Abstract
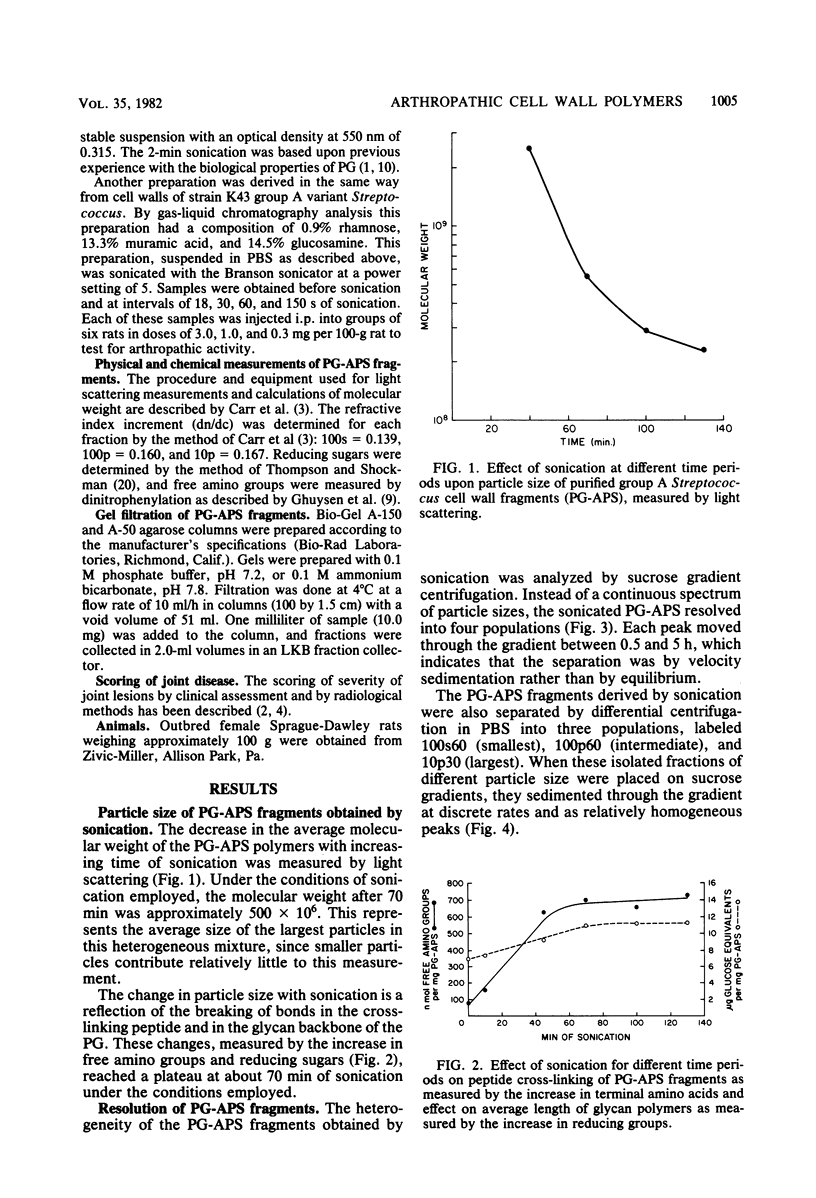
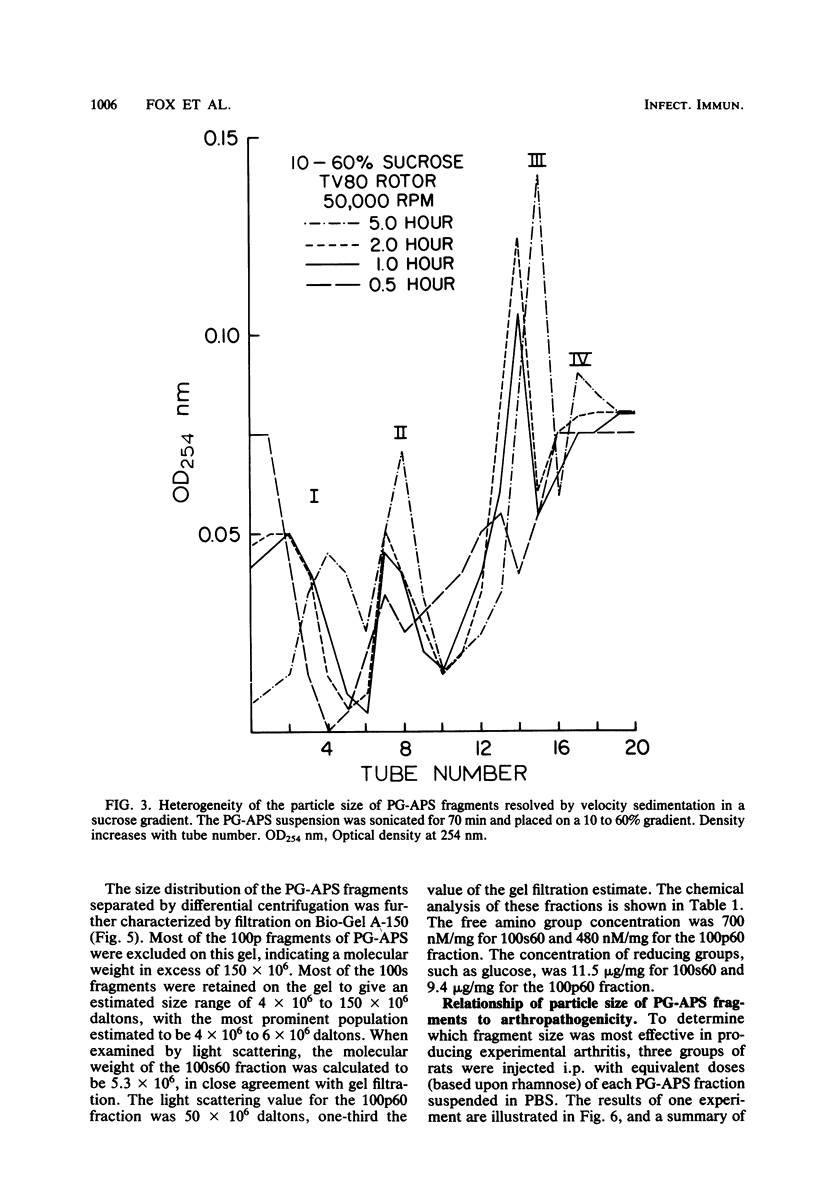
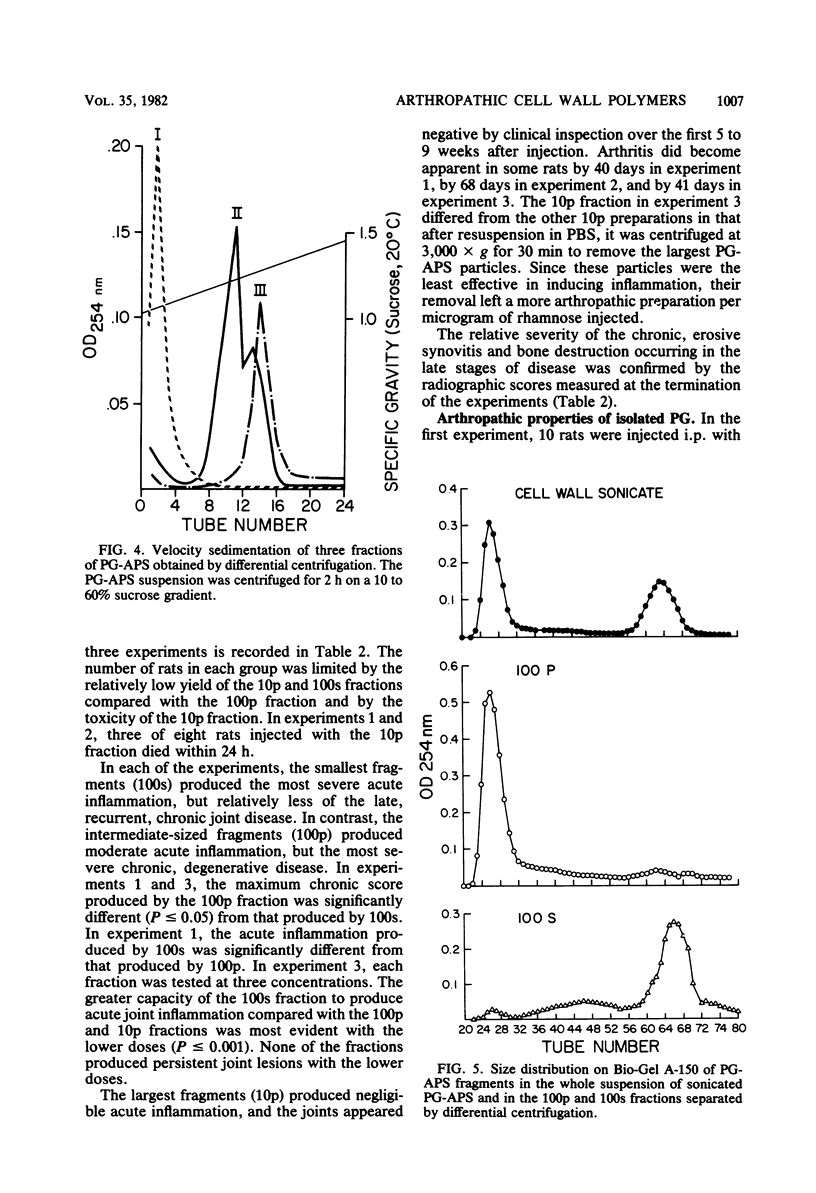
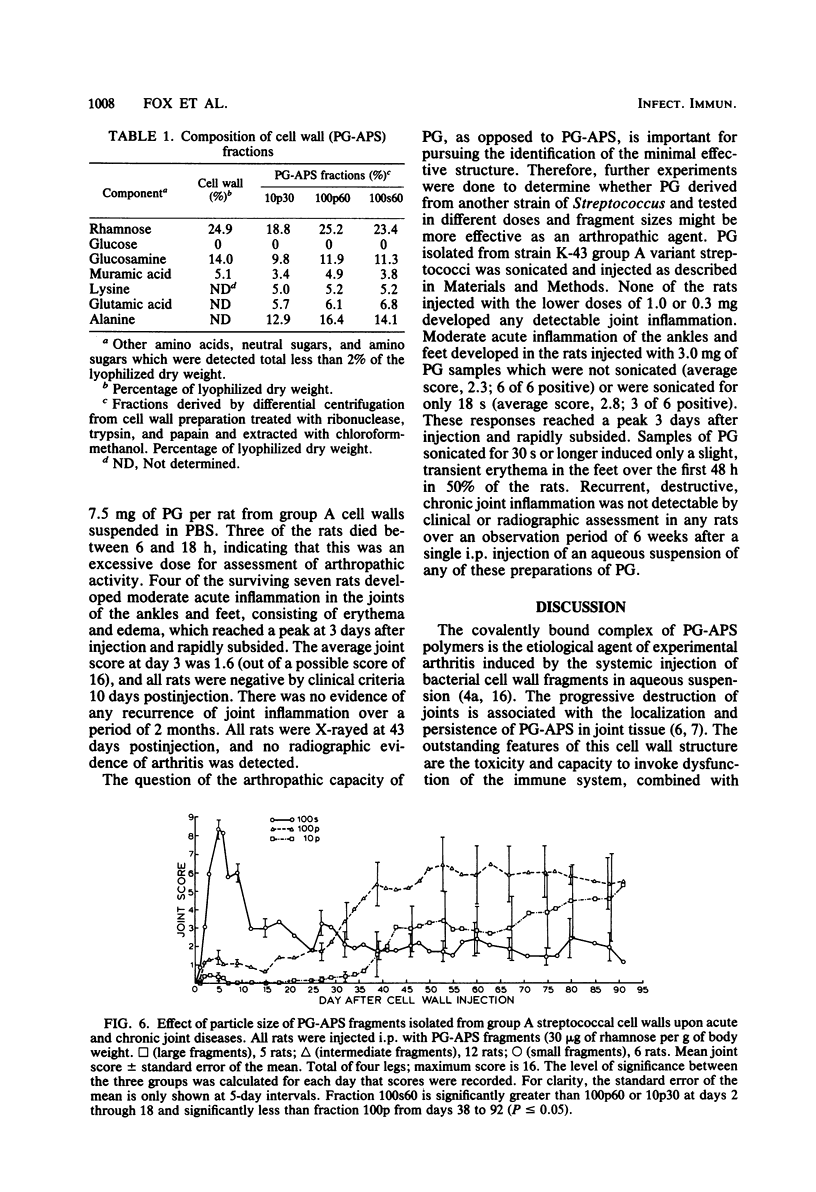
The covalently bound polymers of peptidoglycan and group-specific polysaccharide (PG-APS) were isolated from the cell walls of group A streptococci. Arthritis was induced in rats with a single intraperitoneal injection of an aqueous suspension of PG-APS fragments derived by sonication. The joint lesions induced with this polydisperse suspension followed a bimodal pattern consisting of an acute phase, which reached a peak 5 days after injection and then receded, followed by a chronic, remittent, erosive arthritis lasting several months. The relative severities of the acute and chronic phases could be manipulated by selection of the size of PG-APS fragments. The fragments of PG-APS obtained by sonic treatment were resolved on the basis of size into three major populations by sucrose gradient or differential centrifugation. Based upon light scattering and gel filtration, the average molecular weight of the largest family of fragments was estimated to be about 500 × 106, the intermediate fragments were 50 × 106 daltons, and the predominant size in the smallest population was 5.3 × 106 daltons. The larger fragments induced negligible acute inflammation, but chronic disease became apparent 5 to 9 weeks after injection. The smallest fragments induced the most severe acute inflammation, with relatively little late, chronic joint disease. The particles of intermediate size induced moderate acute inflammation and the most severe chronic, erosive joint lesions. A single injection of fragments of the isolated peptidoglycan moiety of the PG-APS induced only a moderate acute inflammation of joints, with no apparent capacity to maintain the injury and induce chronic disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdulla E. M., Schwab J. H. Biological properties of streptococcal cell-wall particles. 3. Dermonecrotic reaction to cell-wall mucopeptides. J Bacteriol. 1966 Jan;91(1):374–383. doi: 10.1128/jb.91.1.374-383.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderle S. K., Greenblatt J. J., Cromartie W. J., Clark R., Schwab J. H. Modulation of the susceptibility of inbred and outbred rats to arthritis induced by cell walls of group A streptococci. Infect Immun. 1979 Aug;25(2):484–490. doi: 10.1128/iai.25.2.484-490.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M. E., Jr, Shen L. L., Hermans J. Mass-length ratio of fibrin fibers from gel permeation and light scattering. Biopolymers. 1977 Jan;16(1):1–15. doi: 10.1002/bip.1977.360160102. [DOI] [PubMed] [Google Scholar]
- Clark R. L., Cuttino J. T., Jr, Anderle S. K., Cromartie W. J., Schwab J. H. Radiologic analysis of arthritis in rats after systemic injection of streptococcal cell walls. Arthritis Rheum. 1979 Jan;22(1):25–35. doi: 10.1002/art.1780220105. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980 Aug;100(2):383–402. [PMC free article] [PubMed] [Google Scholar]
- Fox A., Schwab J. H., Cochran T. Muramic acid detection in mammalian tissues by gas-liquid chromatography-mass spectrometry. Infect Immun. 1980 Aug;29(2):526–531. doi: 10.1128/iai.29.2.526-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian S. H., Schwab J. H., Cromartie W. J. Relation of rheumatic-like cardiac lesions of the mouse to localization of group A streptococcal cell walls. J Exp Med. 1969 Jan 1;129(1):37–49. doi: 10.1084/jem.129.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian S. H., Schwab J. H. Persistence of group a streptococcal cell walls related to chronic inflammation of rabbit dermal connective tissue. J Exp Med. 1967 Jun 1;125(6):1137–1148. doi: 10.1084/jem.125.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERSON B. S., SCHWAB J. H., CROMARTIE W. J. Relation of particle size of C polysaccharide complexes of group A streptococci to toxic effects on connective tissue. J Exp Med. 1960 Nov 1;112:751–764. doi: 10.1084/jem.112.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWAB J. H., GOODER H., MAXTED W. R. Further studies on toxic C polysaccharide complexes of the beta-haemolytic streptococci. Br J Exp Pathol. 1962 Apr;43:181–188. [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Brown R. R. Modification of antigenic structure in vivo: quantitative studies on the processing of streptococcal cell wall antigens in mice. J Immunol. 1968 Nov;101(5):930–938. [PubMed] [Google Scholar]
- Schwab J. H., Ohanian S. H. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967 Nov;94(5):1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Shockman G. D. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal Biochem. 1968 Feb;22(2):260–268. doi: 10.1016/0003-2697(68)90315-1. [DOI] [PubMed] [Google Scholar]


