Abstract
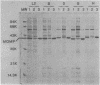
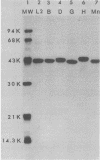
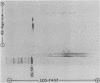
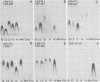
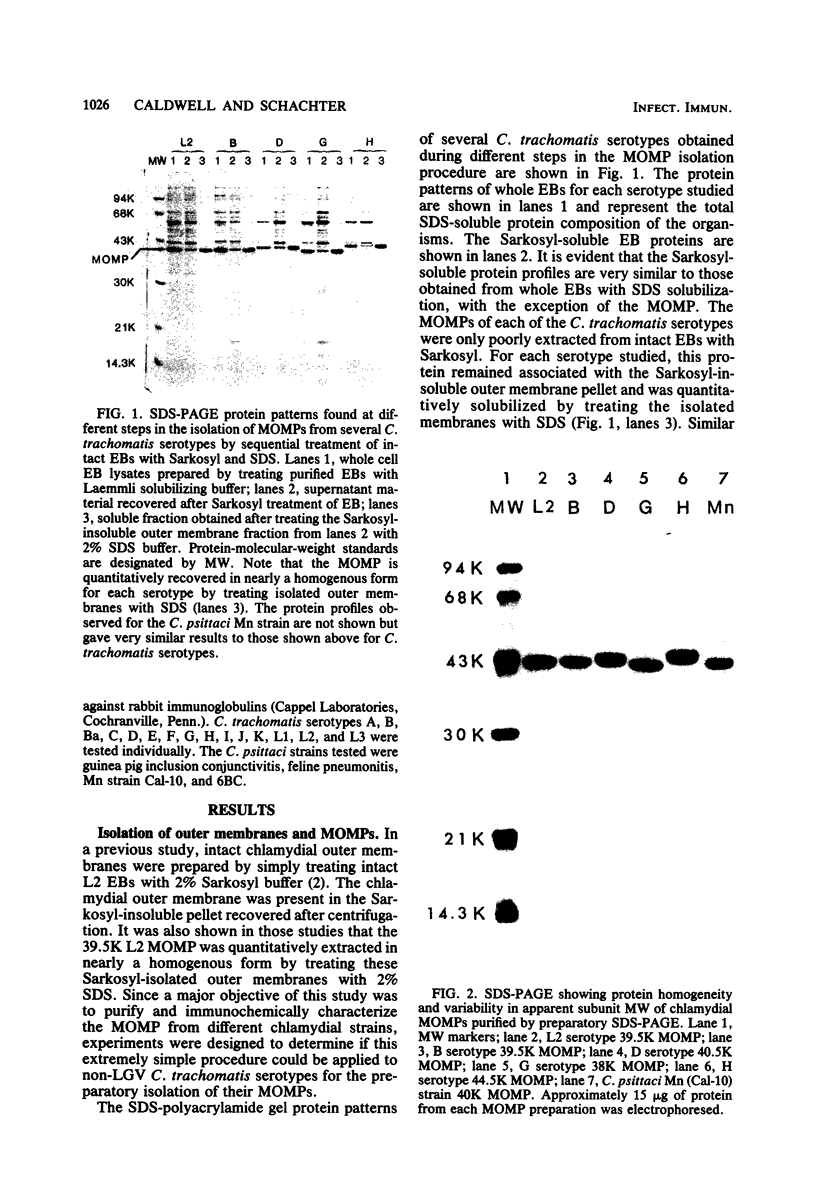
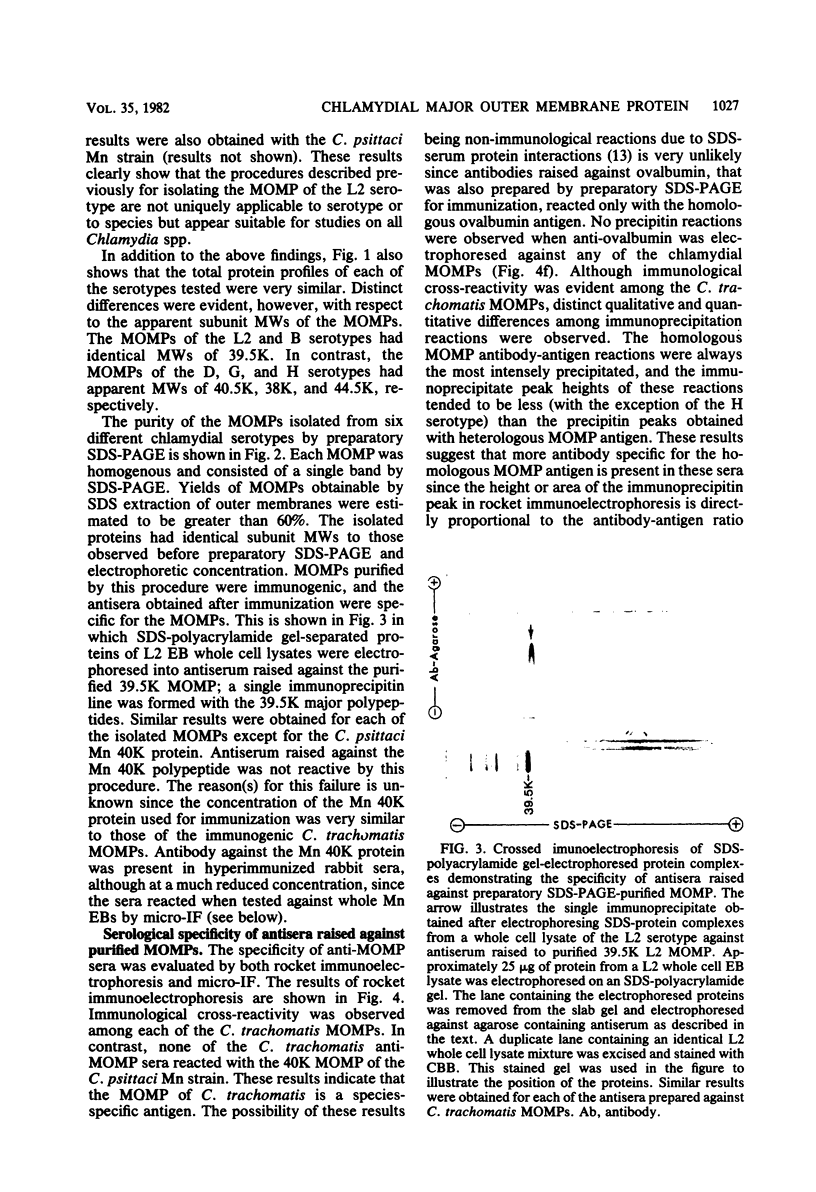
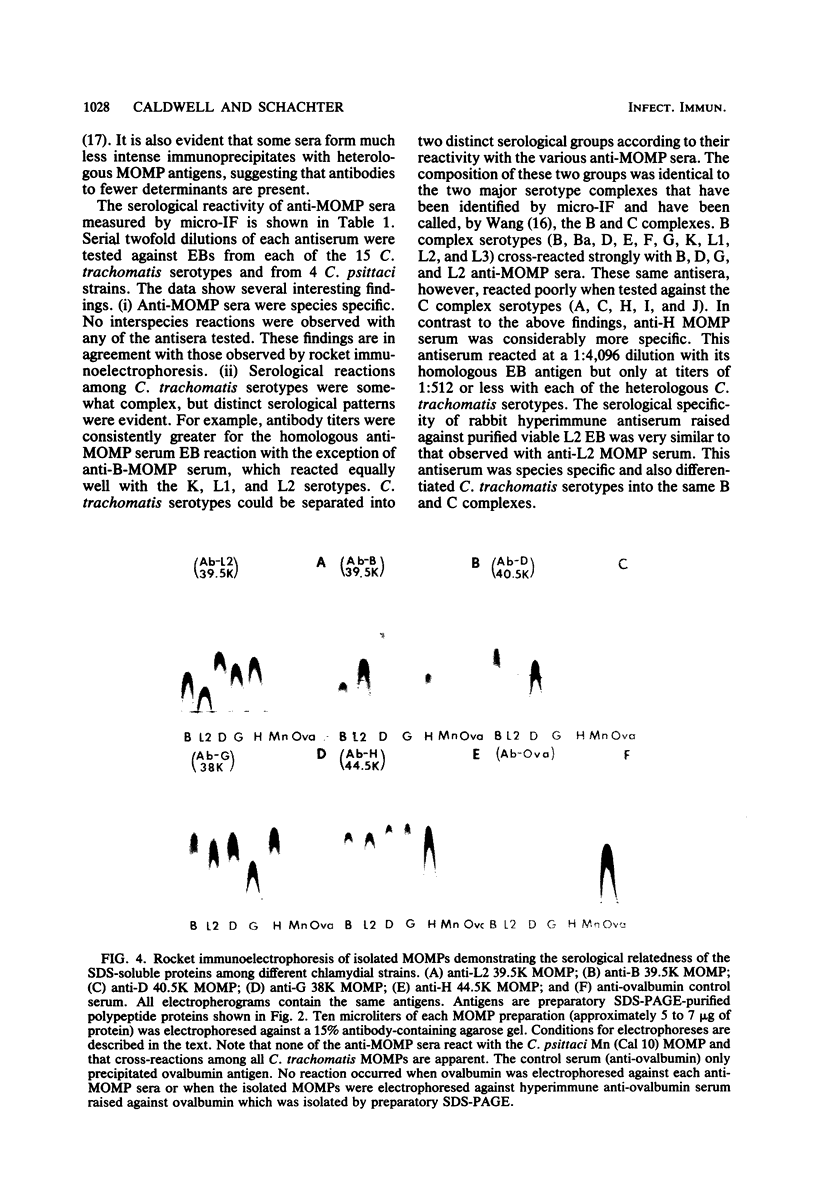
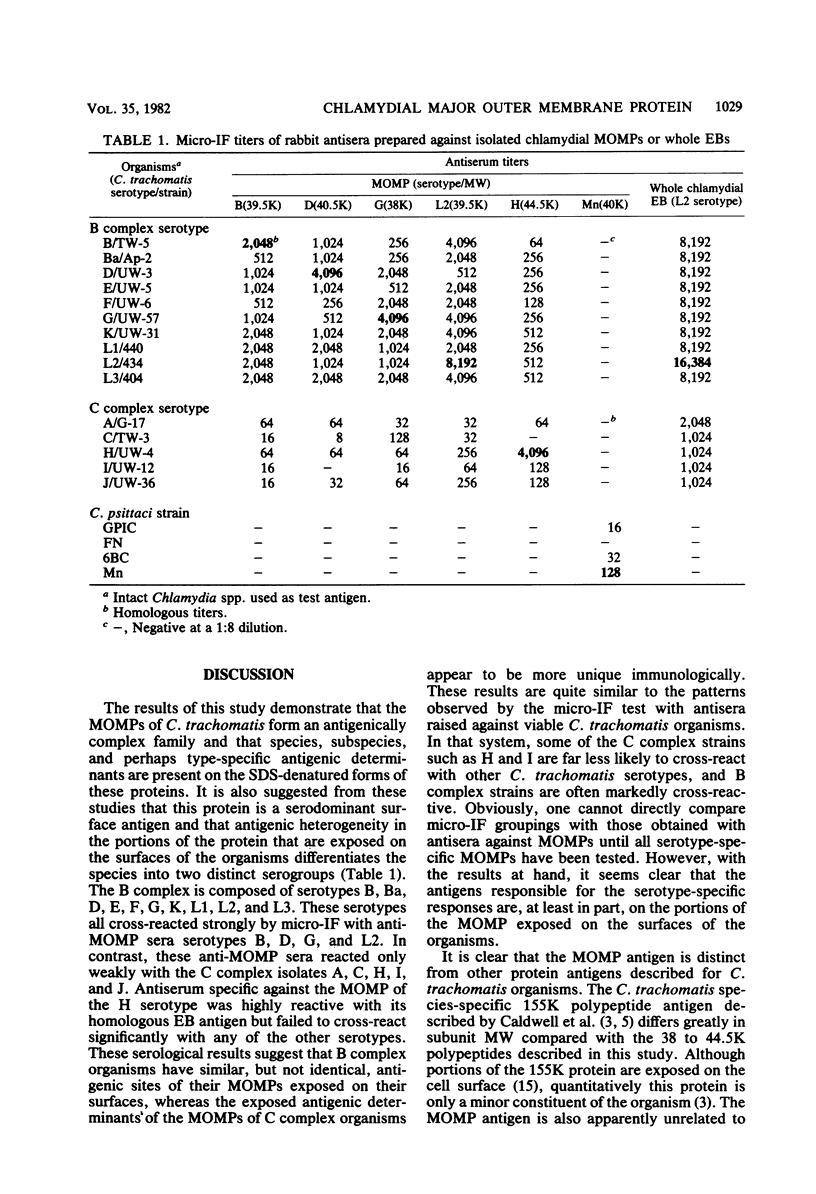
The major outer membrane proteins (MOMPs) of several Chlamydia trachomatis serotypes (B, D, G, H, and L2) and of the C. psittaci meningopneumonitis strain were purified by preparatory sodium dodecyl sulfate-(SDS)-polyacrylamide gel electrophoresis. The isolated SDS-polypeptide complexes, which varied in their apparent subunit molecular weights, were used as immunogens to raise hyperimmune rabbit antisera. The specificities of these antisera were determined both by rocket immunoelectrophoresis with the soluble SDS-polypeptide complex as antigen and by micro-immunofluorescence with whole organisms. By rocket immunoelectrophoresis, each of the soluble C. trachomatis MOMPs was immunologically related; however, no immunological cross-reactions occurred with the C. psittaci meningopneumonitis polypeptide, indicating that the MOMPs are antigenically distinct among members of these two chlamydial species. The same antisera were highly reactive with intact organisms by micro-immunfluorescence, demonstrating that at least some of the antibodies raised with SDS-polypeptide complexes reacted with native antigenic sites of these surface proteins. By micro-immunofluorescence, anti-MOMP sera remained species specific; but, unlike the results observed by rocket immunoelectrophoresis, distinct differences in the reactivity and specificity of these antisera were observed among C. trachomatis serotypes. C. trachomatis isolates were separated into two distinct serogroups on the basis of their reactivity with anti-MOMP sera. B complex organisms (B, Ba, D, E, F, G, K, L1, L2, and L3) all reacted strongly with anti-MOMP sera of the B, D, G, and L2 serotypes. In contrast, these same antisera were poorly reactive with the C complex serotypes A, C, H, I, and J. Anti-H MOMP serum was the most serospecific, since high-antibody titers were found only against the homologous H serotype organism. These findings indicate that MOMPs of different strains of C. trachomatis are antigenically complex and that antigenic heterogeneity exists among the surface-exposed portions of the protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kuo C. C., Kenny G. E. Antigenic analysis of Chlamydiae by two-dimensional immunoelectrophoresis. I. Antigenic heterogeneity between C. trachomatis and C. psittaci. J Immunol. 1975 Oct;115(4):963–968. [PubMed] [Google Scholar]
- Caldwell H. D., Kuo C. C., Kenny G. E. Antigenic analysis of Chlamydiae by two-dimensional immunoelectrophoresis. II. A trachoma-LGV-specific antigen. J Immunol. 1975 Oct;115(4):969–975. [PubMed] [Google Scholar]
- Caldwell H. D., Kuo C. C. Purification of a Chlamydia trachomatis-specific antigen by immunoadsorption with monospecific antibody. J Immunol. 1977 Feb;118(2):437–441. [PubMed] [Google Scholar]
- Chua N. H., Blomberg F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J Biol Chem. 1979 Jan 10;254(1):215–223. [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Hatch T. P., Vance D. W., Jr, Al-Hossainy E. Identification of a major envelope protein in Chlamydia spp. J Bacteriol. 1981 Apr;146(1):426–429. doi: 10.1128/jb.146.1.426-429.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourihan J. T., Rota T. R., MacDonald A. B. Isolation and purification of a type-specific antigen from Chlamydia trachomatis propagated in cell culture utilizing molecular shift chromatography. J Immunol. 1980 May;124(5):2399–2404. [PubMed] [Google Scholar]
- Kingsbury D. T., Weiss E. Lack of deoxyribonucleic acid homology between species of the genus Chlamydia. J Bacteriol. 1968 Oct;96(4):1421–1423. doi: 10.1128/jb.96.4.1421-1423.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Palmer E. L., Martin M. L., Hierholzer J. C., Ziegler D. W. Nonspecific precipitation of serum proteins by sodium lauryl sulfate in agar diffusion and immunoelectrophoresis. Appl Microbiol. 1971 May;21(5):903–906. doi: 10.1128/am.21.5.903-906.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., MacDonald A. B. Isolation of a type-specific antigen from Chlamydia trachomatis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol. 1979 Jan;122(1):136–139. [PubMed] [Google Scholar]
- Salari S. H., Ward M. E. Polypeptide composition of Chlamydia trachomatis. J Gen Microbiol. 1981 Apr;123(2):197–207. doi: 10.1099/00221287-123-2-197. [DOI] [PubMed] [Google Scholar]
- Weeke B. A manual of quantitative immunoelectrophoresis. Methods and applications. 1. General remarks on principles, equipment, reagents and procedures. Scand J Immunol Suppl. 1973;1:15–35. doi: 10.1111/j.1365-3083.1973.tb03776.x. [DOI] [PubMed] [Google Scholar]