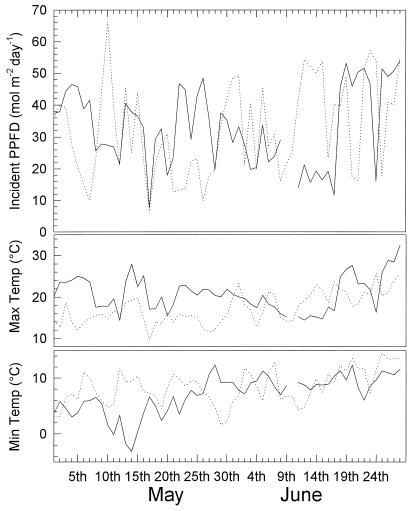
The daily maximum and minimum temperatures and the daily integrated photon flux experienced by the maize crops during May and June in 1994 and 1995 are shown in Figure 1. In 1994 both minimum and maximum temperatures increased during this period. In 1995, although there was an increase in minimum temperature, maximum temperature did not show a consistent increase during May. Light levels fluctuated markedly throughout the experimental period in both years and demonstrate the variable climatic conditions experienced by the crops.
Figure 1.
Daily integrated PPFD incident on the canopy and the maximum and minimum air temperatures at the field site during May and June 1994 (broken line) and 1995 (solid line).
Relationship between Electron Transport and CO2 Assimilation
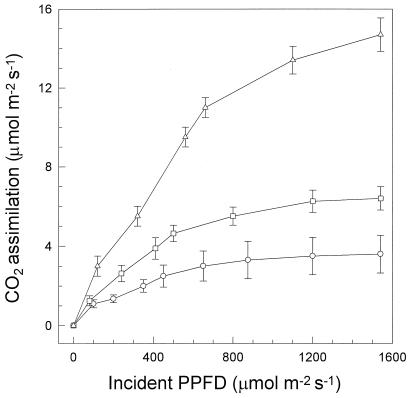
Consistent with the findings of Stirling et al. (1994), the ability of leaves to assimilate CO2 was depressed in May, when periods of chilling were experienced, and increased markedly as temperature increased through June, as illustrated by the changes in the representative photosynthetic light-response curve of leaves harvested in May and June 1995 (Fig. 2).
Figure 2.
Response of CO2 assimilation, corrected for dark respiratory losses, to incident PPFD for the youngest mature leaf of a maize crop sampled on May 16 (○), May 23 (□), and June 26 (▵) of 1994. ses of three leaves are shown.
An effective way to examine the relationship between linear electron transport and CO2 assimilation in maize leaves is to determine the φCO2 and φPSII over the range of PPFDs used to determine a photosynthetic light-response curve (Genty et al., 1989; Edwards and Baker, 1993). Previously, plots of φPSII against φCO2 for mature maize leaves over a wide range of PPFDs and environmental conditions showed a remarkable correlation between the two parameters, with the ratio of φPSII/φCO2 remaining constant within a range of approximately 11 to 13 (Edwards and Baker, 1993). A value of 12 for φPSII/φCO2 implies that six electrons must be transported through PSII for each molecule of CO2 assimilated (Edwards and Baker, 1993).
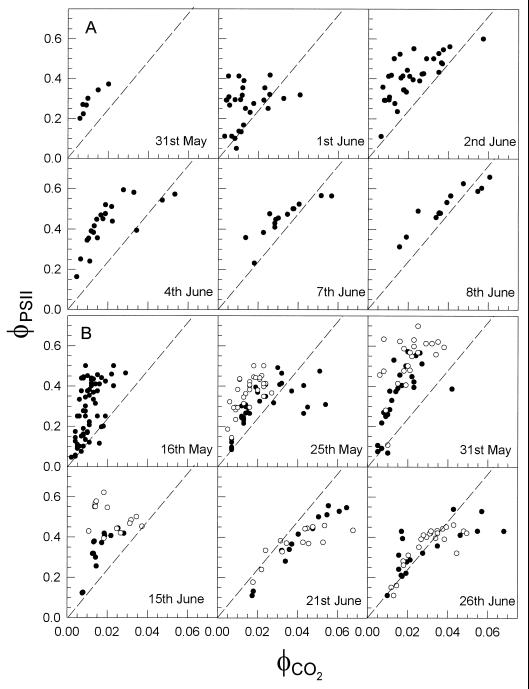
Measurements of φPSII and φCO2 at a range of PPFDs between 150 and 1500 μmol m−2 s−1 were made on maize leaves throughout May and June in 1994 and 1995. Plots of φPSII against φCO2 were then constructed from data collected from all of the leaves monitored on any given sampling day. Representative examples of such plots for days in 1994 and 1995 are shown in Figure 3. The predicted relationship between φPSII and φCO2 (φPSII/φCO2 = 12) for mature maize leaves is shown by the dashed lines in Figure 3. The majority of leaves harvested during May and June exhibit a relationship between φPSII and φCO2 that deviates from that predicted for mature maize leaves. It is only toward the end of June in 1995 that the data points on the φPSII against φCO2 plot fall close to the predicted line (Fig. 3).
Figure 3.
Relationship between φPSII and φCO2 for leaves harvested on selected days during May and June of 1994 (A) and 1995 (B). In 1995 some leaves were measured in an atmosphere containing 2% O2 (○); all other data (•) were obtained from leaves in an atmosphere containing 21% O2. The dashed lines indicate the expected relationships for mature, nonstressed maize leaves (see text).
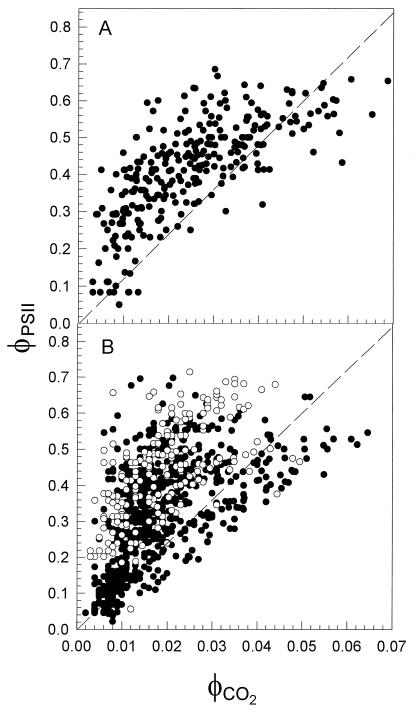
In some cases, toward the end of June, a number of points determined at the lower PPFDs (high φCO2 values) fell below the line (Fig. 3). Such deviations at low PPFDs from the predicted linear relationship have been reported in a range of species and may be associated with the errors involved with correcting CO2 assimilation rates for respiratory losses using dark respiration rates (Edwards and Baker, 1993). During May and early to mid-June, the majority of data points fell well above the predicted line, thus demonstrating an unusually high φPSII/φCO2. The magnitude of the deviations of φPSII/φCO2 from the predicted value is perhaps most clearly demonstrated by plotting φPSII against φCO2 for all leaves monitored in May and June of 1994 and 1995 (Fig. 4). It can be seen clearly from these plots that the majority of the data points fall above the line for the predicted relationship of φPSII and φCO2 in mature maize leaves and that many data points fall considerably above the predicted line. This would suggest that frequently during May and June the rate of electron transport through PSII is in considerable excess of that required to sustain the observed rate of CO2 assimilation.
Figure 4.
Relationship between φPSII and φCO2 for all leaves analyzed during May and June of 1994 (A) and 1995 (B). In 1995 some leaves were measured in an atmosphere containing 2% O2 (○); all other data (•) were obtained from leaves in 21% O2. The dashed lines indicate the expected relationships for mature, nonstressed maize leaves (see text).
If during the period of early crop development the proportion of electron equivalents resulting from PSII photochemical activity that are used for CO2 assimilation in leaves is considerably lower than that found in nonstressed leaves, then, clearly, sinks other than CO2 assimilation for the products of electron transport must be operating. A prime candidate for an alternative electron acceptor is O2, either via a Mehler reaction or photorespiration. Although it is commonly assumed that nonstressed, mature maize leaves do not exhibit any significant level of photorespiration due to the high CO2 concentration in the bundle-sheath cells, it is possible that under chilling stress conditions the CO2-concentrating mechanism may not operate efficiently, and therefore the CO2 concentration would be considerably lower and may permit oxygenation of ribulose 1,5-bisphosphate by Rubisco. This possibility was examined by reducing the O2 concentration from 21 to 2% in the atmosphere of leaves when φPSII andφCO2 were being measured in 1995. This reduction in O2 concentration had no major effect on the relationship between φPSII and φCO2 (Figs. 3 and 4) and is an indication that the onset of photorespiration during periods of low temperature could not account for the additional sink for electron equivalents.
Active O2 Species Metabolism
If increased Mehler-APX cycle activity accounts for the additional sink for electrons during May and early June, then increased activities of active O2 and radical-scavenging enzymes and levels of antioxidants might be expected. The maximum extractable activities of APX, DHAR, GTR, MDHAR, and SOD were determined at 25°C for leaves harvested on May 16, May 23, and June 26, 1995 (Table I). When the enzyme activities were expressed on the basis of leaf area APX and DHAR decrease from May to June, SOD increased and GTR and MDHAR showed no significant changes. However, if the enzyme activities are expressed on the basis of chlorophyll content a very different picture emerges. The activities of all of the enzymes decreased significantly from mid May to the end of June: APX and DHAR by more than 70%, GTR and MDHAR by approximately 60%, and SOD by 50% (Table I).
Table I.
Antioxidant enzyme activities assayed at 25°C and associated antioxidant contents extracted from maize leaves harvested from the field on May 16, May 23, and June 26, 1995
| Parameter | Activity
|
|||||
|---|---|---|---|---|---|---|
| Per unit of
area
|
Per unit of Chla
|
|||||
| May 16 (first leaf) | May 23 (third leaf) | June 26 (sixth leaf) | May 16 (first leaf) | May 23 (third leaf) | June 26 (sixth leaf) | |
| mg m−2 | ||||||
| Total Chl | 96 ± 20 | 144 ± 29 | 272 ± 30 | |||
| μmol m−2 s−1 | μmol mg−1 Chl s−1 | |||||
| APX | 100.8 ± 1.1 | 119.1 ± 8.0 | 79.0 ± 4.3 | 1.05 ± 0.01 | 0.83 ± 0.06 | 0.29 ± 0.02 |
| DHAR | 7.8 ± 2.0 | 6.2 ± 1.4 | 2.1 ± 0.2 | 0.081 ± 0.02 | 0.043 ± 0.010 | 0.008 ± 0.001 |
| GTR | 11.4 ± 1.1 | 14.0 ± 0.8 | 12.2 ± 1.2 | 0.12 ± 0.01 | 0.097 ± 0.006 | 0.045 ± 0.004 |
| MDHAR | 13.8 ± 1.5 | 13.1 ± 0.3 | 14.4 ± 1.2 | 0.14 ± 0.02 | 0.091 ± 0.002 | 0.053 ± 0.004 |
| units m−2 s−1 | units mg−1 Chl s−1 | |||||
| SOD | 39.7 ± 2.8 | 53.0 ± 2.6 | 57.3 ± 5.8 | 0.41 ± 0.03 | 0.37 ± 0.02 | 0.21 ± 0.02 |
| μmol m−2 | μmol mg−1 Chl s−1 | |||||
| Total ascorbate | 162 ± 20 | 180 ± 22 | 190 ± 30 | 1.69 ± 0.21 | 1.25 ± 0.14 | 0.70 ± 0.11 |
| mmol m−2 | nmol mg−1 Chl | |||||
| α-Tocopherol | 2.04 ± 0.89 | 2.01 ± 0.07 | 3.716 ± 0.13 | 21.2 ± 8.4 | 13.9 ± 0.5 | 13.7 ± 0.5 |
Data are the means ± se of four independent replicates.
Chl, Chlorophyll
It has been reported that the activities of enzymes involved in scavenging active O2 species and radicals in maize leaves can be extremely temperature sensitive (Jahnke et al., 1991). In the context of the protection from photoxidation in the field it was important to determine whether the enzymes assayed maintained a high activity at a representative field temperature. Consequently, the enzyme extracts were all assayed at 14°C (Table II). Unlike the situation when the enzymes were assayed at 25°C (Table I), APX, DHAR, GTR, and MDHAR activities on a unit leaf area basis all decreased from May to late June. The activity of SOD showed no significant change.
Table II.
Antioxidant enzyme activities assayed at 14°C after extraction from maize leaves harvested from the field on May 16, May 23, and June 26, 1995.
| Parameter | Activity
|
|||||
|---|---|---|---|---|---|---|
| Per unit of area
|
Per unit of
Chla
|
|||||
| May 16 (first leaf) | May 23 (third leaf) | June 26 (sixth leaf) | May 16 (first leaf) | May 23 (third leaf) | June 26 (sixth leaf) | |
| μmol m−2 s−1 | μmol mg−1 chl s−1 | |||||
| APX | 78.2 ± 10.2 | 83.8 ± 7.0 | 60.4 ± 10.4 | 0.81 ± 0.11 | 0.58 ± 0.05 | 0.22 ± 0.04 |
| DHAR | 4.4 ± 1.3 | 8.7 ± 1.3 | All nonenzymic | 0.046 ± 0.010 | 0.060 ± 0.001 | All nonenzymic |
| (0.130 ± 0.0030 | (5 × 10−4 ± 1 × 10−5) | |||||
| GTR | 5.1 ± 0.1 | 4.8 ± 1.2 | 3.8 ± 0.8 | 0.053 ± 0.001 | 0.033 ± 0.008 | 0.014 ± 0.003 |
| MDHAR | 8.6 ± 0.5 | 8.8 ± 1.1 | 7.2 ± 1.6 | 0.090 ± 0.005 | 0.061 ± 0.008 | 0.026 ± 0.006 |
| units m−2 s−1 | units mg−1 chl s−1 | |||||
| SOD | 45.6 ± 9.2 | 49.2 ± 2.6 | 52.6 ± 11.4 | 0.48 ± 0.10 | 0.34 ± 0.02 | 0.19 ± 0.04 |
Data are the means ± se of four independent replicates.
Chl, Chlorophyll.
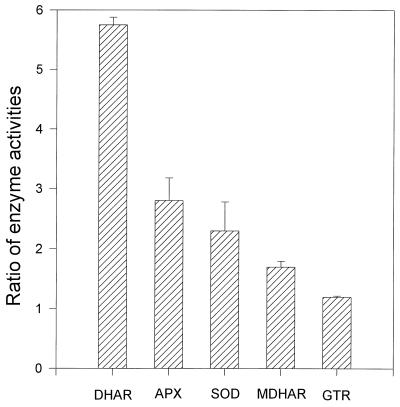
When the activities at 14°C (a temperature better reflecting the day temperature that the leaves in the field might experience in mid-May) were expressed on a unit chlorophyll basis, all enzymes exhibited very large decreases in activity from May to late June. By comparing the activities of each enzyme per unit chlorophyll in extracts made from leaves harvested on May 16 and assayed at 14°C with those from leaves harvested on June 26 and assayed at 25°C (Fig. 5), we could evaluate the magnitude of the change in potential activity of the enzymes operating in leaves in the field from mid-May, when temperatures are low, to the end of June, when temperatures are higher. The activity of DHAR in mid-May was almost 6-fold that in late June, and the activities of APX, MDHAR, and SOD were elevated approximately 2- to 3-fold, whereas there was a much smaller enhancement of GTR activity.
Figure 5.
Ratio of activities of enzymes assayed at 14°C for leaves harvested on May 16 to the activities assayed at 25°C for leaves harvested on May 26, 1995. Data are given for APX, DHAR, GTR, MDHAR, and SOD. ses of three replicates are shown.
Two key antioxidants in chloroplasts involved in active O2 and radical scavenging are ascorbate and α-tocopherol. The level of ascorbate on a unit leaf area basis did not change significantly throughout May and June; however, on a unit chlorophyll basis it decreased by more than 50% (Table I). The level of α-tocopherol actually increased per unit area from May to late June; however, a 35% decrease occurred during this period when expressed per unit chlorophyll.
Lipid peroxidation by LOX in leaves generates both singlet oxygen and superoxide anion radicals (Lynch and Thompson, 1984). LOX is an enzyme normally involved in wound responses in tissues and catalyzes the reaction of O2 with free, polyunsaturated fatty acids to form conjugated lipid hydroperoxides. It is quite possible that LOX could be an important initiator of oxidative damage under chilling-stress conditions. Extractable LOX activity was greater for leaves harvested on May 16 compared with June 26 (Table III). The elevated LOX activity in mid-May is consistent with the higher MDA content in the leaves on May 16 compared with June 26 (Table III); MDA is a product of lipid peroxidation.
Table III.
Relative LOX activity and extent of lipid peroxidation, as monitored by MDA levels, from maize leaves harvested from the field on May 16, May 23, and June 26, 1995
| Date | LOX Activity | MDA Content |
|---|---|---|
| nmol mg−1 chlorophyll | ||
| May 16 (first leaf) | 542 ± 26 | 111 ± 10 |
| June 26 (sixth leaf) | 864 ± 120 | 71 ± 13 |
Data are the means ± se of four independent replicates.
DISCUSSION
The data presented in Figures 3 and 4 demonstrate that during the early growth season maize leaves exhibit values of φPSII/φCO2 that are considerably greater than would be expected for mature, nonstressed leaves. In extreme cases this ratio was increased by a factor of 3.5, implying that 21 electrons are transported through PSII for each molecule of CO2 assimilated, compared with 6 electrons in nonstressed leaves. It would appear that electron sinks other than CO2 must be operating to sustain such high φPSII/φCO2 values. Initially it was thought that photorespiration may be a significant sink for electrons in leaves experiencing low temperatures because chilling may inhibit the CO2-concentrating mechanism in the bundle-sheath cells. This would result in a decreased CO2 concentration and allow oxygenation of ribulose 1,5-bisphosphate by Rubisco. However, when leaves with high φPSII/φCO2 values were exposed to an atmosphere containing 2% O2 to inhibit photorespiration, no significant decreases in φPSII were observed (Figs. 3 and 4). Consequently, photorespiration can be ruled out as a major sink for electrons in these leaves.
Direct reduction of O2 via a Mehler reaction is an obvious candidate for dealing with the increase in electron flux relative to CO2 assimilation in chill-stressed leaves. The increased activities per unit chlorophyll of APX, DHAR, GTR, MDHAR, and SOD, coupled with the increased levels of the antioxidants ascorbate and α-tocopherol, in leaves in mid-May compared with late June (Tables I and II) would be consistent with this hypothesis. However, when the enzyme activities and antioxidant contents are expressed on the basis of unit leaf area, only small differences are found between mid-May and late June (Tables I and II). Because the rate of generation of active O2 species via the Mehler reaction in chilled leaves will be related to the rate of light capture by the antennae pigments, the enzyme activities and antioxidant contents expressed on a chlorophyll basis, rather than on an area basis, may be more relevant to the issue of changing electron sinks. However, comparison of the rates of the enzyme activities on a chlorophyll basis from leaves harvested in mid-May and late June, assayed at temperatures similar to those experienced in the field during the day (Fig. 5), does not give overwhelming support for the Mehler reaction acting as the major sink for electrons. APX, DHAR, MDHAR, and SOD all exhibit 2-fold increases in activity in mid-May compared with late June, but GTR activity is increased by only approximately 15%.
It would appear from the elevated LOX activity and MDA content in leaves in mid-May compared with late June (Table III) that photosynthetic-reducing equivalents are not the only source of oxidative stress during periods of low temperatures. LOX activity is normally associated with peroxidation of lipids during leaf senescence and in response to plant tissue wounding (Kar and Feierabend, 1984; Lynch and Thompson, 1984; Thompson et al., 1987; Croft et al., 1993; Saravitz and Siedow, 1996). The higher LOX activity in maize leaves during periods of low temperatures might suggest that increased LOX synthesis is a leaf response to chilling stress. Alternatively, increased LOX activity may be a response to increased lipid peroxidation produced as a result of chill-induced photo-oxidative events. In either case, LOX-mediated peroxidation of membrane lipids is likely to make a significant contribution to the oxidative damage occurring in chill-stressed leaves.
Aside from the Mehler reaction, there are two other possible metabolic explanations for why the ratio of φPSII/φCO2 is increased when leaves are exposed to periods of low temperatures. It is well established that for C4 plants to maintain a high CO2 concentration in the bundle sheath leakage of CO2 from the bundle sheath to the mesophyll must be compensated for by overcycling of the C4-acid cycle relative to the net rate of C assimilation (Hatch, 1987; Furbank et al., 1990). An additional energy requirement is associated with this overcycling, because ATP is required for PEP synthesis (Furbank et al., 1990). It has been estimated that in mature, nonstressed C4 leaves the C4-acid cycle runs 25% faster than the net rate of photosynthesis (Farquhar, 1983; Evans et al., 1986; Henderson et al., 1992). If the rate of overcycling relative to net photosynthetic C assimilation were to increase at chilling temperatures, then this would result in an increase in φPSII/φCO2.
Another factor that could modify φPSII/φCO2 is the rate of operation of a Q cycle around the Cyt b/f complex relative to linear photosynthetic electron transport. Operation of a Q cycle can potentially increase the ratio of ATP to NADPH produced by linear electron transport (Ort, 1986), which could modify the quantum yield of CO2 assimilation. Furbank et al. (1990) estimated that in the absence of a Q cycle increasing the C4-acid overcycling from 0 to 100% of total C assimilation would increase the quantum requirement of C4 photosynthesis from approximately 18 to 24. When a Q cycle is operating, this change from no C4-acid overcycling to 100% C4-acid overcycling would increase the quantum requirement from 12 to 15. Operation of a Q cycle was estimated to decrease the quantum requirement from 18 to 12 if no C4-acid overcycling occurred and from 24 to 15 at 100% overcycling.
From the calculations of Furbank et al. (1990) it can be seen that a C4 leaf at optimal growth temperature, operating a Q cycle but having no C4-acid overcycling, will have a quantum requirement for CO2 assimilation of approximately 12, which would increase to 24 if the Q cycle ceased to operate and 100% C4-acid overcycling occurred. If we assume the most extreme, but highly unlikely, scenario that maize leaves that have developed at optimal growth temperatures operate a Q cycle but have no C4-acid cycling and that in leaves that have developed at chilling temperatures the Q cycle ceases to operate and 100% C4-acid cycling occurs, then a doubling of the quantum requirement for C4 photosynthesis from 12 to 24 would occur at chilling temperatures. Clearly, this doubling of the quantum requirement would not be sufficient to account for the observed 3.5-fold increase in φPSII/φCO2 (and the quantum requirement for CO2 assimilation) when maize leaves experience low temperatures in the field. Although Q-cycle operation and C4-acid cycling may be factors that could be modified by chilling, even the most extreme changes in these activities could not account for the observed increases in φPSII/φCO2.
It is possible that the high φPSII/φCO2 values for leaves experiencing low temperatures may be due to inaccuracies in the measurement of φPSII and φCO2. To determine φCO2, the rate of respiratory CO2 evolution in the light is estimated from the dark respiration rate. If the rate of respiration in the light relative to that in the dark increases at low temperatures, then this would result in an underestimation of CO2 assimilation in chilled leaves and an elevated φPSII/φCO2. However, it seems unlikely that the magnitude of any such chill-induced changes in respiratory activity in the light would be sufficiently large to account for the large increases in φPSII/φCO2.
A potential source of error in the estimation of φPSII is the overestimation of Fm′, which would lead to an overestimation of φPSII. Kramer et al. (1995) showed that the saturating light pulse (0.5 s at a PPFD of approximately 10,000 μmol m−2 s−1) used to reduce QA during the measurement of φPSII will also reduce the plastoquinone pool, which will result in a loss of the quenching due to oxidized plastoquinone prior to applying the saturating light pulse. However, this error will be significant only when leaves have highly oxidized plastoquinone pools at steady-state photosynthesis, as would be found at low PPFDs, and would then only result in overestimations of φPSII of less than 10%. All of the φPSII measurements in this study were made on leaves exposed to PPFDs between 150 and 2000 μmol m−2 s−1. Over this PPFD range qP is low (data not shown), indicating that QA is not highly oxidized. As there is a close relationship between the redox state of QA, as estimated from qP, and the plastoquinone pool, errors due to quenching by oxidized plastoquinone did not affect significantly the φPSII/φCO2 values obtained in this study.
It is possible that differences in the optical properties of the maize leaves at different times could result in differences in φPSII/φCO2. In maize leaves at high PPFDs, measurement of φPSII using fluorescence excitation of 560 and 660 nm produces different values, but this is not the case at low PPFDs (Kingston-Smith et al., 1997). This has been attributed to the differential penetration of the 560 and 660 nm radiation into the leaf. Because measurements of φPSII at high PPFDs using 560 and 660 nm of fluorescence excitation for maize leaves harvested from a field plot throughout May and June in 1997 produced similar results (within 10%, data not shown), it is unlikely that such errors are important in the context of the high φPSII/φCO2 values observed during periods of chilling.
This study demonstrates that φPSII/φCO2 is considerably elevated when maize leaves are exposed to low temperatures in the field. Although chill-induced inhibition of a Q cycle, an increase in the overcycling of C4 acids, and possible errors in measurement of φPSII and φCO2 would produce increases in this ratio, these factors cannot account for the magnitude of the increases observed. The chill-induced increases in φPSII/φCO2 imply that the rate of linear electron transport relative to CO2 assimilation increases and alternative electron acceptors to CO2 must become available. Increased levels of active O2- and radical-scavenging enzymes and levels of antioxidants in the chilled leaves suggest that O2, via a Mehler reaction, is a candidate for such an alternative electron acceptor.
Abbreviations:
- APX
ascorbate peroxidase
- DHAR
dehydroascorbate reductase
- DTPA
diethylenetriaminepentaacetic acid
- GTR
glutathione reductase
- LOX
lipoxygenase
- MDA
malondialdehyde
- MDHAR
monodehydroascorbate reductase
- NBT
nitroblue tetrazolium
- φCO2
quantum efficiency of CO2 assimilation
- φPSII
relative quantum efficiency of PSII electron transport
- qP
photochemical quenching coefficient
- SOD
superoxide dismutase
Footnotes
This work was supported by grants from the Biotechnology and Biological Sciences Research Council of the United Kingdom and from the Commission of the European Communities (grant no. AIRI-CT92-0205 to N.R.B.).
LITERATURE CITED
- Andrews JR, Fryer MJ, Baker NR. Characterization of chilling effects on photosynthetic performance of maize crops during early season growth using chlorophyll fluorescence. J Exp Bot. 1995;46:1195–1203. [Google Scholar]
- Asada K. Radical production and scavenging in the chloroplasts. In: Baker NR, editor. Photosynthesis and the Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 123–150. [Google Scholar]
- Baker NR, Nie G-Y. Chilling sensitivity of photosynthesis in maize. In: Bajaj YPS, editor. Biotechnology in Agriculture and Forestry, Vol 25: Maize. Berlin: Springer-Verlag; 1994. pp. 465–481. [Google Scholar]
- Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Cheeseman JM (1994) Depressions of photosynthesis in mangrove canopies. In NR Baker, JR Bowyer, eds, Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field. Bios Scientific Publishers, Oxford, UK, pp 377–389
- Croft KPC, Juttner F, Slusarenko AJ. Volatile products of the lipoxygenase pathway evolved from Phaseolus vulgaris (L.) leaves inoculated with Pseudomonas syringae pv phaseolivola. Plant Physiol. 1993;101:13–24. doi: 10.1104/pp.101.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GE, Baker NR. Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynth Res. 1993;37:89–92. doi: 10.1007/BF02187468. [DOI] [PubMed] [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol. 1986;13:281–292. [Google Scholar]
- Farquhar GD. On the nature of carbon isotope discrimination in C4 species. Aust J Plant Physiol. 1983;10:205–226. [Google Scholar]
- Fryer MJ, Oxborough K, Martin B, Ort DR, Baker NR. Factors associated with depression of photosynthetic quantum efficiency in maize at low growth temperature. Plant Physiol. 1995;108:761–767. doi: 10.1104/pp.108.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Jenkins CLD, Hatch MD. C4 photosynthesis: quantum requirement, C4 acid overcycling and Q-cycle involvement. Aust J Plant Physiol. 1990;17:1–7. [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Grossman S, Zakut R . Determination of the activity of lipoxygenase (lipoxidase) Methods Biochem Anal. 1978;25:303–329. doi: 10.1002/9780470110454.ch5. [DOI] [PubMed] [Google Scholar]
- Haldimann P, Fracheboud Y, Stamp P. Photosynthetic performance and resistance to photoinhibition of Zea mays L. leaves grown at sub-optimal temperature. Plant Cell Environ. 1996;19:85–92. [Google Scholar]
- Hatch MD. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta. 1987;895:81–106. [Google Scholar]
- Henderson SA, von Caemmerer S, Farquhar GD. Short-term measurements of carbon isotope discrimination in several C4 species. Aust J Plant Physiol. 1992;19:263–285. [Google Scholar]
- Hipkins MF, Baker NR. Spectroscopy. In: Hipkins M, Baker NR, editors. Photosynthesis: Energy Transduction. A Practical Approach. Oxford, UK: IRL Press; 1986. pp. 51–101. [Google Scholar]
- Hodges DM, Andrews CJ, Johnson DA, Hamilton RI. Antioxidant enzyme responses to chilling stress in differentially sensitive inbred maize lines. J Exp Bot. 1997;48:1105–1113. [Google Scholar]
- Hodgson RAJ, Raison JK. Lipid peroxidation and superoxide dismutase activity in relation to photoinhibition induced by chilling in moderate light. Planta. 1991;185:215–219. doi: 10.1007/BF00194063. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Nakano Y, Asada K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in regulation of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984;25:385–395. [Google Scholar]
- Hull MR (1990) The activity of active oxygen scavenging and C4 cycle enzymes in relation to photosynthesis of two Zea genotypes at chilling temperatures. PhD thesis. University of Essex, Colchester, UK
- Jahnke LS, Hull MR, Long SP. Chilling stress and oxygen metabolizing enzymes in Zea mays and Zea diploperennis. Plant Cell Environ. 1991;14:97–104. [Google Scholar]
- Kar M, Feierabend J. Metabolism of activated oxygen in detached wheat and rye leaves and its relevance to the initiation of senescence. Planta. 1984;160:385–391. doi: 10.1007/BF00429753. [DOI] [PubMed] [Google Scholar]
- Kingston-Smith AH, Harbinson J, Williams J, Foyer CH. Characterization of chilling sensitivity in maize. II. Effect of chilling on carbon assimilation, enzyme activation and photosynthetic electron transport in the absence of photoinhibition. Plant Physiol. 1997;114:1039–1046. doi: 10.1104/pp.114.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DM, DiMarco G, Loreto F. Contribution of plastoquinone quenching to saturation pulse-induced rise of chlorophyll fluorescence in leaves. In: Mathis P, editor. Photosynthesis: From Light to Biosphere, Vol I. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 147–150. [Google Scholar]
- Lynch DV, Thompson JE. Lipoxygenase-mediated production of superoxide anion in senescing plant tissue. FEBS Lett. 1984;173:251–254. [Google Scholar]
- Massacci A, Battistelli A, Loreto F. Effect of drought stress on photosynthetic characteristics, growth and sugar accumulation of field-grown sweet sorghum. Aust J Plant Physiol. 1996;23:331–340. [Google Scholar]
- Massacci A, Iannelli MA, Pietrini F, Loreto F. The effect of growth at low temperature on photosynthetic characteristics and mechanisms of photoprotection of maize leaves. J Exp Bot. 1995;46:119–127. [Google Scholar]
- Miedema P. The effects of low temperatures on Zea mays. Adv Agron. 1982;35:93–128. [Google Scholar]
- Nie G-Y, Baker NR. Modifications to thylakoid composition during development of maize leaves at low growth temperatures. Plant Physiol. 1991;95:184–191. doi: 10.1104/pp.95.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie G-Y, Long SP, Baker NR. The effects of development at sub-optimal growth temperatures on photosynthetic capacity and susceptibility to chilling-dependent photoinhibition in Zea mays. Physiol Plant. 1992;85:554–560. [Google Scholar]
- Nie G-Y, Robertson EJ, Fryer MJ, Leech RM, Baker NR. Response of the photosynthetic apparatus in maize leaves grown at low temperature to transfer to normal growth temperature. Plant Cell Environ. 1995;18:1–12. [Google Scholar]
- Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol. 1979;62:3–10. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- Ort DR (1986) Energy transduction in oxygenic photosynthesis. LA Staehelin, CJ Arntzen, eds, Photosynthesis III, Encyclopedia of Plant Physiology, New Series, Vol 19. Springer-Verlag, New York, pp 143–196
- Ortiz-Lopez A, Nie G-Y, Ort DR, Baker NR. The involvement of the photoinhibition of photosystem II and impaired membrane energization in the reduced quantum yield of carbon assimilation in chilled maize. Planta. 1990;181:78–84. doi: 10.1007/BF00202327. [DOI] [PubMed] [Google Scholar]
- Saravitz DM, Siedow JN. The differential expression of wound-inducible lipoxygenase genes in soybean leaves. Plant Physiol. 1996;110:187–299. doi: 10.1104/pp.110.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaedle M, Bassham JA. Chloroplast glutathione reductase. Plant Physiol. 1977;59:1011–1012. doi: 10.1104/pp.59.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling CM, Aguilera C, Baker NR, Long SP. Changes in the photosynthetic light response curve during leaf development of field grown maize with implications for modelling canopy photosynthesis. Photosynth Res. 1994;42:217–225. doi: 10.1007/BF00018264. [DOI] [PubMed] [Google Scholar]
- Stirling CM, Nie G-Y, Aquilera C, Nugawela A, Long SP, Baker NR. Photosynthetic productivity of an immature maize crop: changes in quantum yield of CO2 assimilation, conversion efficiency and thylakoid proteins. Plant Cell Environ. 1991;14:947–954. [Google Scholar]
- Thompson JE, Legge RL, Barber RF. The role of free radicals in senescence and wounding. New Phytol. 1987;105:317–344. doi: 10.1111/j.1469-8137.1987.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Tiley GED, Warbots IB (1975) Crop establishment. In GM Milburn, ed, Grower's Handbook, Ed 3. Home-Grown Cereals Authority, London, pp 18–25