Abstract
Techniques of compartmental (efflux) and kinetic influx analyses with the radiotracer 13NH4+ were used to examine the adaptation to hypoxia (15, 35, and 50% O2 saturation) of root N uptake and metabolism in 3-week-old hydroponically grown rice (Oryza sativa L., cv IR72) seedlings. A time-dependence study of NH4+ influx into rice roots after onset of hypoxia (15% O2) revealed an initial increase in the first 1 to 2.5 h after treatment imposition, followed by a decline to less than 50% of influx in control plants by 4 d. Efflux analyses conducted 0, 1, 3, and 5 d after the treatment confirmed this adaptation pattern of NH4+ uptake. Half-lives for NH4+ exchange with subcellular compartments, cytoplasmic NH4+ concentrations, and efflux (as percentage of influx) were unaffected by hypoxia. However, significant differences were observed in the relative amounts of N allocated to NH4+ assimilation and the vacuole versus translocation to the shoot. Kinetic experiments conducted at 100, 50, 35, and 15% O2 saturation showed no significant change in the Km value for NH4+ uptake with varying O2 supply. However, Vmax was 42% higher than controls at 50% O2 saturation, unchanged at 35%, and 10% lower than controls at 15% O2. The significance of these flux adaptations is discussed.
More than 70% of the world′s rice (Oryza sativa L.) is produced in intensively cultivated, irrigated lowland systems in Asia (International Rice Research Institute, 1997). In these systems N is generally the main factor limiting the realization of yield potentials (Kropf et al., 1993; Cassman et al., 1997). As a consequence, large amounts of mineral N fertilizers are used. According to one estimate, 7 × 106 metric tons of N is applied each year to the 74 × 106 ha of irrigated rice in Asia (Cassman and Pingali, 1995). However, unless the application of N fertilizer is timed precisely to match plant demand (Cassman et al., 1998), less than 50% of fertilizer N is usually recovered by the crop, because of high rates of loss through ammonia volatilization and denitrification (Craswell and Vlek, 1979; Vlek and Byrnes, 1986; Cassman et al., 1993). Clearly, the capacity of the root system to capture N in competition with these processes is critical. Mathematical modeling of the uptake process (Kirk and Solivas, 1997) shows that, under typical field conditions and following the initial flush of available N after fertilization, N absorption from the soil is rate limiting.
In flooded lowland rice soils, where the bulk of the soil is hypoxic to anaerobic, the main form of plant-available N is NH4+ (Sasakawa and Yamamoto, 1978; Yu, 1985). This is in marked contrast to most (aerobic) agricultural soils, where NO3− is the predominant inorganic N species (Kronzucker et al., 1995b). There have been reports that NH4+ is the preferred N species taken up by rice (Bonner, 1946; Fried et al., 1965; Shen, 1969; Dijkshoorn and Ismunadji, 1972a, 1972b; Yoneyama and Kumazawa, 1974, 1975; Sasakawa and Yamamoto, 1978; Ancheng et al., 1993; Wang et al., 1993a, 1993b) and that NH4+ is superior to NO3− in terms of fertilizer efficiency (Craswell and Vlek, 1979). Information regarding NH4+ uptake capacity and affinity under hypoxic conditions is scarce, however (Sasakawa and Yamamoto, 1978; Youngdahl et al., 1982; Wang et al., 1993b). It is not known how intracellular compartmentation and metabolic processing of NH4+ are affected by lowered O2 tensions (Wang et al., 1993a). With the goal of developing new rice varieties, which might be more efficient in N extraction from paddy soils (Kirk and Kronzucker, 1998), information concerning N uptake and metabolism under more realistic conditions is needed. In this paper we report a study of the adaptation of flux parameters for NH4+ to hypoxic growth conditions in roots of rice, using 13NH4+ as a tracer and combining techniques of kinetic flux and compartmental analyses.
MATERIALS AND METHODS
Plant Growth Conditions
Rice (Oryza sativa L., cv IR72) seeds were surface sterilized in 5% NaOCl for 10 min, then rinsed several times with deionized water, and left to soak in aerated, deionized water at 30°C in a water bath for 48 h. The partially germinated seeds were then placed onto plastic mesh mounted on Plexiglas discs (Rhom Co., Ltd., New York). The discs were transferred to 40-L hydroponic Plexiglas tanks (see below) located in walk-in controlled-environment growth chambers. The growth chambers were maintained at 30 ± 2°C and 70% RH and set to a 12-h day/12-h night photoperiod. A photon flux of approximately 500 μmol m−2 s−1 measured at plant level (with an LI-189 light meter and an LI-190SA quantum sensor, Li-Cor, Lincoln, NE) was provided by fluorescent lamps (215 W, 1500, F96T12/CW/VHO, Philips, Mahwah, NJ).
Nutrient Solutions
Rice seedlings were cultivated in hydroponic medium contained in 40-L Plexiglas tanks. Deionized, distilled water and reagent-grade chemicals were used in the preparation of all nutrient solutions. NH4+ was provided as the only source of N in the form of (NH4)2SO4. Other nutrient salts added were as follows: K2SO4 (1 mm), MgSO4 (2 mm), CaCl2 (1 mm), NaH2PO4 (300 μm), Fe-EDTA (100 μm), MnCl2 (9 μm), (Na)6Mo7O24 (25 μm), H3BO3 (20 μm), ZnSO4 (1.5 μm), and CuSO4 (1.5 μm). The complete solution was maintained from germination onward.
Nutrient solutions in tanks were continuously mixed via electric circulating pumps (circulator model IC-2, Brinkmann). Continuous infusion of the concentrated nutrient stock solution via peristaltic pumps (Technicon Proportioning Pump II, Technicon Instrument, Tarrytown, NY) allowed steady-state control of nutrient concentrations in the tanks. Solutions were checked daily for [NH4+], measured using a Philips PU 8820 UV/VIS spectrophotometer, according to the method of Solorzano (1969); [K+], measured flame-photometrically (using an Instrumentation Laboratory Photometer, model 443, Lexington, MA); pH, measured with a microprocessor-based pocket-sized pH meter (pH Testr2 model 59000–20; Cole Parmer, Chicago, IL) and maintained at 6.5 ± 0.3 by addition of powdered CaCO3; and [O2], measured using a biological O2 monitor (YSI model 53, Yellow Springs Instruments, Yellow Springs, OH) equipped with an O2 electrode (YSI 5331 Oxygen Probe, Yellow Springs Instruments). Nutrient solutions were degassed prior to filling of the tanks. O2 concentrations of 7.5, 3.75, 2.6, and 1.1 μg mL−1 were maintained by infusion of N2 gas (Praxair, Mississauga, Ontario, Canada) via aquarium stones placed at various solution depths (5, 10, and 15 cm; tanks were covered and the overall solution depth was 17 cm). The minimum O2 concentration attainable with this method was 1.1. μg mL−1 (15% of saturation).
Measurement of Fluxes
The radiotracer 13N (half-life = 9.96 min) was produced by the Tri-University Meson Facility cyclotron at the University of British Columbia (Vancouver, Canada) by proton irradiation of water. This procedure produced mostly 13NO3−, with high radiochemical purity (Kronzucker et al., 1995b). The irradiated solutions (approximately 700–740 MBq) were supplied in sealed 20-mL glass vials. Procedures for the removal of radiocontaminants and conversion of 13NO3− to 13NH4+ using Devarda's alloy were as described in detail elsewhere (Kronzucker et al., 1995a, 1995b, 1995c). A volume of 20 to 100 mL of 13NH4+-containing stock solution was prepared in a fumehood and was transferred to the controlled-environment chambers where experiments were carried out. All uptake solutions were premixed, and, in influx experiments, these were contained in individual 500-mL plastic vessels behind lead shielding. The chemical composition of the uptake solution was identical to the growth solution in the hydroponic tanks (see above) and contained NH4+ at the desired concentrations (Figs. 2 and 3). Tracer was then added by syringe to the individual uptake vessels.
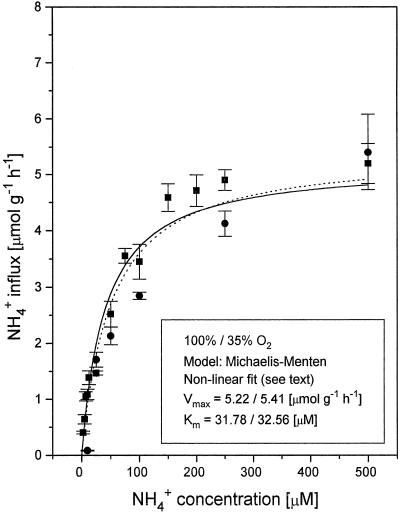
Figure 2.
Steady-state NH4+ influx into roots of intact cv IR-72 rice seedlings as a function of [NH4+]o in the high-affinity transport range (2.5–500 μm) at 100 and 35% O2. ▪ (solid line for isotherm fit), Control plants at 100% O2 provision; and • (dotted line for isotherm fit), plants exposed to 35% O2 for 7 d prior to the influx determinations. Kinetic analysis according to Cornish-Bowden and Wharton (see text) was used for the derivation of Vmax and Km values. Data are means ± se (n ≥ 12).
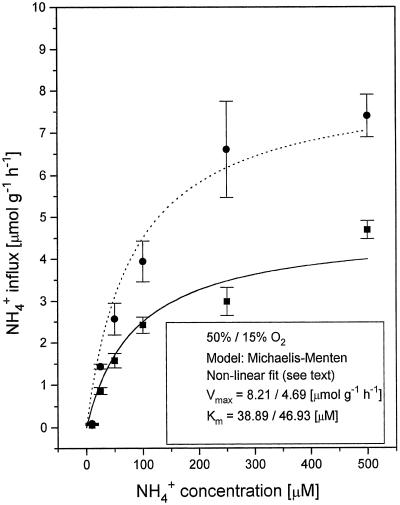
Figure 3.
Steady-state NH4+ influx into roots of intact cv IR-72 rice seedlings as a function of [NH4+]o in the high-affinity transport range (2.5–500 μm) at 50 and 15% O2. • (dotted line for isotherm fit), Plants exposed to 50% O2; and ▪ (solid line for isotherm fit), plants exposed to 15% O2. See Figure 2.
At the start of influx experiments, rice seedlings were transferred from the hydroponic growth tanks to prewash solutions in 1-L vessels for 5 min prior to immersion of the intact seedling roots in the labeled uptake solutions. This protocol minimized perturbation and allowed the roots to equilibrate to the exact solution temperature and to the solution composition used during influx. The roots were then exposed to tracer for 10 min. Immediately following the 10 min of isotope loading, roots were dipped into nonlabeled solutions for 5 s to minimize carry-over of label by the root surface to the desorption solution. Roots were then desorbed in unlabeled solution, which was otherwise chemically identical, for 3 min to desorb 13NH4+ contained in the Donnan free space. The duration of these steps was based on the t1/2 of NH4+ for the root surface, the Donnan free space, and the cytoplasm, as determined by efflux analysis (see below; Kronzucker et al., 1995c, 1995e). An exposure time of 10 min to 13NH4+ was chosen, since the contribution of tracer efflux from the cytoplasm can be expected to be negligible during this time (Kronzucker et al., 1995d; A.D.M. Glass, H.J. Kronzucker, and M.Y. Siddiqi, unpublished results).
Following desorption, seedling roots were excised from the shoots, the roots were spun in a low-speed centrifuge for 30 s to remove surface liquid, and the fresh weights of roots and shoots were determined. The plant organs were then introduced into 20-mL scintillation vials, and the radioactivities of roots and shoots were determined in a γ-counter (Minaxi δ, series Auto-γ 5000, Packard, Meriden, CT), measuring the 511-kV positron-electron annihilation radiation generated by recombination of ambient electrons and β+ particles emitted from 13N. Using the specific activity (13N/[13N + 14N] [disintegrations per micromole]) of the loading solution and the total fresh root weight of each seedling, we calculated NH4+ fluxes and expressed the results in micromoles per gram fresh weight per hour.
Efflux experiments were performed essentially as described elsewhere (Kronzucker et al., 1995b, 1995d, 1995e) under the same conditions as the influx experiments. Roots of intact rice seedlings were immersed for 45 to 60 min in 120-mL darkened plastic beakers containing the 13NH4+-labeled solution. Steady-state conditions, with respect to all nutrients as well as O2 tensions, were maintained throughout growth, loading, and elution. A 60-min loading period was chosen on the basis of the t1/2 for the cytoplasmic phase being approximately 14 min (see “Results and Discussion”). Therefore, 60 min of exposure to tracer ensured that the specific activity of the cytoplasm was approximately 95% of that in the loading solution (Kronzucker et al., 1995e). Following loading with 13NH4+, seedlings were transferred to “efflux funnels” (Wang et al., 1993a), and the roots were eluted with 20-mL aliquots of nonradioactive solution after varying intervals. These intervals ranged from 5 s to 2 min, over an experimental duration of 22 min. Eluates from a total of 25 intervals were collected separately, and the radioactivities of 20-mL samples from each eluate were determined (using a Minaxi δ counter, series Auto-γ 5000). After the final elution, roots and shoots were excised, introduced into scintillation vials and also counted for γ activity.
Data Analysis
All experiments were replicated three to five times. Each experimental treatment consisted of four replicates for influx experiments and two replicates for efflux experiments. Data from several experiments were pooled (n ≥ 6 in efflux experiments; n ≥ 12 in influx experiments) for calculations of means and ses. These values were used for plotting time-dependence curves and uptake isotherms, as well as for calculating Vmax and Km values. The least-squares method by Cornish-Bowden and Wharton, based on the Michaelis-Menten equation, was used to obtain Vmax and Km estimates for the saturable high-affinity transport system isotherms (Kronzucker et al., 1995d, 1996). The calculation of t1/2, fluxes and pool sizes in efflux analyses were as described in detail elsewhere (Kronzucker et al., 1995a, 1995b, 1995c, 1995e). All fluxes are expressed in micromoles of NH4+ per gram root fresh weight per hour.
Symbols used for fluxes are as follows: φco, efflux from the cytoplasm, obtained from the rate of 13N release from the cytoplasm at time 0 divided by the specific activity of the loading solution; φnet, net flux, obtained directly from the accumulation of 13N in the plants at the end of the loading period; φoc, unidirectional influx, calculated from φnet + φco; φxylem, flux of 13N to the shoot, obtained directly from count accumulation in the shoot at the end of the elution period; and φvac./ass., combined fluxes to ammonium assimilation and to the vacuole, resulting from φnet − φxylem.
RESULTS AND DISCUSSION
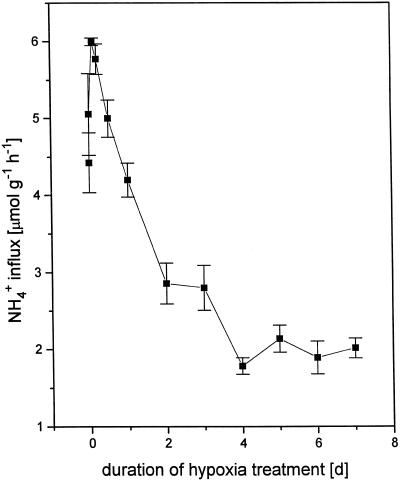
We found that NH4+ influx across the root plasmalemma responded within hours to the imposition of hypoxia (Fig. 1). Whereas in fully oxygenated plants NH4+ influx, measured at 100 μm [NH4+]o, was 4.31 (± 0.39) μmol g−1 h−1, an increase in influx of approximately 35% was apparent after 1 to 2.5 h of hypoxia (15% O2 saturation, i.e. approximately 1.1 μg mL−1). Even though evolutionary adaptation strategies to deal with restricted O2 supply in the rooting zone differ markedly between species and are poorly understood (Drew, 1990; Crawford, 1992), some rapid cellular responses appear to be universal. In particular, cytosolic acidification at the onset of hypoxia has been documented in several species, including rice (Roberts et al., 1985; Hoffman et al., 1986). This is believed to be due to lactic acid production preceding a switch to fermentative metabolism, as well as, in some cases, to proton leakage from the vacuole (Menegus et al., 1989, 1991). In species susceptible to damage from O2 deprivation, this cytoplasmic acidosis is pronounced (as much as 0.8 pH unit) and not fully reversible. By contrast, in hypoxia-tolerant plants, it is of a relatively lesser magnitude (≤ 0.4 pH unit in rice) and is followed by alkalinization of both the cytoplasm and the vacuole (Menegus et al., 1991). In fact, in rice cytosolic acidosis is complete after as little as 10 min of O2 withdrawal and is sustained for no more than 4 h (Menegus et al., 1991), i.e. within an interval of time corresponding to our observed up-regulation of NH4+ influx into rice roots.
Figure 1.
NH4+ influx into roots of intact cv IR-72 rice seedlings as a function of increasing time of O2 deprivation. Seedlings were cultivated hydroponically at 100 μm [NH4+]o for 3 weeks. Plants were exposed for varying periods to 15% O2 (growth and control at 100%). Data are means ± se (n ≥ 12).
Several reports suggest that in hypoxia-tolerant plants, cytosolic alkalinization following the initial acidosis appears to involve metabolic H+ consumption through glutamate and Arg decarboxylation, leading to the formation of either γ-aminobutyric acid or polyamines such as putrescine, respectively (Reggiani et al., 1989, 1990, 1993; Reggiani, 1994; Aurisano et al., 1995). Polyamines in turn have been shown to stimulate plasmalemma H+-ATPase activity (Reggiani et al., 1992). Thus, the observed higher N acquisition rates may be consistent with the N requirements associated with pH regulation during the first hours under conditions of O2 restriction. Also, higher NH4+ influx might meet the needs of an apparently generally increased N metabolism under O2-restriction conditions (Reggiani et al., 1988, 1989). Increased N acquisition might be operating in parallel to the documented N remobilization by degradation of storage proteins (Reggiani et al., 1988). Ultimately, i.e. under prolonged O2 stress, such up-regulation responses of N uptake must be compromised by restrictions in ATP supply (Reggiani et al., 1985).
In our study, following the initial increase, NH4+ influx declined to an apparent steady-state value of approximately 2 μmol g−1 h−1 by 4 d. To ensure steady-state conditions in subsequent kinetic experiments, 7 d of hypoxia pretreatment was therefore used prior to labeling with 13N.
From the kinetics of NH4+ influx at different O2 tensions (Figs. 2 and 3), it is evident that rice can maintain substantial influx of N even at 15% O2. Vmax for fully oxygenated plants was 5.22 μmol g−1 h−1 (±0.48), with a Km of 31.78 μm (±11.8). Km values did not change significantly with varying O2 supply; Vmax was unchanged at 35%, approximately 10% lower at 15% and 42% higher at 50% saturation. The increase of NH4+ influx observed at 50% O2 is interesting and appears to be another manifestation of increased N demand under O2 stress, realized in up-regulated N uptake. Apparently, below 35% O2 rice roots are no longer able to up-regulate NH4+ influx. However, N acquisition rates remain considerable. The maintenance of appreciable N uptake rates in deoxygenated hydroponic systems has been reported previously for Japonica rice (Sasakawa and Yamamoto, 1978).
Compartmental analysis (efflux analysis) was used in the present study to examine in greater detail the adaptation of component fluxes and subcellular compartmentation of NH4+ to hypoxia over a period of 5 d. Experiments were conducted at 100 μm [NH4+]o and with hypoxia treatment at 15% O2 imposed for 0, 1, 3, and 5 d. Efflux analysis requires that plants be at a quasi-steady state with respect to ambient conditions and that the plant's physiological status should not change during the experimental probing. Thus, we could not examine the effects of very short times of exposure to lowered O2 tensions via efflux analysis (Kronzucker et al., 1995a). Semilogarithmic plots of the rate of 13NH4+ release from cv IR-72 roots versus time of elution showed three distinct phases of 13N efflux (Wang et al., 1993a; Kronzucker et al., 1995c, 1995e). All three efflux phases could be described adequately by first-order kinetics (r2 ≥ 0.95). Eluates representative of each efflux phase were passed through cation-exchange resins (analytical grade AG 50 W-X 8 cation-exchange resin, 200–400 mesh, Na+ form, Bio-Rad; Kronzucker et al., 1995c), and it was confirmed that ≥99.2% of the 13N was positively charged. Since the concentration of positively charged amino acids in 3-week-old rice roots is typically less than 5% of total amino acid concentrations (Yoneyama and Kumazawa, 1974; Wang et al., 1993a) and the metabolic pool of assimilation products can be expected to be labeled more slowly than the cytoplasmic NH4+ pool (Macklon et al., 1990), the contribution of the N species other than 13NH4+ to the pool of effluxing 13N was considered negligible.
Based on previous 13NH4+-efflux studies in which extensive compartment identification tests were carried out (Kronzucker et al., 1995e), the three phases of NH4+ exchange in the present study could be interpreted as a surface film of NH4+ adhering to the roots (including the water free space), the Donnan free space, and the cytoplasm. This is in keeping with previously published tentative compartment assignments in similar 13N studies (Kronzucker et al., 1995e, and refs. therein). For the three compartments in the present study t1/2 values were approximately 2 to 3 s, 30 s, and 14 min, respectively. These estimates are very close to those reported by Wang et al. (1993a) for rice, except for t1/2 of the cytoplasmic phase, which was significantly longer in our study (14 as opposed to 7 min). However, 10 to 14 min for NH4+ was also found in other species, such as spruce (Kronzucker et al., 1995c, 1995e), other tree species, Arabidopsis, and barley (Hordeum vulgare L.) (A.D.M. Glass, H.J. Kronzucker, X.-J. Min, and M.Y. Siddiqi, unpublished results).
Under all conditions efflux analysis revealed high cytoplasmic NH4+ concentrations, in the range from 15 to 20 mm (at 100 μm [NH4+]o). This has also been reported by Wang et al. (1993a) for rice and is similar to results obtained in spruce, which is known to be better adapted to NH4+ uptake than NO3− (Kronzucker et al., 1997). The high cytoplasmic NH4+ levels raise interesting questions with respect to NH4+ toxicity (Givan, 1979). It has long been assumed that high intracellular NH4+ concentrations are incompatible with physiological functioning for various reasons (Magalhaes and Fernandes, 1995), especially in species such as barley, wheat, pea, or tomato, which show pronounced symptoms of NH4+ toxicity when grown on NH4+ as the sole N source (Kronzucker et al., 1995c; Magalhaes and Fernandes, 1995; Bligny et al., 1997). It is unclear why NH4+ is not toxic in rice and spruce. There are also implications pertaining to some traditional assumptions regarding substrate limitation for enzymic NH4+-processing reactions alternative to Gln synthetase, in particular glutamate dehydrogenase and Asn synthase (Cedar and Schwartz, 1969a, 1969b; Oaks and Ross, 1984). Our results suggest that genetic engineering, through overexpression of genes that code for such enzymes in rice, might well be a useful technological approach to increasing N-utilization capacity by enhancing metabolic processing of the freely available NH4+.
Efflux analysis essentially confirmed results from our time-dependence influx study (Table I). Estimates from compartmental analysis for unidirectional influx were 3.97 μmol g−1 h−1 for fully oxygenated controls, 4.02 after 1 d of hypoxia, 2.78 after 3 d, and 2.12 after 5 d. Efflux, as a percentage of influx, was approximately 15% to 25% and as such was not significantly different between treatments. Significant differences were seen, however, in the amounts of N allocated to NH4+ assimilation and the vacuole (φvac./ass.) and to the shoot (φxylem). φvac./ass. was 52% of incoming N in controls and approximately 45, 34.5, and 62% after 1, 3, and 5 d, respectively, whereas φxylem was approximately 25, 31, 50, and 22% of incoming N, respectively. These observed shifts in the allocation pattern of N may reflect a redirection of N metabolism during adaptation to hypoxia. Significant changes in amino acid profiles in rice under hypoxic/anaerobic conditions have been documented by Reggiani et al. ([1988]; also see above). These workers also found a substantial accumulation of polyamines and speculated that these compounds play a critical role in triggering shoot elongation beyond the flooded zone (Reggiani et al., 1989). The changes we observed in 13N transfer between the root and shoot might indicate the transfer of such N compounds. The exact role of these N shifts in the context of adaptation to hypoxia is unknown.
Table I.
Component fluxes of NH4+ as estimated from compartmental analysis (for derivation of flux parameters and symbols, see text)
| Pretreatment O2 | NH4+ Fluxes
|
||||
|---|---|---|---|---|---|
| φoc | φco | φnet | φvac./ass. | φxylem | |
| μmol g−1 h−1 | |||||
| 100% (control) | 3.97 ± 0.42 | 0.95 ± 0.17 (23) | 3.02 ± 0.36 (77) | 2.04 ± 0.13 (52) | 0.97 ± 0.07 (25) |
| 15% (1 d) | 4.02 ± 0.37 | 0.96 ± 0.11 (24) | 3.06 ± 0.33 (76) | 1.81 ± 0.04 (45) | 1.25 ± 0.17 (31) |
| 15% (3 d) | 2.78 ± 0.41 | 0.43 ± 0.02 (16) | 2.35 ± 0.38 (84) | 0.96 ± 0.09 (34) | 1.39 ± 0.08 (50) |
| 15% (5 d) | 2.12 ± 0.19 | 0.35 ± 0.02 (17) | 1.77 ± 0.14 (83) | 1.31 ± 0.07 (62) | 0.46 ± 0.11 (21) |
Rice seedlings were grown under steady-state nutritional conditions for 3 weeks prior to conducting efflux experiments. For each flux component, the respective percentage of influx is indicated in parentheses. Data are means ± se (n ≥ 6).
CONCLUSIONS
(a) The capacity for NH4+ acquisition in rice seedlings in the vegetative stage remains high, even at very low O2 concentrations (approximately 1 μg mL−1). Both up- and down-regulation of NH4+ influx were observed as rice seedlings adapted to hypoxic conditions. These involve only changes in Vmax for NH4+ influx, whereas uptake affinity for NH4+ (i.e. Km) is unchanged. (b) An up-regulatory response in NH4+ uptake in the initial phases (first few hours) of hypoxia appears to occur in response to cytoplasmic acidosis in rice. It is speculated that additional N is supplied through plasma membrane influx to satisfy the requirements for pH restoration, as related to the production of N compounds, such as polyamines or γ-aminobutyric acid. (c) Reproducible changes in N allocation between different compartments inside root cells and the shoot occur in response to hypoxia. (d) [NH4+]cyt under hypoxic as well as fully aerated conditions are high and at a given external concentration appear to be maintained within a defined range (15–20 mm at 0.1 mm [NH4+]o). At the cellular level such a high [NH4+]cyt illustrates the unique ability of rice plants to tolerate NH4+ as the sole N source, and they point to the possibility of engineering transgenic rice plants with higher N-utilization capacity by overexpressing genes coding for NH4+-assimilation enzymes, such as Asn synthetase or glutamate dehydrogenase. This is presently being pursued at the International Rice Research Institute.
ACKNOWLEDGMENTS
For provision of 13N we wish to thank M. Adam, T. Hurtado, and T. Ruth at the particle acceleration facility Tri-university Meson Facility at the University of British Columbia. Our thanks also to D.T. Britto, Y. Erner, X.-J. Min, J.K. Schjoerring, and D. Zhuo for essential assistance with 13N experiments.
Abbreviations:
- [NH4+]cyt
cytoplasmic NH4+ concentration
- [NH4+]o
NH4+ concentration in the external solution
- φ
symbol for NH4+ flux (see Methods for subscripts denoting component fluxes)
- t1/2
half-life of exchange
Footnotes
This work was supported by funds from the New Frontier project grant to the International Rice Research Institute and by a National Sciences and Engineering Research Council of Canada grant to A.D.M.G.
LITERATURE CITED
- Ancheng L, Jianming X, Xiaoe Y. Effect of nitrogen (NH4NO3) supply on absorption of ammonium and nitrate by conventional and hybrid rice during reproductive growth. In: Barrow NJ, editor. Plant Nutrition—From Genetic Engineering to Field Practice. Wageningen, The Netherlands: Kluwer Academic Publishers; 1993. pp. 537–540. [Google Scholar]
- Aurisano N, Bertani A, Reggiani R. Anaerobic accumulation of 4-aminobutyrate in rice seedlings; causes and significance. Phytochemistry. 1995;38:1147–1150. [Google Scholar]
- Bligny R, Gout E, Kaiser W, Heber U, Walker D, Douce R. pH regulation in acid-stressed leaves of pea plants grown in the presence of nitrate or ammonium salts: studies involving 31P-NMR spectroscopy and chlorophyll fluorescence. Biochim Biophys Acta. 1997;1320:142–152. [Google Scholar]
- Bonner J. The role of organic matter, especially manure, in the nutrition of rice. Bot Gaz. 1946;107:267–278. [Google Scholar]
- Cassman KG, Kropff MJ, Gaunt J, Peng S (1993) Nitrogen use efficiency of rice reconsidered: what are the key constraints? Plant Soil 155/156: 359–362
- Cassman KG, Peng S, Olk DC, Ladha JK, Reichardt W, Dobermann A, Singh U (1998) Opportunities for increased nitrogen use efficiency from improved resource management in irrigated rice systems. Field Crops Res (in press)
- Cassman KG, Puigali PL. Intensification of irrigated rice systems: learning from the past to meet future challenges. Geo J. 1995;35:299–305. [Google Scholar]
- Cedar H, Schwartz JH. The asparagine synthetase of Escherichia coli. I. Biosynthetic role of the enzyme, purification, and characterization of the reaction products. J Biol Chem. 1969a;244:4112–4121. [PubMed] [Google Scholar]
- Cedar H, Schwartz JH. The asparagine synthetase of Escherichia coli. II. Studies on mechanism. J Biol Chem. 1969b;244:4122–4127. [PubMed] [Google Scholar]
- Craswell ET, Vlek PLG (1979) Fate of fertilizer nitrogen applied to wetland rice. In Nitrogen and Rice. International Rice Research Institute, Los Banos, Philippines, pp 174–192
- Crawford RMM. Oxygen availability as an ecological limit to plant distribution. Adv Ecol Res. 1992;23:93–185. [Google Scholar]
- Dijkshoorn W, Ismunadji M. Nitrogen nutrition of rice plants measured by growth and nutrient content in pot experiments. 2. Uptake of ammonium and nitrate from waterlogged soil. Neth J Agric Sci. 1972a;20:44–57. [Google Scholar]
- Dijkshoorn W, Ismunadji M. Nitrogen nutrition of rice plants measured by growth and nutrient content in pot experiments. 3. Changes during growth. Neth J Agric Sci. 1972b;20:133–144. [Google Scholar]
- Drew MC. Sensing soil oxygen. Plant Cell Environ. 1990;13:681–693. [Google Scholar]
- Fried MF, Zsoldos F, Vose PB, Shatokhin IL. Characterizing the NO3− and NH4+ uptake process of rice roots by use of 15N-labelled NH4NO3. Physiol Plant. 1965;18:313–320. [Google Scholar]
- Givan CV. Metabolic detoxification of ammonia in tissues of higher plants. Phytochemistry. 1979;18:375–382. [Google Scholar]
- Hoffman NE, Bent AF, Hanson AD. Induction of lactate dehydrogenase isozymes by oxygen deficit in barley root tissue. Plant Physiol. 1986;82:658–663. doi: 10.1104/pp.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Rice Research Institute (1997) Rice Almanac, Ed 2. International Rice Research Institute, Manila, Philippines
- Kirk GJD, Kronzucker HJ. Roots and N acquisition. In: Thiyagarajan TM, ten Berge HFM, editors. Rice—Nitrogen Relations. Wageningen, The Netherlands: Kluwer Academic Publishers; 1998. (in press) [Google Scholar]
- Kirk GJD, Solivas JL (1997) On the extent to which root properties and transport through the soil limit nitrogen uptake by lowland rice. Eur J Soil Sci (in press)
- Kronzucker HJ, Glass ADM, Siddiqi MY. Nitrate induction in spruce: an approach using compartmental analysis. Planta. 1995a;196:683–690. [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Compartmentation and flux characteristics of nitrate in spruce. Planta. 1995b;196:674–682. [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Compartmentation and flux characteristics of ammonium in spruce. Planta. 1995c;196:691–698. [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Kinetics of NO3− influx in spruce. Plant Physiol. 1995d;109:319–326. doi: 10.1104/pp.109.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Analysis of 13NH4+-efflux in spruce roots. A test case for compartment identification in efflux analysis. Plant Physiol. 1995e;109:481–490. doi: 10.1104/pp.109.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Kinetics of NH4+ influx in spruce. Plant Physiol. 1996;110:773–779. doi: 10.1104/pp.110.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature. 1997;385:59–61. [Google Scholar]
- Kropf MJ, Cassman KG, Van Laar HH, Peng S. Nitrogen and yield potential of irrigated rice. In: Barrow NJ, editor. Plant Nutrition—From Genetic Engineering to Field Practice. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 533–536. [Google Scholar]
- Macklon AES, Ron MM, Sim A. Cortical cell fluxes of ammonium and nitrate in excised root segments of Allium cepa L; studies using 15N. J Exp Bot. 1990;41:359–370. [Google Scholar]
- Magalhaes JR, Fernandes MS (1995) Nitrate versus ammonium nutrition and plant growth. In HS Srivastava, RP Singh, eds, Nitrogen Nutrition in Higher Plants. Associated Publishers, New Delhi, India, pp 131–143
- Menegus F, Cattaruzza L, Chersi A, Fronza G. Differences in the anaerobic lactate-succinate production and in the changes of cell sap pH for plants with high and low resistance to anoxia. Plant Physiol. 1989;90:29–32. doi: 10.1104/pp.90.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegus F, Cattaruzza L, Mattana M, Beffagna N, Ragg E. Response to anoxia in rice and wheat seedlings. Plant Physiol. 1991;95:760–767. doi: 10.1104/pp.95.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks A, Ross DW. Asparagine synthetase in Zea mays. Can J Bot. 1984;62:68–73. [Google Scholar]
- Reggiani R. Purification and synthesis under anaerobic conditions of rice arginine decarboxylase. Plant Cell Physiol. 1994;35:1245–1249. [Google Scholar]
- Reggiani R, Aurisano N, Mattana M, Bertani A. Effect of potassium ions on polyamine levels in wheat seedlings under anoxia. J Plant Physiol. 1993;142:94–98. [Google Scholar]
- Reggiani R, Brambilla I, Bertani A. Effect of exogenous nitrate on anaerobic metabolism in excised rice roots. II. Fermentative activity and adenylic energy charge. J Exp Bot. 1985;36:1698–1704. [Google Scholar]
- Reggiani R, Cantu CA, Brambilla I, Bertani A. Accumulation and interconversion of amino acids in rice roots under anoxia. Plant Cell Physiol. 1988;29:981–987. [Google Scholar]
- Reggiani R, Giussani P, Bertani A. Relationship between the accumulation of putrescine and the tolerance to oxygen-deficient stress in Gramineae seedlings. Plant Cell Physiol. 1990;31:489–494. [Google Scholar]
- Reggiani R, Hochkoeppler A, Bertani A. Polyamines and anaerobic elongation of rice coleoptile. Plant Cell Physiol. 1989;30:893–898. [Google Scholar]
- Reggiani R, Zaina S, Bertani A. Plasmalemma ATPase in rice coleoptiles: stimulation by putrescine and polyamines. Phytochemistry. 1992;31:417–420. [Google Scholar]
- Roberts JKM, Andrade FH, Anderson IC. Further evidence that cytoplasmic acidosis is a determinant of flooding intolerance in plants. Plant Physiol. 1985;77:492–494. doi: 10.1104/pp.77.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa H, Yamamoto Y. Comparison of the uptake of nitrate and ammonium by rice seedlings. Influences of light, temperature, oxygen concentration, exogenous sucrose, and metabolic inhibitors. Plant Physiol. 1978;62:665–669. doi: 10.1104/pp.62.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen TC. Induction of nitrate reductase and the preferential assimilation of ammonium in germinating rice seedlings. Plant Physiol. 1969;44:1650–1655. doi: 10.1104/pp.44.11.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorzano L. Determination of ammonia in natural waters by the phenol-hypochlorite method. Limnol Oceanogr. 1969;14:799–801. [Google Scholar]
- Vlek PLG, Byrnes BH. The efficacy and loss of fertilizer N in lowland rice. Fert Res. 1986;9:131–147. [Google Scholar]
- Wang MY, Siddiqi MY, Ruth TJ, Glass ADM. Ammonium uptake by rice roots. I. Fluxes and subcellular distribution of 13NH4+ Plant Physiol. 1993a;103:1249–1258. doi: 10.1104/pp.103.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Siddiqi MY, Ruth TJ, Glass ADM. Ammonium uptake by rice roots. II. Kinetics of 13NH4+ influx across the plasmalemma. Plant Physiol. 1993b;103:1259–1267. doi: 10.1104/pp.103.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama T, Kumazawa K. A kinetic study of the assimilation of 15N-labeled ammonium in rice seedling roots. Plant Cell Physiol. 1974;15:655–661. [Google Scholar]
- Yoneyama T, Kumazawa K. A kinetic study of the assimilation of 15N-labeled nitrate in rice seedling roots. Plant Cell Physiol. 1975;16:21–26. [Google Scholar]
- Youngdahl LJ, Pacheco R, Street JJ, Vlek PLG. The kinetics of ammonium and nitrate: uptake by young rice plants. Plant Soil. 1982;69:225–232. [Google Scholar]
- Yu T-R (1985) Soil and plants. In T-R Yu, ed, Physical Chemistry of Paddy Soils. Science Press, Beijing, China, pp 197–217