Abstract
Background
Phlebotomine sand flies are the vectors of the leishmaniases, parasitic diseases caused by Leishmania spp. Little is known about the prevalence and diversity of sand fly microflora colonizing the midgut or the cuticle. Particularly, there is little information on the fungal diversity. This information is important for development of vector control strategies.
Methodology/Principal Findings
Five sand fly species: Phlebotomus papatasi, P. sergenti, P. kandelakii, P. perfiliewi and P. halepensis were caught in Bileh Savar and Kaleybar in North-Western Iran that are located in endemic foci of visceral leishmaniasis. A total of 35 specimens were processed. Bacterial and fungal strains were identified by routine microbiological methods. We characterized 39 fungal isolates from the cuticle and/or the midgut. They belong to six different genera including Penicillium (17 isolates), Aspergillus (14), Acremonium (5), Fusarium (1), Geotrichum (1) and Candida (1). We identified 33 Gram-negative bacteria: Serratia marcescens (9 isolates), Enterobacter cloacae (6), Pseudomonas fluorescens (6), Klebsiella ozaenae (4), Acinetobacter sp. (3), Escherichia coli (3), Asaia sp. (1) and Pantoea sp. (1) as well as Gram-positive bacteria Bacillus subtilis (5) and Micrococcus luteus (5) in 10 isolates.
Conclusion/Significance
Our study provides new data on the microbiotic diversity of field-collected sand flies and for the first time, evidence of the presence of Asaia sp. in sand flies. We have also found a link between physiological stages (unfed, fresh fed, semi gravid and gravid) of sand flies and number of bacteria that they carry. Interestingly Pantoea sp. and Klebsiella ozaenae have been isolated in Old World sand fly species. The presence of latter species on sand fly cuticle and in the female midgut suggests a role for this arthropod in dissemination of these pathogenic bacteria in endemic areas. Further experiments are required to clearly delineate the vectorial role (passive or active) of sand flies.
Introduction
Phlebotomine sand flies are the natural exclusive vectors of leishmaniases, a group of parasitic diseases caused by protozoan kinetoplastid flagellates belonging to the genus Leishmania. They affect about 12 million people in many countries located in Mediterranean, tropical and sub-tropical regions [1], [2].
Sand flies harbor a huge variety of microorganisms, and not all are resident in the gut [3].They can also originate from the sand flies’ external environment. In nature, adult Phlebotomine sand flies can colonize highly divergent environments e.g. tropical forests, temperate regions and deserts, in wild, domestic or anthropized biotopes. Adult sand flies usually remain close (less than one kilometer) from their larval development sites [4]. The sites where larval development take place are usually a mixture of animal faeces and mud which are found in both wild (rodent burrows, forest floors, caves) and anthropized biotopes (villages, animal shelters) [5], [6]. Larvae feed on the decomposing organic materials in these sites and the adults can therefore acquire a part of their microflora during their larval development. Furthermore, male and female sand flies feed daily on natural sugars, especially nectars or sap secretions and drink water from plants [7]. These sugars are the main source of carbohydrates for adults. Additionally, females require a blood-meal to complement their diet, during the maturation of their eggs and completion of the gonotrophic cycle [8], [9], [10]. During these feeding events, they can also acquire various microorganisms including bacteria (e.g. Bartonella bacilliformis), fungi, Phleboviruses or other trypanosomatidae and co-colonization by human pathogenic and non pathogenic species of Leishmania i.e L. turanica, L. gerbilli and L. major [11], [12]. Beside the lumen of the gut, the diversity of microorganism present on the cuticle that is acquired independently of their breeding, feeding and resting places might also be highly informative for sand fly biology.
The majority of studies dealing with the microflora that colonizes gut of hematophagous insects were performed on mosquitoes [13]. However, very little is known about sand fly microflora and its’ possible impact on the biology (including longevity), reproduction and sand fly-pathogen interaction. This information is important for the development of new strategies for vector control. To date, a few investigations focused on the midgut bacterial flora have been carried out on Lutzomyia longipalpis, Phlebotomus papatasi, P. tobbi, P. argentipes, P. duboscqi and Sergentomyia spp. only [14], [15], [16], [17], [18], [19], [20].
Visceral leishmaniasis or Kala-azar, is a life-threatening parasitic infection, caused by L. infantum in Iran. The two provinces of Azarbaijan-e-sharqi and Ardabil used in the present study are two out of four main endemic foci of VL in Iran. Two others including Fars and Bushehr provinces are located in the south of the country. Several investigations have been carried out on the population composition and Leishmania infection of sand flies in different localities within these provinces. P. perfiliewi transcaucasicus and P. kandelakii have been reported as the proven vectors of VL in Azarbaijan-e-sharqi and Ardabil [21], [22]. Parvizi et al. 2008 [23] and Sanei Dehkordi et al. 2011 [24] have reported P. perfiliewi as the proven vector of VL in Kaleybar and Bileh Savar counties.
The aim of our study was to investigate the diversity of the microbial flora including bacteria and fungi that colonize both the cuticle and midgut in wild populations of sand flies through a culture dependent methodology. Specimens of sand flies investigated were prevalent in the endemic foci of visceral leishmaniasis in North-Western Iran and are representative of the sand fly diversity in this area.
Materials and Methods
Study Area
Sampling was carried out in August 2011 from rural regions of Kaleybar (38°58′ N 47°13′ E) and Bileh Savar (39°03′ N 48°31′ E) in Azarbaijan Sharqi and Ardabil provinces. These provinces are well-known endemic foci of visceral leishmaniasis in North-Western Iran. Five CDC miniature light traps were used in each sampling site and placed in houses, animal shelters, yards and rodents’ burrows. All light traps were sterilized by ethanol full spraying just before use and installed before sunset and remained functional throughout the night until the next morning.
Processing of Phlebotomine Sand Flies
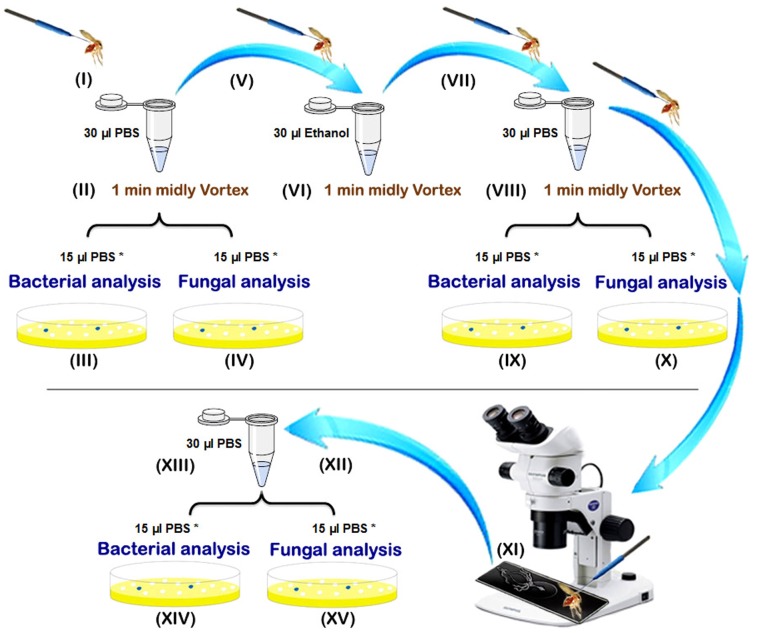
Sand flies caught alive were processed individually using sterile single-use materials and reagents as summarized in the Figure 1. Sand flies were killed using cold shock (I) and males and females, subdivided in unfed, fresh fed (having red fresh blood non digested in their gut), semi gravid (having some eggs and a part of digested dark red to brown blood in their gut) and gravid specimens (with an abdomen full of eggs), were processed individually according the following protocol. Each individual sand fly was placed in a 1.5 ml microtube containing 30 µl PBS (Phosphate Buffered Saline). Samples were mildly vortex for 1 minute (II) and then 15 µl of the PBS was taken for bacterial isolation (III) and another 15 µl of the PBS for fungal characterization (IV) of the cuticle microflora. Each sand fly specimen was then transferred using sterile entomological micro needles to a new sterile microtube containing 30 µl absolute ethanol (V). After 1 min of mild vortex mixing (VI), the sand fly specimens were transferred to a new sterile microtube and washed with 30 µl of PBS (VII). After vortexing as previously described (VIII), 15 µl of the PBS was used for bacterial anlysis (IX) and 15 µl for fungal analysis (X) in order to check the sterilization of the cuticle. The sand flies were then removed from the microtubes and dissected under a stereomicrosope on a sterile microscopic slide with a drop of sterile PBS using sterile entomological micro needles. After the sand fly’s head was removed, the digestive tract was isolated on the microscopic slide (XI), washed with 30 µl PBS into a new sterile microtube (XII), and crushed using a glass pestle (XIII). Then, 15 µl of the PBS was used for bacterial analysis (XIV) and 15 µl for fungal analysis (XV) of the gut microflora.
Figure 1. Preparation steps of the sand fly processing for fungal and bacterial analyses.
*: include primary volume (15 µl) which were diluted up to 100 µl for microbial assessments.
Mycological Identification
The PBS extracts from the cuticle and gut (IV and XV) were diluted with PBS to give a final volume of 100 µl and these diluted solutions were spread on to Sabouraud Dextrose Agar (Peptone 1%, Glucose 2%, Agar-agar 1.5%; Merck, Germany) and Potato Dextrose Agar (Potato infusion 20%, Dextrose 2%, Agar 2%) plates and incubated for 2 weeks at 25°C. The plates were periodically checked for fungal colonies. Identification of fungal isolates was performed according to a combination of macro- and microscopic morphology. Yeasts were identified by the use of the chromogenic medium Chromagar Candida ID2 (BioMérieux, France).
Bacterial Identification
The 15 µl PBS solutions from steps III and XIV were also diluted to a final volume of 100 µl by the addition of PBS. Fifty µl of each were then used for a bacterial colony count assessment. To determine the number of colony forming units (CFU), the samples were serially diluted 10 times (from 11 to 110) and aliquots of 100 µl were transferred on the PCA: Plate Count Agar (105463, E. Merck Co.; Darmstadt, Germany). Plates were then incubated at 35°C for 48 h. Total colony counts were recorded for each dilution and the average for every sample was calculated.
The remaining 50 µl of each starting extract was then transferred into BHI broth (Brain Heart Infusion broth) medium and incubated at 37°C for 24 hours. Then, bacteria were plated on BHI agar, XLD (Xylose-Lysine-Desoxycholate), Hektoen enteric agar, MacConkey agar and blood agar media containing Amphotericin B (2 µg/ml) and incubated at 37°C for 24–48 hours.
The initial identification of bacterial species was based on the colony characteristics (involving colony size, shape, color, margin, opacity, elevation and consistency) and the morphology of isolates based on Gram’s staining procedure.
Finally, the API identification kit (API 20E, BioMerieux) was used for final identification of Gram-negative bacteria. The identification of Gram-positive bacteria was performed using the API Staph, API 20 Strep and API50CH B following the manufacturer’s recommendations.
Statistical Analysis
Pearson Chi-Square and Scheffe’s tests based on the colony counting was performed using SPSS software ver. 18 to detect statistical differences in bacterial populations isolated from cuticle and midgut of (i) P. papatasi versus all other species using Pearson Chi-Square test and (ii) to compare males with unfed females, unfed females with freshly fed females, freshly fed females with semi-gravid females and semi-gravid females with gravid females using Scheffe’s test.
Results
Prevalence of Micro-organisms in Field Caught Sand Flies
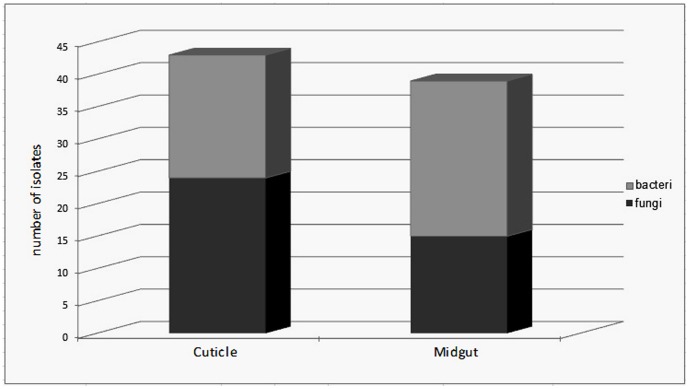
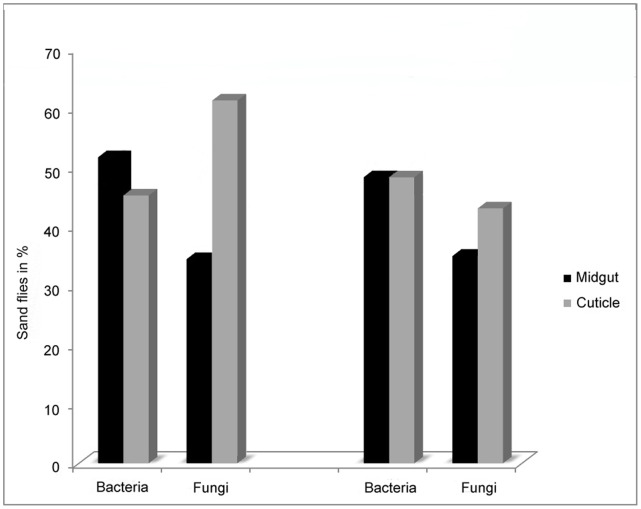
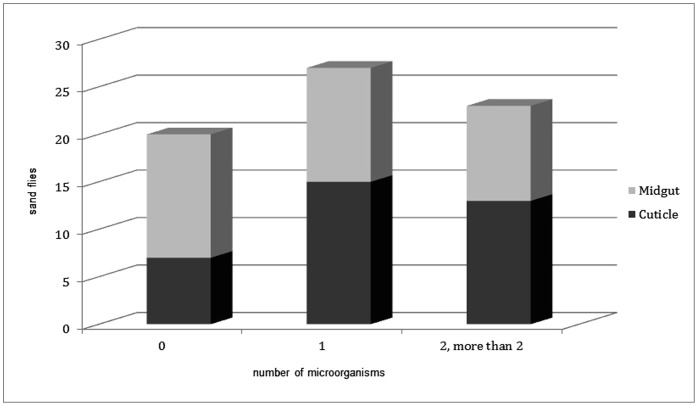
The aim of the study was to investigate the diversity of the bacterial and fungal strains that Phlebotomine sand flies carry with a culture dependent method. A total of 35 sand flies, 9 males and 26 females, belonging to five species (P. papatasi. P. sergenti, P. kandelakii, P. perfiliewi and P. halepensis) were collected. Sand flies were trapped in two different habitats, animal shelter (21) and outdoors (14). Samples were dissected and microorganisms were collected according to the procedure described in Figure 1. The sterilization efficiency was controlled during the whole procedure, see step VIII in Figure 1 and all samples correctly controlled (without any bacterial and fungal growing) for this step were included in the study and processed further. This methodology allowed us to readily isolate and identify the microorganisms present on the cuticle of the insect and those colonizing their midgut. Among the 35 processed sand flies, only 4 of them (3 males and 1 female) were negative for both bacteria and fungi on their cuticle or in their midgut. Among the 31 sand flies that bore microorganisms, slightly more microorganisms could be isolated from the cuticle than from their midgut. Interestingly, fungi were isolated more frequently on the cuticle than in the midgut and conversely bacteria were isolated more frequently from midgut than from cuticle (see Figure 2). Surprisingly, 37% of the midgut samples obtained from P. papatasi carried at least one fungal species whereas 63% of the cuticle samples originating from P. papatasi bore at least one fungal species. Such differences were not observed for the cuticle nor when looking at the presence of bacteria on the cuticle or in the sand fly midgut (see Figure 3). No differences were observed between males and females (data not shown), although this can not be statistically evaluated, because of the low number of male samples processed (9) as compared to females (26). The proportion of sand flies carrying no, one or multiple microorganisms (bacteria and/or fungi) in their midgut appears to be roughly the same (see Figure 4). By contrast, we observed that mono infection by bacteria and/or fungi is more frequent than no or multiple infection at the level of their cuticle. Finally, among the processed females, more bacterial strains are present on the cuticle and in the midgut of gravid compared to other physiological stages. The prevalence of bacteria increased progressively (except on the cuticle of semi-gravid females) with the advancement of the gonotrophic cycle (see Table 1).
Figure 2. Mean number of bacteria and fungi isolated from cuticle or midgut of sand flies.
These microorganisms were processed as described in the material and methods and the diversity of bacteria and fungi was ascertained by culture dependent methods.
Figure 3. Frequency of bacterial or fungal isolation from the midgut or the cuticle of sand flies.
P. papatasi appears on the left panel, and of the other species tested, on the right panel (i. e. P. sergenti, P. perfiliewi, P. kandelakii and P. halepensis).
Figure 4. Mean number of sand flies carrying no, one or multiple microorganisms on their cuticle or midgut.
Table 1. Synthesis of the average number of bacteria (plain) and fungal (bold) strains from the cuticle and the midgut of sand flies, according to sex, species and habitats.
| Species | County | Habitat | No. | Cuticle | ||||
| M | F | |||||||
| UnFed | Freshly Fed | Semi Gravid | Gravid | |||||
| P. papatasi | Bileh Savar(Goon Papagh) | A.Sh | 3 | neg.neg. | neg. Penicillium sp.1 | Pantoea sp.(−)neg. | ||
| Outdoor | 3 | Bacillus subtilis (+)neg. | neg. Penicillium sp.1 Aspergillus terreus | Micrococcus luteus (+) Aspergillus sp. | ||||
| Bileh Savar(Gog Tapeh) | A.Sh | 3 | neg.Penicillium sp.1 | Serratia marcescens (−) neg. | neg.Aspergillus nidulans | |||
| Outdoor | 3 | Bacillus subtilis (+)Fusarium sp. | neg.neg. | Klebsiella ozaenae (−)neg. | ||||
| Kaleybar(Khane Khosro) | A.Sh | 4 | neg.Penicillium sp.1 A spegillus fumigatus | Acinetobacter sp. (−)Klebsiella ozaenae (−) neg. | neg. Penicillium sp.2 | Pseudomonas fluorescens (−) Penicillium sp.2 | ||
| Outdoor | 3 | Micrococcus luteus (+) Acremonium sp. | neg. Aspergillus flavus | neg. Penicillium sp.1 | ||||
| P. sergenti | Bileh Savar(Goon Papagh) | A.Sh | 1 | Pseudomonas fluorescens (−)Micrococcus luteus (+) neg. | ||||
| Outdoor | 1 | Asaia sp. (−) Penicillium sp.1 Aspergillus sp. | ||||||
| Bileh Savar(Gog Tapeh) | A.Sh | 1 | neg. neg. | |||||
| Kaleybar(Khane Khosro) | A.Sh | 1 | Serratia marcescens (−)Aspergillus sp. Geotrichum sp. | |||||
| P. kandelakii | Kaleybar(Khane Khosro) | A.Sh | 1 | Serratia marcescens (−)neg. | ||||
| Outdoor | 1 | neg.Penicillium sp.1 | ||||||
| P. perfiliewi | Bileh Savar(Goon Papagh) | A.Sh | 2 | neg.neg. | Serratia marcescens (−) Penicillium sp.3 | |||
| Bileh Savar(Gog Tapeh) | Outdoor | 1 | neg. Penicillium sp.1 Aspergillus flavus | |||||
| Kaleybar(Khane Khosro) | A.Sh | 1 | Pseudomonas fluorescens (−) neg. | |||||
| P. halepensis | Bileh Savar(Goon Papagh) | A.Sh | 1 | neg. Acremonium sp. | ||||
| Bileh Savar(Gog Tapeh) | Outdoor | 1 | neg.neg. | |||||
| Kaleybar(Khane Khosro) | A.Sh | 3 | neg.neg. | Bacillus subtilis (+)neg. | Serratia marcescens (−) Penicillium sp.3 | |||
| Outdoor | 1 | neg. neg. | ||||||
| Total | 35 | 0.560.44 | 0.330.33 | 0.50 0.67 | 0.43 0.57 | 0.570.71 |
neg.: negative M: Male F: Female UF: UnFed FF: Freshly Fed SG: Semi Gravid G: Gravid A.sh: Animal shelter (+): Gram-positive bacteria (−): Gram-negative bacteria.
Colony forming unit.
Mean Number of Bacterial Colonies Per Individuals
The individual results are presented in Table 1. Because the number of sampled sand flies is not in equilibrium (include more P. papatasi than all other species), we have analyzed the data in two groups: P. papatasi (n = 19) and species belonging to another species (n = 16).
First of all, cuticle and midgut isolated from P. papatasi produced a significantly, lower number of colonies than the other species for cuticle (<chi>2 = 1592, P<0.05) and midgut (<chi>2 = 11500, P<0.05) respectively.
In addition, the average number of colonies obtained from cuticle of P. papatasi was higher in females than males and depended on the physiological state of the insects, the semi gravid females producing a higher number of colonies (2.6×102 for the males and 2.6×103 for the freshly fed females, 2×104 for the semi-gravid females and 2.6×104 for the gravid ones). The same trends were observed for the other species (mean = 3×102 for the males, 3.5×102 for the unfed females, 1×103 for the freshly fed females, 1.7×104 for the semi-gravid females and 3×104 for the gravid ones). A similar observation performed on the midgut of P. papatasi showed that the numbers of colonies were: 1.5×102 for the males, 3×102 for the unfed females, 2.7×103 for the freshly fed females, 1.8×104 for the semi-gravid females and 2×105 for the gravid ones. By comparison the colonies obtained for the other species were: 1.1×103 for the males, 3×103 for the unfed females, 3×103 for the semi-gravid females and 8.7×104 for the gravid ones. Differences in quantities of bacteria in males and the different physiological stages of females were also analyzed using Scheffe’s test. There was no significant differences in the numbers of colonies derived from the cuticle of P. papatasi and other groups for males-unfed females, unfed-freshly fed physiological stages (P>0.0125) whereas they were significant for freshly fed-semi gravid and semi gravid-gravid (P<0.0125).
There were no significant differences in bacterial colonies isolated from the midgut for males-unfed, unfed-freshly fed, freshly fed-semi gravid physiological stages (P>0.0125) and only it was significant between semi gravid and gravid females (P<0.0125).
It seems therefore that species, sex and physiology have some influences on the bacterial colonization of the cuticle and the midgut of sand flies.
Diversity of Micro-organisms Isolated
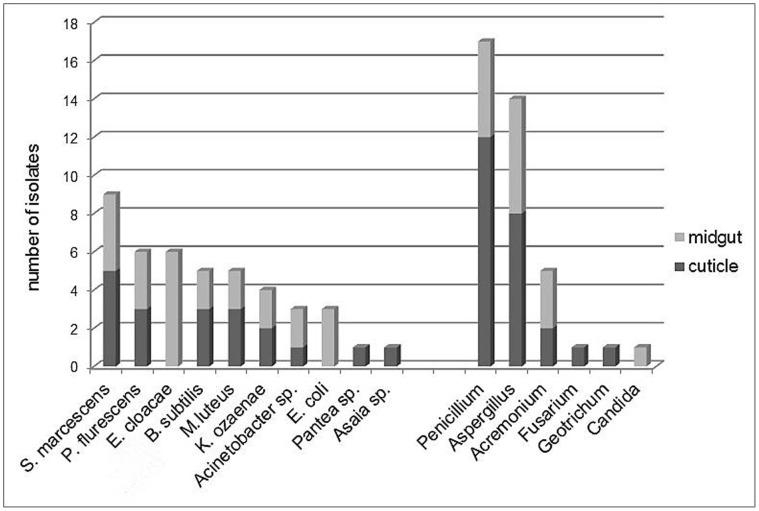
A total of 39 fungal strains belonging to six genera and 43 bacterial strains were isolated from the 35 processed Phlebotomine sand flies. Penicillium (17 isolates) and Aspergillus (14 isolates) occurred more frequently in sand flies than the other genera that were present, i.e. Acremonium (5 isolates), and Fusarium (1 isolate), Geotrichum (1 isolate) and Candida (1 isolate) (Figure 5).
Figure 5. Origin and frequency of isolation of the different microorganisms.
The Penicillium genera isolated in the present study showed different morphological features, suggesting that three different species were present but we were unable to identify at the species level. Consequently, we called them Penicillium sp.1, Penicillium sp.2 and Penicillium sp.3 respectively.
The bacterial isolates corresponded to ten bacterial taxa: Serratia marcescens (9 isolates), Pseudomonas fluorescens (6 isolates), Klebsiella ozaenae (4 isolates), Acinetobacter sp. (3 isolates), Bacillus subtilis (5 isolates) and Micrococcus luteus (5 isolates) isolated from both cuticle and midgut. Enterobacter cloacae (6 isolates) and Escherichia coli (3 isolates) as well as Candida albicans were isolated only from midgut whereas some other were specifically isolated from the cuticle like the bacteria belonging to Asaia sp. (1 isolate) and Pantea sp. (1 isolate) as well as the fungi including Geotrichum and Fusarium (see Figure 5).
Discussion
In Phlebotomine sand flies, studies carried out on the gut flora of the wild or laboratory reared Lutzomyia longipalpis, P. papatasi, P. tobbi, P. argentipes, P. duboscqi and Sergentomyia spp. have demonstrated the presence of huge diversity of bacterial strains that belong to the genera Acinetobacter, Bacillus, Brevibacterium, Burkholderia, Cellulomonas, Chloroflexi, Citrobacter, Enterobacter, Escherichia, Flavimonas, Gordonia, Klebsiella, Maltophila, Microbacterium, Micrococcus, Morganella, Ochrobactrum, Oligella, Pantoea, Pseudomonas, Serratia, Shigella, Sphingobacterium, Staphylococcus, Stenotrophomonas, Streptococcus and Weeksella [14], [15], [16], [17], [19], [20], [25].In agreement with these investigations, bacteria belonging to the genera Acinetobacter, Bacillus, Enterobacter, Escherichia, Klebsiella, Micrococcus, Pseudomonas and Serratia were isolated from our samples. E. cloacae being the most common bacteria isolated from the sand flies’ gut. This result is in agreement with other studies carried out on sand flies [15], [16], [17], [19], [20], [26,]. Asaia sp. that has never been previously reported from sand flies has been isolated in the present study. We also report the isolation of K. ozaenae from both cuticle and midgut of four females. This bacterium was previously reported once from the midgut of Lutzomyia longipalpis, the vector of visceral leishmaniasis in the Americas [1].
Several studies have reported a higher prevalence of Gram-negative bacteria than Gram-positive ones in the gut of different vector insects [19], [20], [25], [27]. The lower prevalence of Gram-positive bacteria as compared to Gram-negative ones is due to antimicrobial activity against M. luteus and B. subtilis [20], [28] which make them less susceptible to colonization by Gram-positive bacteria. This is in agreement with our observation that the majority of the bacterial strains isolated in the present study were Gram-negative bacteria (76%) mainly Enterobacter, Serratia and Pseudomonas. Some studies have investigated a correlation between the presence of midgut bacteria and the development of parasites in flies. A high concentration of bacteria (mainly Gram-negative) in the midgut of mosquitoes as well as sand flies was reported to either completely or partly influence the development of parasites [2], [20], [29], [30]. In mosquitoes, a wide range of bacterial strains such as Serratia, Klebsiella, Acinetobacter, Micrococcus, Escherichia, Enterobacter, Micrococcus, Pseudomonas, Staphylococcus were pointed out as being symbionts in gut flora [31], [32], [33]. There is general agreement on the important role played by the gut microbial flora on the development of pathogens in the midgut of the insect. Wolbachia are common and widespread cytoplasmically inherited bacteria, found in reproductive tissues of arthropods, including Phlebotomine sand flies [34], [35]. Its interactions with its hosts are often complex and have evolved to be symbiotic rather than parasitic [34], [35], [36]. The lack of Wolbachia isolated in the present study might be due to the isolation and characterization methodology that we have used.
Several studies have reported the inhibitory activity of Gram-negative bacteria on the development of parasites in the mosquitoes’ gut [28], [31], [33], [37], [38], [39]. In sand flies, a study [19] reported a high prevalence of microbial infection in the digestive tract of laboratory reared Phlebotomus papatasi females and hypothesized they could have a negative effect on Leishmania transmission in endemic areas. However, these studies have not clearly identified the causal mechanisms explaining the impact of microbial infection on the intravectorial development of Leishmania. It is as yet impossible to know whether the microflora of sand flies could affect the development of Leishmania or not. Because of the low prevalence of Leishmania spp. in the sand flies’ gut, we could not explore this link in the present study but future studies on colonized species should be done to clarify the relationship. Interestingly, promastigotes of Leishmania in culture grow with difficulty when competing with bacteria. In the same way, bacteria can interfere with the development of promastigotes in the digestive tract of sand flies probably by competing for nutrients and reducing the pH [14]. In nature, despite the probable well-balanced associations between some bacteria and sand flies, there could be natural selective pressure involving some species of bacteria, Leishmania and their vectors.
Only a few publications that have aimed to identify fungal diversity are available and the majority of these are from work on mosquitoes [39], [40] only one [19] has been carried out on Phlebotomine sand flies. That study showed that only Aspergillus sclerotiorum and Saccharomyces cerevisiae were present in sand flies. In our study, we did not isolate and identify these two species, but we isolated and identified other species belonging to the Aspergillus genera (A. flavus, A. fumigatus, A. nidulans, A. terreus), Penicillium, Geotrichum, Fusarium, Acremonium and Candida. It was not possible for us to explain the role of these fungi which we isolated from the sand fly cuticle and/or midgut or reach any conclusion about any potential pathogenic effect or interaction with Leishmania. However, Adler and Theodor [41] and Schlein et al. [19] have proposed that female P. papatasi infected by fungal strains were significantly more resistant to Leishmania major infection.
The eggs of Phlebotomine sand flies are laid in soil that is rich in organic matter and the larvae pass through four instars in the soil before pupation and adult emergence. Consequently, the local soil environment and animal stools may play an important role in the colonization capacity of the sand flies, with microorganisms geographically specific encountered at the oviposition sites or during sugar meal feeding [42].
A large number of soil and environmental strains including Acinetobacter sp., Asaia sp., B. subtilis, M. luteus, P. fluorescens, Pantoea sp. and S. marcescens and also intestinal strains such as E. cloacae, E. coli and K. ozaenae were identified in the present study. These bacterial species belong to aerobic and facultative anaerobic Gram negative bacteria (Acinetobacter sp., Asaia sp., E. cloacae, E. coli, K. ozaenae, Pantoea sp., P. fluorescens and S. marcescens) and Gram positive bacteria (B. subtilis and M. luteus). According to the ecology of the bacteria and fungi isolated in the present study, it is probable that E. coli, Enterobacter, Klebsiella, Pantoea, S. marcescens or C. albicans, which all are intestinal microorganisms, have contaminated the sand flies during their larval stages. The telluric species like Acinetobacter, Asaia, Bacillus subtilis, M. luteus and Pseudomonas spp. could have contaminated the larvae, but also the adults in their resting places. The ubiquitous Aspergillus and Penicillium which are also telluric microorganisms, have probably contaminated adult sand flies.
According to our findings, the average number of counted bacterial strains in females with different physiological digestive stages has increased progressively from unfed to gravid females, like the prevalence of bacteria and fungi in the midgut as well as on the cuticle of female sand flies (Table 1). The same phenomenon has been also observed on mosquitoes [14], [20]. The authors of these studies suggested that the variation of the bacterial species in mosquitoes gut increased during the 24–48 hours following the blood feeding. This hypothesis could be applied to the Phlebotomine sand flies that we processed in this study. Another explanation could be that the life span of females is longer than that of males [35], [43]. Therefore, it seems probable that females may encounter more fungal and bacterial contaminants during their life span.
The present paper constitutes an interesting pilot study with new findings on the isolation of bacteria and fungi on the cuticle and in the gut of sand flies. It also suggests interesting trends like an increasing number of bacterial strains and colonies depending on the physiological stage of Phlebotomine sand flies. However, these data have to be confirmed in the future by further studies carried out on more specimens.
Acknowledgments
The authors are grateful to Parisa Rezanejad (Tehran University-Biostatistics Department) for her help in statistical analysis. They thank Sylvette Gobert (Reims University) and James Gordon Hamilton (Keele University) for proofreading this manuscript.
Funding Statement
No current external funding sources for this study.
References
- 1. Oliveira SMP, Moraes BA, Gonçalves CA, Giordano-Dias CM, D’almeida JM, et al. (2000) Prevalence of microbiota in the digestive tract of wild females of Lutzomyia longipalpis Lutz & Neiva, 1912 (Diptera: Psychodidae). Rev Soc Bras Med Trop 33: 319–322. [DOI] [PubMed] [Google Scholar]
- 2.WHO Media centre website. Available: http://www.who.int/entity/mediacentre/factsheets/fs117/en/index.html. Accessed 2012 Jan.
- 3. McCarthy CB, Diambra LA, Rivera Pomar RV (2011) Metagenomic Analysis of Taxa Associated with Lutzomyia longipalpis, Vector of Visceral Leishmaniasis, Using an Unbiased High-Throughput Approach. PLoS Negl Trop Dis 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Killick-Kendrick R, Wilkes TJ, Bailly M, Bailly I, Righton LA (1986) Preliminary field observations on the flight speed of a Phlebotomine sand fly. Trans R Soci Trop Med Hyg 80: 138–142. [DOI] [PubMed] [Google Scholar]
- 5. Ferro C, Pardo R, Torres M, Morrison A (1997) Larval microhabitats of Lutzomyia longipalpis (Diptera: Psychodidae) in an endemic focus of visceral leishmaniasis in Colombia. J Med Entomol 34: 719–728. [DOI] [PubMed] [Google Scholar]
- 6. Ireri LN, Kongoro J, Ngure P, Sum KS, Tonui W (2011) Insecticidal properties of Pyrethrin formulation against immature stages of Phlebotomine sand flies (Diptera: Psychodedae). J entomol 8: 581–587. [Google Scholar]
- 7. Schlein Y, Jacobson R, Muller GC (2001) Sand fly feeding on noxious plants: a potential method for the control of leishmaniasis. Am J Trop Med Hyg 65: 300–303. [DOI] [PubMed] [Google Scholar]
- 8. Samie M, Wallbanks KR, Moore JS, Molineux DH (1990) Glycosidase activity in the sand fly Phlebotomus papatasi . Comp Biochem Physiol 96: 577–579. [Google Scholar]
- 9. Schlein Y (1986) Sand fly diet and Leishmania . Parasitol Today 2: 175–177. [DOI] [PubMed] [Google Scholar]
- 10. SchleinY, Warburg A (1986) Phytophagy and the feeding cycle of Phlebotomus papatasi (Diptera: Psychodidae) under experimental conditions. J Med Entomol 23: 11–15. [DOI] [PubMed] [Google Scholar]
- 11. Rassi Y, Oshaghi MA, Mohammadi-Azani S, Abaie MR, Rafizadeh S, et al. (2011) Molecular Detection of Leishmania Infection Due to Leishmania major and Leishmania turanica in the Vectors and Reservoir Host in Iran. Vector Borne Zoonotic Dis 11: 145–150. [DOI] [PubMed] [Google Scholar]
- 12. Strelkova MV, Eliseev LN, Ponirovsky EN, Dergacheva TI, Evans DA (2001) Mixed leishmanial infections in Rhombomys opimus: a key to the persistence of Leishmania major from one transmission season to the next. Ann Trop Med Parasit 95: 811–819. [DOI] [PubMed] [Google Scholar]
- 13. Dillon RJ, Dillon VM (2004) The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol 49: 71–92. [DOI] [PubMed] [Google Scholar]
- 14. Dillion RJ, El Kordy E, Lane RP (1996) The prevalence of a microbiota in the digestive tract of Phlebotomus papatasi . Ann Trop Med Parasitol 90: 669–673. [DOI] [PubMed] [Google Scholar]
- 15. Gouveia C, Asensi MD, Zahner V, Rangel EF, Oliveira SM (2008) Study on the bacterial midgut microbiota associated to different Brazilian populations of Lutzomyia longipalpis (Lutz & Neiva) (Diptera: Psychodidae). Neotrop Entomol 37: 597–601. [DOI] [PubMed] [Google Scholar]
- 16. Hillesland H, Read A, Subhadra B, Hurwitz I, McKelvey R, et al. (2008) Identification of Aerobic Gut Bacteria from the Kala Azar Vector, Phlebotomus argentipes: A Platform for Potential Paratransgenic Manipulation of Sand flies. Am J Trop Med Hyg 79: 881–886. [PubMed] [Google Scholar]
- 17. Rajendran P, Modi GB (1982) Bacterial flora of sand fly gut (Diptera: Psychodidae). Indian J Public Health 26: 49–52. [PubMed] [Google Scholar]
- 18. Rodrigo S, Salvatore T (2003) Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae): a review. An Acad Bras Cienc 75: 301–330. [DOI] [PubMed] [Google Scholar]
- 19. Schlein Y, Polacheck I, Yuva B (1985) Mycoses, bacterial infections and antibacterial activity in sand flies (Psychodidae) and their possible role in the transmission of leishmaniasis, Parasitology. 90: 57–66. [DOI] [PubMed] [Google Scholar]
- 20. Volf P, Kiewengova A, Nemec A (2002) Bacterial colonization in the gut of Phlebotomus duboscqi (Diptera: Psychodidae): Transtadial passage and the role of female diet. Folia Parasitol 49: 73–77. [DOI] [PubMed] [Google Scholar]
- 21. Rassi Y, Javadian E, Nadim A, Zahraii A, Vatandoost H, et al. (2005) Phlebotomus (Larroussius) kandelakii the principal and proven vector of visceral leishmaniasis in north west of Iran. Pak J Biol Sci 8: 1802–1806. [Google Scholar]
- 22. Oshaghi MA, Maleki Ravasan N, Hide M, Javadian E, Rassi Y, et al. (2009) Phelebotomus perfiliewi transcaucasicus is circulating both Leishmania donovani and L.infantum in north-west Iran. Exp Parasitol 123: 218–25. [DOI] [PubMed] [Google Scholar]
- 23. Parvizi P, Mazloumi-Gavgani AS, Davies CR, Courtenay O, Ready PD (2008) Two Leishmania species circulating in the Kaleybar focus of infantile visceral leishmaniasis, northwest Iran: implications for deltamethrin dog collar intervention. Trans R Soc Trop Med Hyg 102: 891–7. [DOI] [PubMed] [Google Scholar]
- 24. Sanei Dehkordi A, Rassi Y, Oshaghi MA, Abai MR, Rafizadeh S, et al. (2011) Molecular Detection of Leishmania infantum in Naturally Infected Phlebotomus perfiliewi transcaucasicus in Bilesavar District, Northwestern Iran. Iran J Arthropod-Borne Dis 5: 20–27. [PMC free article] [PubMed] [Google Scholar]
- 25. Midori O, Braig HR, Munstermann L, Ferro C, O’neill S (2001) Wolbachia infections of Phlebotomine sand flies (Diptera : Psychodidae). Med Ent J 38: 237–241. [DOI] [PubMed] [Google Scholar]
- 26. Oliveira SMP, Moraes BA, Gonçalves CA, Giordano-Dias CM, D’almeida JM, et al. (1998) Prevalência da microbiota no trato digestivo de fêmeas de Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae) provenientes do campo. Rev Soc Bras Med Trop 33: 319–322. [DOI] [PubMed] [Google Scholar]
- 27. DeMaio J, Pumpuni CB, Kent M, Beier JC (1996) The midgut bacterial flora of wild Aedes triseriatus, Culex pipens and Psorophora columbiae mosquitoes. Am J Trop Med Hyg 54: 219–223. [DOI] [PubMed] [Google Scholar]
- 28.Kiewegova A (1999) Antimikrobialni activity Phlebotomus duboscqi. Unpubl M.Sc. Thesis, Charles University, Prague, 81pp.
- 29. Guernaoui S, Garcia D, Gazanion E, Ouhdouch Y, Boumezzough A, et al. (2011) Bacterial flora as indicated by PCR-temperature gradient gel electrophoresis (TGGE) of 16S rDNA gene fragments from isolated guts of Phlebotomine sand flies (Diptera: Psychodidae). J Vec Ecol 36: 144–147. [DOI] [PubMed] [Google Scholar]
- 30.Tanada Y, Kaya HK (1993) Associations between insects and nonpathogenics microorganisms. In Y. Tanada & H.K. Kaya (eds.), Insect pathology, p.12–51. Academic Press, New York, 666p.
- 31. Gonzalez-Ceron L, Santillan F, Rodríguez MH, Mendez D, Hernández-Avila JE (2003) Bacteria in midguts of field collected Anopheles albimanus block Plasmodium vivax sporogonic development. J Med Entomol 40: 371–4. [DOI] [PubMed] [Google Scholar]
- 32. Pumpuni CB, Beier MS, Nataro JP, Guers LD, Davis JR (1993) Plasmodium falciparum - Inhibition of sporogonic development in Anopheles stephensi by Gram-negative bacteria. Exp Parasitol 77: 195–199. [DOI] [PubMed] [Google Scholar]
- 33. Seitz HM, Maier WA, Rottok M, Becker-Feldmann H (1987) Concomitant infections of Anopheles stephensi with Plasmodium berghei and Serratia marcescens: additive detri-mental effects. Zentralbl Bakteriol Mikrobiol Hyg 266: 155–166. [DOI] [PubMed] [Google Scholar]
- 34. Benlarbi M, Ready PD (2003) Host-specific Wolbachia strains in widespread populations of Phlebotomus perniciosus and P. papatasi (Diptera: Psychodidae) and prospects for driving genes into these vectors of Leishmania. . Bull Entomol Res 93: 383–391. [DOI] [PubMed] [Google Scholar]
- 35. Merritt RW, Dadd RH, Walker ED (1992) Feeding-behavior, natural food, and nutritional relationships of larval mosquitos. Annu Rev Entomol 37: 349–376. [DOI] [PubMed] [Google Scholar]
- 36. Werren JH, Guo L, Windsor DW (1995) Distribution of Wolbachia among neotropical arthropods. Proc R Soc London Ser 262: 197–204. [Google Scholar]
- 37. Aguilar R, Jedlicka AE, Mintz M, Mahairaki V, Scott AL, et al. (2005) Global gene expression analysis of Anopheles gambiae responses to microbial challenge. Insect Biochem Mol Biol 35: 709–19. [DOI] [PubMed] [Google Scholar]
- 38. Jadin J, Vincke IH, Dunjic A, Delville JP, Wery M, et al. (1966) Role of Pseudomonas in the sporogenesis of the hematozoon of malaria in the mosquito In French. Bull Soc Pathol Exot Filiales 59: 514–525. [PubMed] [Google Scholar]
- 39. Beier MS, Pumpuni CB, Beier JC, Davis JR (1994) Effects of para-aminobenzoic acid, insulin and gentamycin on Plasmodium falciparum development in Anopheline mosquitoes (Diptera: Culicidae). J Med Entomol 31: 561–565. [DOI] [PubMed] [Google Scholar]
- 40. St. Leger RJ, Screen SE, Shams-Pirzadeh B (2000) Lack of Host Specialization in Aspergillus flavus . Appl Environ Microbiol 66: 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adler S, Theodor O (1929) Attempts to transmit Leishmania tropica: the transmission of L. tropica by Phlebotomus sergenti . Ann Trop Med Parasitol 23: 1–18. [Google Scholar]
- 42. Pavlovich SG, Rockett CL (2000) Color, bacteria, and mosquito eggs as ovipositional attractants for Aedes aegypti and Aedes albopictus (Diptera: Culicidae). Great Lake Entomol 33: 141–153. [Google Scholar]
- 43. Addis CJ (1945) Laboratory rearing and life cycle of Phlebotomus (Dampfomyia) anthophorus Addis (Diptera: Psychodidae). J Parasitol 31: 319–322. [Google Scholar]