Abstract
Macroporous chitosan spheres encapsulating superparamagnetic iron oxide nanoparticles were synthesized by a facile and effective one-step fabrication process. Ferro-gels containing ferrous cations, ferric cations and chitosan were dropped into a sodium hydroxide solution through a syringe pump. In addition, a sodium hydroxide solution was employed for both gelation (chitosan) and co-precipitation (ferrous cations and ferric cations) of the ferro-gels. The results showed that the in-situ co-precipitation of ferro-ions gave rise to a radial morphology with non-spheroid macro pores (large cavities) inside the chitosan spheres. The particle size of iron oxide can be adjusted from 2.5 nm to 5.4 nm by tuning the concentration of the sodium hydroxide solution. Using Fourier Transform Infrared Spectroscopy and X-ray diffraction spectra, the synthesized nanoparticles were illustrated as Fe3O4 nanoparticles. In addition, the prepared macroporous chitosan spheres presented a super-paramagnetic behaviour at room temperature with a saturation magnetization value as high as ca. 18 emu/g. The cytotoxicity was estimated using cell viability by incubating doses (0∼1000 µg/mL) of the macroporous chitosan spheres. The result showed good viability (above 80%) with alginate chitosan particles below 1000 µg/mL, indicating that macroporous chitosan spheres were potentially useful for biomedical applications in the future.
Introduction
Porous spheres have several, extremely valuable therapeutic and biotechnological applications [1], [2], including cell immobilization, drug delivery, and as a packing material in chromatography [3]–[6]. Macroporous structures are especially important to spheres to improve their performance [1], [2], [7]. For example, large pores can increase the drug permeability of the spheres in drug delivery, significantly increase their specific surface area, allow them to be used as culture systems for growing adherent cells, be used as water remediation in high diffusion rates, or be used in the separation of large biomolecules, etc [8]–[16].
Chitosan itself is eco-friendly due to the properties of non-toxic, biodegradable and bio-compatible, and has wide applications on medicine, pharmacy, and environmental protection [17]–[19]. Highly porous chitosan beads or scaffolds are especially useful for bone tissue engineering, drug delivery, and heavy metal adsorption [20]–[22]. Furthermore, macroporous chitosan structure has special advantages in some applications [23]–[25]. For example, Xi and Wu employed macroporous chitosan-coated silica gel to accommodate the accessibility of the protein adsorption [23]. Wu et al. employed macroporous chitosan-silica gel beads for the immobilized affinity chromatographic (IMAC) absorbents [24]. Li et al. fabricated spherical chitosan with macro reticular structure on removal of heavy metals from wastewaters [25]. In addition, incorporating iron oxide nanoparticles and macroporous chitosan matrices exhibits specific mechanical and functional properties, that can lead to wide variety of applications, such as recyclable heavy metal removal, magnetic-induced tumor therapy, responsive drug release, magnetic resonance imaging (MRI) enhancement, etc [26]–[28].
The preparation of chitosan particles with/without iron oxide nanoparticles have been investigated in the literatures, such as emulsification, ionotropic gelation, reverse micellar, solvent evaporation, spray drying, freeze-drying, coacervation, sieving, porogen leaching out, alkaline gelation, and other techniques [17]–[32]. However, most of the pores in these above mentioned studies were spherical. To the best of our knowledge, the synthesis of a patterned, macroporous structure (e.g. radial-like and interconnected large cavities) has rarely been discussed in the literature.
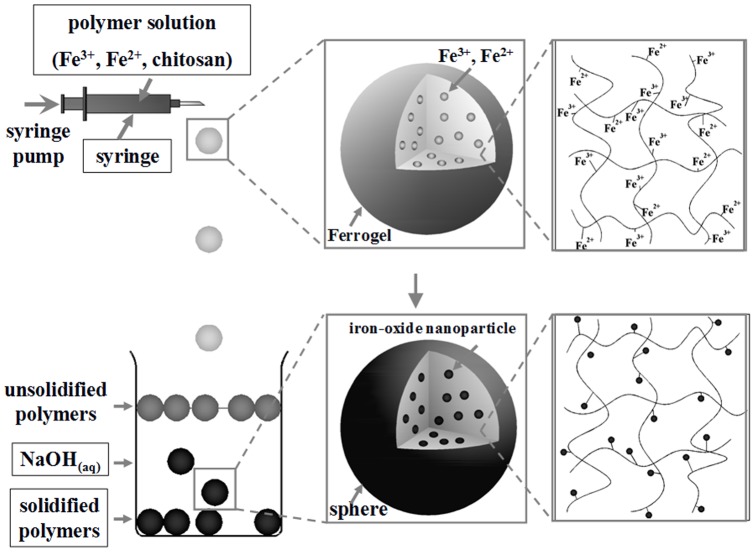
Herein we reported an approach for novel macroporous chitosan spheres with a non-spheroid, patterned (i.e. radial-like) morphology. Figure 1 shows the one step process for macroporous chitosan spheres production through in-situ co-precipitation and the gelation of ferro-gels. Due to the abundant amino groups, chitosan has a good capability for the uptake of ferrous and ferric cations via the chelating or ion exchange mechanisms [33]. Ferrous and ferric cations were mixed with a chitosan solution to form the ferro-gel solution. Subsequently the ferro-gel was dropped into a NaOH solution for simultaneous chitosan solidification and iron cations co-precipitation and form the iron oxide nanoparticles embedded chitosan spheres. The color of the solution changed quickly from yellow-orange to black, indicating that iron oxide nanoparticles are formed through the co-precipitation reaction [34]. The strategy of the iron oxide nanoparticles synthesis is based on a ferro-solution containing Fe2+ and Fe3+ dissolved in 1∶2 molar ratios.
Figure 1. Schematic of the formation process of macroporous chitosan spheres.
Experimental Section
Materials
Chitosan (molecular weight: 150,000), iron(II) chloride tetrahydrate (FeCl2•4H2O, 99%), iron (III) chloride hexahydrate (FeCl3•6H2O, 98%), and sodium hydroxide (NaOH) were purchased from SIGMA, J. T. Baker, Alfa Aesar and Mallinckrodt, respectively, and used as received without further processing.
Synthesis of the chitosan spheres
Chitosan (0.1 g, dissolved in 2 mL of 1%, v/v CH3COOH solution) were prepared and dropped into NaOH solutions of various concentrations (5, 20, 30, and 50 wt %) respectively, by means of a syringe and pump. After 10 minutes, chitosan spheres were observed. Spheres were collected by centrifugation, and were washed twice with 30 mL dd-H2O to remove any alkali. The spheres were then dried (EYELA FDU-1100) in vacuum at −54°C for 24 hours. The dried particles were stored at 4°C until use.
Synthesis of the Fe3O4-chitosan composite spheres
Chitosan (0.1 g, dissolved in 2 mL of 1%, v/v CH3COOH solution), FeCl2•4H2O (0.0224 g, dissolved in 0.5 mL of 2N HCl solution) and FeCl3•6H2O (0.0448 g, dissolved in 0.5 mL of 2N HCl solution) were mixed through constant stirring for 30 minutes, until a ferro-gel solution was obtained. The ferro-gel solution was then dropped into NaOH solutions of various concentrations (5, 20, 30, and 50 wt %) respectively, by means of a syringe and pump. After 10 minutes, Fe3O4-chitosan composite spheres having a black color were observed. Spheres were collected by centrifugation, and were washed twice with 30 mL dd-H2O to remove any alkali. The spheres were then dried (EYELA FDU-1100) in vacuum at −54°C for 24 hours. The dried particles were stored at 4°C until use.
Characterization
Size distributions of the various droplets samples were obtained from the random sampling of about 50 individual spheres so as to minimize selection bias. An inverted microscope system, including an optical microscope (BX60, Olympus, Japan) and a digital camera (DP70, Olympus, Japan), were employed for imaging. The average diameter of the spheres, expressed as mean ± standard deviation, was obtained from the photomicrograph. Statistical analysis was performed on the size distribution using an unpaired t-test. X-ray diffraction (XRD, D8 Advance, PANalytical X'PERT PRO) patterns were obtained at room temperature by using Cu K-α radiation (λ = 1.5406 Å) with a range of 2θ = 20°∼80°, and a scanning rate of 0.05 s−1. Fourier transform infrared spectroscopy (FTIR) spectra were recorded with a Spectrum RXI FTIR Spectrometer, using KBr pellets, in the range of 400∼4000 cm−1, with a resolution of 4 cm−1. The morphology of the composites was analyzed using a scanning electron microscope (SEM, Hitachi, S-2700, Japan), and a transmission electron microscope (TEM, FEI Tecnai G2 20 S-Twin, EDS: METEK). The magnetic properties were measured with a superconducting quantum interference device (SQUID, MPMS-XL7) and were evaluated in terms of saturation magnetization and coercivity. The lyophilized microcapsules were placed in a capsule and wrapped with Teflon to prevent leakage. The magnetic susceptibility was measured with a magnetic field of 60 KOe at 300 K. The zero-field-cooled (ZFC) and field-cooled (FC) magnetization curves were measured at a temperature ranging between 5 and 300 K at a 500 Orested applied field.
Cell viability analysis
The viability of the control and the treated cells were evaluated using an MTT (3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay with human glioblastoma U87MG, human breast adenocarcinoma MCF-7, and glioblastoma multiforme (GBM) cells lines. Cells (1×104/well) were seeded in 96-well microtiter plates containing 100 µL culture medium, permitted to adhere for 18 hours, washed with phosphate buffered saline, and then treated with various Fe3O4-chitosan composite spheres. After 24 hours of treatment, cells were incubated at 37°C in 200 µL MTT solution (1 mg/mL) for 4 hours. After removal of the medium and MTT, 100 µL dimethyl sulfoxide (DMSO) was added to each well, and the assay plate was read at 595 nm using a microplate reader (Thermo ELECTRON CORPORATION MULTISKAN ASCENT). The absorbance of untreated cells was considered as 100%.
Results and Discussion
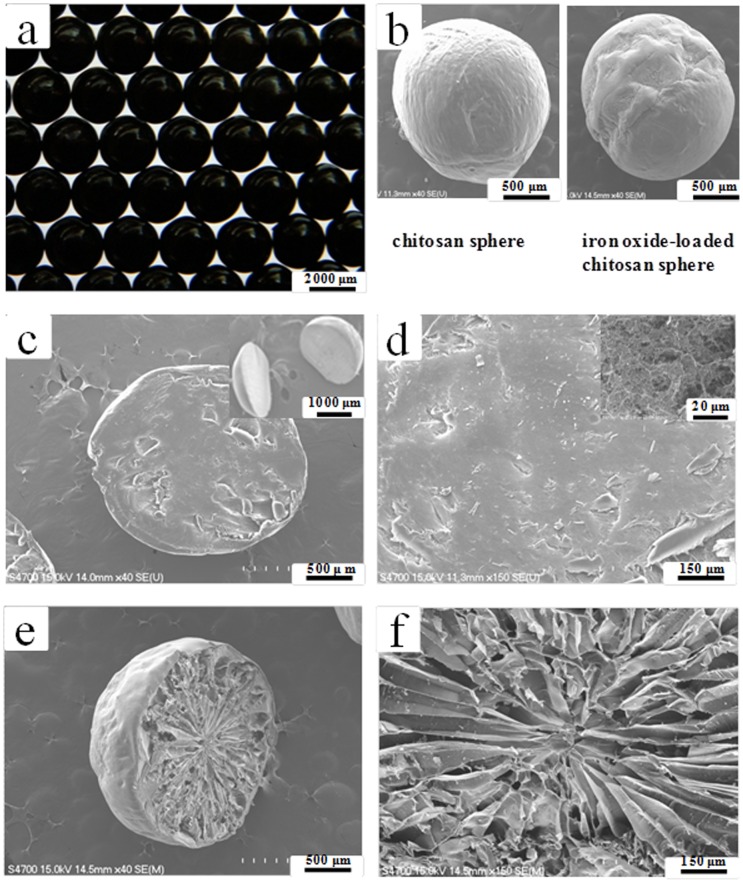
Figure 2a shows a photograph of the prepared iron oxide-loaded chitosan spheres. It is evident that the spheres are uniform in size, measuring 2.67±0.08 mm in diameter. After the drying process, the spheres had shrunk by about 79%. The diameter of the spheres could be controlled from 1 to 5 mm (with a variation of less than 8%) by altering the size of the needles under the relative sample flow rate from 20 to 30 mL/hour. In the future, the diameter of the spheres could be made smaller by employing other conventional droplet generation methods, such as atomization (spraying), emulsification, coacervation, sonication, electrostatic droplets, microfluidic droplets, etc [35]–[38]. It is worth noting that the surface of the iron oxide-loaded chitosan sphere is more irregular than that of pure chitosan spheres ( Fig. 2b ). This finding gave us the motivation to explore the internal structure of the polymer spheres. The pure chitosan sphere (absence of iron oxide nanoparticles) possesses a compact intra structure ( Figs. 2c and 2d ). In contrast, to our surprise, the inner structure of the iron oxide-loaded chitosan sphere shows a radial-patterned-like, non-spheroid morphology of macro pores and interconnected large cavities, as shown in Figs. 2e and 2f .
Figure 2. Morphologies of the synthesyzed chitosan spheres.
(a) An optical image of the iron oxide nanoparticles loaded chitosan spheres. (b) SEM images of chitosan spheres without (left) and with (right) iron oxide nanoparticles. (c) and (d) show the cross-section images of chitosan spheres (without iron oxide nanoparticles). Inset in (c) shows the sliced hemispheres of chitosan sphere. Inset in (d) shows details of the internal structure. (e) and (f) show the cross-section images of iron oxide nanoparticles loaded chitosan spheres.
We presume that this special pattern of intra macroporous structures is due to the interaction of iron-oxide co-precipitation and chitosan gelation. Similar results were obtained by using gold ions for the synthesis of chitosan particles. A cellular-patterned-like cross-section structure and a micro-channeled-like longitudinal structure were achieved through the combination of the ISISA (ice-segregation-induced self-assembly) process and co-precipitation of gold nanoparticles [39]. Hortigüela et al. demonstrated that macro pores size and shape differed depending on the HAuCl4•3H2O concentration in the starting solution. In addition, the ISISA is related to the viscosity of the solution and eventually to the hydrogel strength. Deville et al. demonstrated that ice formation could be used to develop sophisticated porous polymer scaffolds [40]. In addition, Zhang et al. further showed that aligned porous polymer-inorganic composite materials by templating the polymer structure by means of instability formation whilst simultaneously freeze concentrating inorganic nanoparticles [41]. In our experiments, however, the only factor that caused the formation of macroporous structure is the in situ generated iron oxide nanoparticles (please see Figs. 2c vs. 2e ). Without the process of iron oxide nanoparticles formation, the macroporous structure is absent ( Fig. 2c ). To understand the exact mechanism, however, requires further investigation. The proposed route for the generation of chitosan spheres with non-spheroid macro pores is quite novel compared with the other synthetic methods [42]–[45].
Furthermore, the diameter of the iron oxide nanoparticles could be tuned by adjusting the concentration of the NaOH solution. The diameter of the prepared iron oxide spheres were identified by a transmission electron microscope (TEM). The particle size of iron oxide decreases when the NaOH concentration increases, e.g. the mean diameters were 5.4±1.7 nanometers (with 5% NaOH), 4.4±0.9 nanometers (with 20% NaOH), 3.8±0.9 nanometers (with 30% NaOH), and 2.5±0.2 nanometers (with 50% NaOH), respectively (Figs. S1a∼S1d). At the same time, radial macroporous structures were observed under all of the above conditions. In addition, the effects of chitosan concentration and molecular weight on the size of iron-oxide nanoparticles were studied. There was no significant difference among these conditions. The prepared iron oxide particles were in the range of 2.5 nm to 7 nm (Fig. S2). Finally, we will briefly describe the main findings and contributions of this study: (i) a facile and one-step method for fabricating macroporous chitosan spheres was established; (ii) radiating cavities and a non-spheroid structure of macroporous chitosan spheres was obtained; and (iii) size-adjustable iron oxide nanoparticles were synthesized and embedded in the chitosan spheres simultaneously.
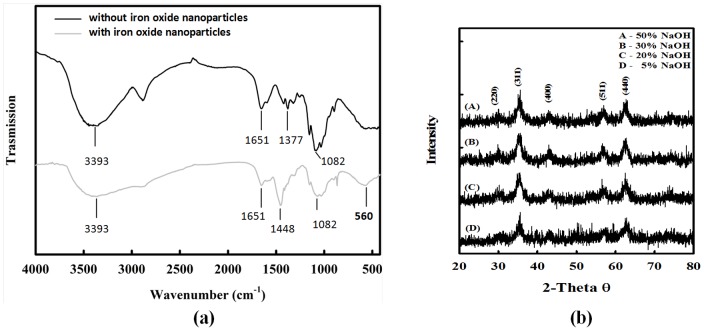
The properties of prepared iron oxide nanoparticles-loaded chitosan spheres were characterized. The fact that these spheres contained iron oxide nanoparticles was determined by an atomic absorption spectrophotometer (AAS). The results show that each chitosan sphere (2.67±0.08 mm in diameter, 5 wt % NaOH solution) contained on average 125.95 mg/mL of ferro ions (e.g. each chitosan sphere contained 1.4 mg of iron oxide nanoparticles). Figure 3a shows the FTIR spectra of chitosan spheres and iron oxide nanoparticle-loaded chitosan spheres (produced from a 5 wt % NaOH solution). Characteristic peaks of chitosan spheres were evident in spectrum at 3393 cm−1 (-OH group), 1651 cm−1 (N-H bending vibration), and 1377 cm−1 (-C-O stretching of primary alcoholic group), respectively. The characteristic absorption peak for iron oxide nanoparticles was observed at ∼560 cm−1 (Fe-O group, iron oxide nanoparticle-loaded chitosan spheres). Similar results were obtained in other researches [26]–[29]. Therefore, it was concluded that the embedded nanoparticles were iron oxide nanoparticles. Figure 3b shows X-ray diffraction (XRD) spectra, which revealed that the synthesized nanoparticles were Fe3O4 phase, since the position and relative intensity of all diffraction peaks of samples were consistent with the crystalline pattern of Fe3O4 phase [29]. In addition, the chitosan joining the synthesis process did not cause any phase changes in the Fe3O4 nanoparticles. The shape of each peak was broadened due to the influence of chitosan polymer [29], [46]–[47].
Figure 3. Characterization of the iron oxide-chitosan composite spheres.
(a) FTIR spectra of chitosan spheres (gray line) and iron oxide nanoparticle-loaded chitosan spheres (black line). (b) X-ray diffraction pattern from iron oxides nanoparticle-loaded chitosan spheres prepared from the NaOH solutions with various concentrations.
The abovementioned results suggest that chitosan spheres have a superparamagnetic property, because of the Fe3O4 nanoparticles. The photograph in Fig. S3 shows that Fe3O4 nanoparticles-loaded chitosan spheres can be attracted and drawn through the solution to the side wall of the vial by an external magnetic field. The magnetic properties were studied using a superconducting quantum interference device (SQUID). The plots of typical magnetization loops of magnetization (M) versus the magnetic field (H) at 300 K are shown in Fig. S4a. The saturation magnetization (Ms), remanent magnetization (Mr), coercivity (Hc) and squareness (Sr = Mr/Ms) were calculated from the plot. Since no remanence and coercivity was observed, it was concluded that the Fe3O4 nanoparticles-loaded chitosan spheres possess superparamagnetic nature at room temperature [48]. From the M–H loops, the saturation magnetization (Ms) was determined to be 18, 17, 15, and 14 emu/g, for the samples prepared from 5, 20, 30, and 50 wt % NaOH solutions, respectively. The saturation magnetization decreased when the NaOH concentration increased. This phenomenon can be explained by the fact that the magnetization of small iron oxide particles decreases as the particle size decreases [49]. Field-cooled (FC) and zero-field-cooled (ZFC) magnetization curves were taken under an external magnetic field of 500 Orested. Figure S4b shows the FC and ZFC curves for the samples prepared from 5, 20, 30, and 50 wt % NaOH solutions, respectively, ranging from 5 to 300 K. The ZFC and FC curves coincided above 40 K and separated below 40 K. The ZFC curve shows a broad peak at Tmax 30±10 K indicative of a characteristic blocking temperature for superparamagnetic particles [50].
Stability of the beads in 37°C water (pH = 7) was tested for three days (72 hours). The experimental setup was shown in Fig. S5a. Figure S5b showed the beads (made from 5%, 20%, 30% and 50% NaOH solution, respectively) placed in the 37°C water at the beginning. Figure S5c showed the same beads after 3 days. Result suggests that the beads did not swell nor dissolve in 37°C water for at least 3 days. This suggests that the beads were stable in aqueous condition for at least 72 hours. In addition, stability in acidic condition was also tested by placing beads in 1% acetic acid (at room temperature). Chitosan beads start to dissolve in 20 minutes (Fig. S6a). The beads made from 5% and 20% NaOH were almost dissolved after 3.5 hours (Fig. S6b). All the beads made from various concentration were dissolved after 6 hours (Fig. S6c). Result shows that the beads were not suitable to be preserved in acidic condition.
The nanoparticle-loaded chitosan spheres were evaluated for pernicious biological properties by MTT assay. The cytotoxicity was estimated using cell viability by incubating doses (0∼1000 µg/mL) of the microparticles with U87MG (BCRC ID: 60360), MCF-7 (BCRC ID: 60436), and GBM (BCRC ID: 60164) cells for 24 hours (Fig. S7). All the cells lines were purchased from Biosource Center and Research Center, Food Insdustry Research and Development Institite, Taiwan. The results showed good viability (above 80%) of a series of Fe3O4-chitosan composite containing up to 1000 µg/mL of micro particles, indicating that the spheres are biologically friendly and can potentially be used in biological and biomedical applications in the future.
Conclusions
Chitosan spheres with non-spheroid, radial-like macro pores were successfully obtained. By employing the in-situ co-precipitation for ferro-gels, the macro pores with radiating cavities were observed. The proposed method provides an alternative way for generating macroporous spheres. In addition, a sodium hydroxide solution was employed for both the gelation (chitosan) and co-precipitation (ferrous cations and ferric cations) of ferro-gels. The results show that the diameter of both the iron oxide nanoparticles and the chitosan were controllable. In addition, the prepared iron oxide nanoparticles loaded chitosan spheres present a superparamagnetic character with good viability (above 80% for chitosan spheres containing iron-oxide up to 1000 µg/mL), indicating that they are eco-friendly and can potentially be used in various biological and biomedical applications, such as magnetic-responsive drug delivery systems, scaffold for bone tissues, ultrasound contrast agent, targeted drug delivery vehicle, and for delivering tumor and/or thrombus destruction materials.
Supporting Information
TEM images of iron oxide nanoparticles-loaded chitosan spheres prepared in various concentrations of NaOH solutions with (a) 5%, (b) 20%, (c) 30%, and (d) 50%, respectively.
(TIF)
The effects among chitosan concentration, chitosan molecular weight on the size of magnetic iron-oxide (MIO) nanoparticles.
(TIF)
Iron oxide nanoparticles-loaded chitosan spheres were attracted to wall of the vial by using an external magnetic field.
(TIF)
Magnetic characteristics of the iron oxide nanoparticles-loaded chitosan spheres. (a) Magnetization plots as a function of the applied field at 300 K. (b) Temperature dependent ZFC-FC magnetization curves measured at 500 Oe.
(TIF)
Stability test of the spheres in 37°C water (pH = 7). (a) shows the experimental setup. Spheres (made from 5%, 20%, 30% and 50% NaOH solution, respectively) deposed in the 37°C water (b) at the beginning, and (c) for 3 days.
(TIF)
Spheres placed in 1% acetic acid for (a) 20 minutes; (b) 3.5 hours; and (c) 6 hours.
(TIF)
Pernicious biological properties test for the nanoparticle-loaded chitosan spheres. (a) The cytotoxicity of iron oxide nanoparticles-loaded chitosan spheres (5.4±1.7 nanometers of iron oxide nanoparticles (with 5% NaOH) inside chitosan spheres) with three cell lines. (b) The MTT assay data of various iron oxide nanoparticles loaded chitosan spheres (MCF-7 cells). Sample A: 5.4±1.7 nanometers of iron oxide nanoparticles (with 5% NaOH) inside chitosan spheres. Sample B: 4.4±0.9 nanometers of iron oxide nanoparticles (with 20% NaOH) inside chitosan spheres. Sample C: 3.8±0.9 nanometers of iron oxide nanoparticles (with 30% NaOH) inside chitosan spheres. Sample D: 2.5±0.2 nanometers of iron oxide nanoparticles (with 50% NaOH) inside chitosan spheres.
(TIF)
Acknowledgments
We thank Mr. Wei-Ting Pan for the discussion and join the research project.
Funding Statement
This work was financially supported by a grant from the National Science Council of Taiwan, R.O.C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kim H, Park H, Lee J, Kim TH, Lee ES, et al. (2011) Highly porous large poly(lactic-co-glycolic acid) microspheres adsorbed with palmityl-acylated exendin-4 as a long-acting inhalation system for treating diabetes. Biomaterials 32: 1685–1693. [DOI] [PubMed] [Google Scholar]
- 2. Tsapis N, Bennett D, Jackson B, Weitz DA, Edwards DA (2002) Trojan particles: Large porous carriers of nanoparticles for drug delivery. Proc Natl Acad Sci U S A 17: 12001–12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edwards DA, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, et al. (1997) Large porous particles for pulmonary drug delivery. Science 276: 1868–1872. [DOI] [PubMed] [Google Scholar]
- 4. Lahooti S, Sefton MV (2000) Effect of an immobilization matrix and capsule membrane permeability on the viability of encapsulated HEK cells. Biomaterials 21: 987–995. [DOI] [PubMed] [Google Scholar]
- 5. Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, et al. (2005) Engineering vascularized skeletal muscle tissue. Nat Biotechnol 23: 879–884. [DOI] [PubMed] [Google Scholar]
- 6. Lee J, Oh YJ, Lee SK, Lee KY (2010) Facile control of porous structures of polymer microspheres using an osmotic agent for pulmonary delivery. J Control Release 146: 61–67. [DOI] [PubMed] [Google Scholar]
- 7. Girod Fullana S, Ternet H, Freche M, Lacout JL, Rodriguez F (2010) Controlled release properties and final macroporosity of a pectin microspheres-calcium phosphate composite bone cement. Acta Biomater 6: 2294–2300. [DOI] [PubMed] [Google Scholar]
- 8. Hwang JR, Sefton MV (1997) Effect of capsule diameter on the permeability to horseradish peroxidase of individual HEMA-MMA microcapsules. J Control Release 49: 217–227. [Google Scholar]
- 9. Güven S, Mehrkens A, Saxer F (2011) Engineering of large osteogenic grafts with rapid engraftment capacity using mesenchymal and endothelial progenitors from human adipose tissue. Biomaterials 32: 5801–5809. [DOI] [PubMed] [Google Scholar]
- 10. Kim TK, Yoon JJ, Lee DS, Park TG (2006) Gas foamed open porous biodegradable polymeric microspheres. Biomaterials 27: 152–159. [DOI] [PubMed] [Google Scholar]
- 11. Davis ME (2002) Ordered porous materials for emerging applications. Nature 417: 813–821. [DOI] [PubMed] [Google Scholar]
- 12. Piao Y, Burns A, Kim J, Wiesner U, Hyeon T (2008) Designed fabrication of silica-based nanostructured particle systems for nanomedicine applications. Adv Funct Mater 18: 3745–3758. [Google Scholar]
- 13. Nakanishi K, Tanaka N (2007) Sol-gel with phase separation. Hierarchically porous materials optimized for high-performance liquid chromatography separations. Acc Chem Res 40: 863–873. [DOI] [PubMed] [Google Scholar]
- 14. Odedra D, Chiu LL, Shoichet M, Radisic M (2011) Endothelial cells guided by immobilized gradients of vascular endothelial growth factor on porous collagen scaffolds. Acta Biomater 7: 3027–3035. [DOI] [PubMed] [Google Scholar]
- 15. Svec F, Fréchet JM (1996) New designs of macroporous polymers and supports: from separation to biocatalysis. Science 273: 205–211. [DOI] [PubMed] [Google Scholar]
- 16. Kim HK, Chung HJ, Park TG (2006) Biodegradable polymeric microspheres with “open/closed” pores for sustained release of human growth hormone. J Control Release 112: 167–174. [DOI] [PubMed] [Google Scholar]
- 17. Costa-Pinto AR, Reis RL, Neves NM (2011) Scaffolds based bone tissue engineering: the role of chitosan. Tissue Eng Part B Rev 17: 331–347. [DOI] [PubMed] [Google Scholar]
- 18. Patel MP, Patel RR, Patel JK (2010) Chitosan mediated targeted drug delivery system: a review. J Pharm Pharm Sci 13: 536–557. [DOI] [PubMed] [Google Scholar]
- 19. Wan Ngah WS, Teong LC, Hanafiah MAKM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohyd Polym 83: 1446–1456. [Google Scholar]
- 20. Cai X, Chen L, Jiang T, Shen X, Hu J, et al. (2011) Facile synthesis of anisotropic porous chitosan/hydroxyapatite scaffolds for bone tissue engineering. J Mater Chem 21: 12015–12025. [Google Scholar]
- 21. Naikwade S, Bajaj A (2009) Preparation and in vitro evaluation of budesonide spray dried microparticles for pulmonary delivery. Sci Pharm 77: 419. [Google Scholar]
- 22. Kanai Y, Oshima T, Baba Y (2008) Synthesis of highly porous chitosan microspheres anchored with 1,2-ethylenedisulfide moiety for the recovery of precious metal ions. Ind Eng Chem Res 47: 3114–3120. [Google Scholar]
- 23. Xi F, Wu J (2004) Macroporous chitosan layer coated on non-porous silica gel as a support for metal chelate affinity chromatographic adsorbent. J Chromatogr A 1057: 41–47. [DOI] [PubMed] [Google Scholar]
- 24. Wu J, Luan M, Zhao J (2006) Trypsin immobilization by direct adsorption on metal ion chelated macroporous chitosan-silica gel beads. Int J Biol Macromol 39: 185–191. [DOI] [PubMed] [Google Scholar]
- 25. Li XL, Li YF, Ye ZF (2011) Preparation of macroporous bead adsorbents based on poly(vinyl alcohol)/chitosan and their adsorption properties for heavy metals from aqueous solution. Chem Eng J 178: 60–68. [Google Scholar]
- 26. Liu XW, Hu QY, Fang Z, Zhang XJ, Zhang BB (2009) Magnetic Chitosan Nanocomposites: A Useful Recyclable Tool for Heavy Metal Ion Removal. Langmuir 25: 3–8. [DOI] [PubMed] [Google Scholar]
- 27. Kievit FM, Zhang M (2011) Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Acc Chem Res 44: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arami H, Stephen Z, Veiseh O, Zhang MQ (2011) Chitosan-coated iron oxide nanoparticles for molecular imaging and drug delivery. Adv Polym Sci 243: 163–184. [Google Scholar]
- 29. Song W, Lima AC, Mano JF (2010) Bioinspired methodology to fabricate hydrogel spheres for multi-applications using superhydrophobic substrates. Soft Matter 6: 5868–5871. [Google Scholar]
- 30. Son JS, Appleford M, Ong JL, Wenke JC, Kim JM, et al. (2011) Porous hydroxyapatite scaffold with three-dimensional localized drug delivery system using biodegradable microspheres. J Control Release 153: 133–140. [DOI] [PubMed] [Google Scholar]
- 31. Yu H, Chen S, Fan X, Quan X, Zhao H, et al. (2010) A structured macroporous silicon/graphene heterojunction for efficient photoconversion. Angew Chem Int Ed 49: 5106–5109. [DOI] [PubMed] [Google Scholar]
- 32. Zhao L, Zhu L, Wang Q, Li J, Zhang C, et al. (2011) Synthesis of composite microgel capsules by ultrasonic spray combined with in situ crosslinking. Soft Matter 7: 6144–6150. [Google Scholar]
- 33. Chang YC, Chen DH (2006) Recovery of gold(III) ions by a chitosan coated magnetic nano-adsorbent. Gold Bull 39: 98–102. [Google Scholar]
- 34. Park J, An K, Hwang Y, Park JG, Noh HJ, et al. (2004) Ultra-large-scale syntheses of monodisperse nanocrystals. Nat Mater 3: 891–895. [DOI] [PubMed] [Google Scholar]
- 35. Huang KS, Lin YS, Yang CH, Tsai CW, Hsu MY (2011) In situ synthesis of twin monodispersed alginate microparticles. Soft Matter 7: 6713–6718. [Google Scholar]
- 36. Wang CY, Yang CH, Lin YS, Chen CH, Huang KS (2012) Anti-inflammatory effect with high intensity focused ultrasound-mediated pulsatile delivery of diclofenac. Biomaterials 33: 1547–1553. [DOI] [PubMed] [Google Scholar]
- 37. Yang CH, Huang KS, Lin YS, Lu K, Tzeng CC, et al. (2009) Microfluidic assisted synthesis of multi-functional polycaprolactone microspheres: incorporation of CdTe quantum dots, Fe3O4 superparamagnetic nanoparticles, and tamoxifen. Lab Chip 9: 961–965. [DOI] [PubMed] [Google Scholar]
- 38. Yang CH, Lin YS, Huang KS, Huang YC, Wang EC, et al. (2009) Microfluidic emulsification and sorting assisted preparation of monodisperse chitosan microparticles. Lab Chip 9: 145–150. [DOI] [PubMed] [Google Scholar]
- 39. Hortigüela MJ, Aranaz I, Gutiérrez MC, Ferrer ML, del Monte F (2011) Chitosan gelation induced by the in situ formation of gold nanoparticles and its processing into macroporous scaffolds. Biomacromolecules 12: 179–186. [DOI] [PubMed] [Google Scholar]
- 40. Deville S, Saiz E, Nalla RK, Tomsia AP (2006) Freezing as a path to build complex composites. Science 311: 515–518. [DOI] [PubMed] [Google Scholar]
- 41. Zhang H, Hussain I, Brust M, Butler MF, Rannard SP, et al. (2005) Aligned two- and three-dimensional structures by directional freezing of polymers and nanoparticles. Nat Mater 4: 787–793. [DOI] [PubMed] [Google Scholar]
- 42. California A, Cardoso VF, Vitor Sencadas CM, Botelho G, Gómez-Ribelles JL, et al. (2011) Tailoring porous structure of ferroelectric poly(vinylidene fluoride-trifluoroethylene) by controlling solvent/polymer ratio and solvent evaporation rate. Eur Polym J 47: 2442–2450. [Google Scholar]
- 43. Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Jiang T, et al. (2010) In situ porous structures: a unique polymer erosion mechanism in biodegradable dipeptide-based polyphosphazene and polyester blends producing matrices for regenerative engineering. Adv Funct Mater 20: 2794–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tokuyama H, Kanehara A (2007) Novel synthesis of macroporous poly(N-isopropylacrylamide) hydrogels using oil-in-water emulsions. Langmuir 23: 11246–11251. [DOI] [PubMed] [Google Scholar]
- 45. Carn F, Steunou N, Livage J, Colin A, Backov R (2005) Tailor-made macroporous vanadium oxide foams. Chem Mater 17: 644649. [Google Scholar]
- 46. Huang KS, Lu K, Yeh CS, Chung SR, Lin CH, et al. (2009) Microfluidic controlling monodisperse microdroplet for 5-fluorouracil loaded genipin-gelatin microcapsules. J Control Release 137: 15–19. [DOI] [PubMed] [Google Scholar]
- 47. Lu LC, Wang CI, Sye WF (2011) Applications of chitosan beads and porous crab shell powder for the removal of 17 organochlorine pesticides (OCPs) in water solution. Carbohyd Polym 83: 1984–1989. [Google Scholar]
- 48. Zhou ZH, Wang J, Liu X, Chan HSO (2001) Synthesis of Fe3O4 nanoparticles from emulsions. J Mater Chem 11: 1704–1709. [Google Scholar]
- 49. Wang Z, Zhu H, Wang X, Yang F, Yang X (2009) One-pot green synthesis of biocompatible arginine-stabilized magnetic nanoparticles. Nanotechnology 20: 465606. [DOI] [PubMed] [Google Scholar]
- 50. Zhang L, Papaefthymiou GC, Ying JY (2001) Synthesis and properties of γ-Fe2O3 nanoclusters within mesoporous aluminosilicate matrices. J Phys Chem B 105: 7414–7423. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TEM images of iron oxide nanoparticles-loaded chitosan spheres prepared in various concentrations of NaOH solutions with (a) 5%, (b) 20%, (c) 30%, and (d) 50%, respectively.
(TIF)
The effects among chitosan concentration, chitosan molecular weight on the size of magnetic iron-oxide (MIO) nanoparticles.
(TIF)
Iron oxide nanoparticles-loaded chitosan spheres were attracted to wall of the vial by using an external magnetic field.
(TIF)
Magnetic characteristics of the iron oxide nanoparticles-loaded chitosan spheres. (a) Magnetization plots as a function of the applied field at 300 K. (b) Temperature dependent ZFC-FC magnetization curves measured at 500 Oe.
(TIF)
Stability test of the spheres in 37°C water (pH = 7). (a) shows the experimental setup. Spheres (made from 5%, 20%, 30% and 50% NaOH solution, respectively) deposed in the 37°C water (b) at the beginning, and (c) for 3 days.
(TIF)
Spheres placed in 1% acetic acid for (a) 20 minutes; (b) 3.5 hours; and (c) 6 hours.
(TIF)
Pernicious biological properties test for the nanoparticle-loaded chitosan spheres. (a) The cytotoxicity of iron oxide nanoparticles-loaded chitosan spheres (5.4±1.7 nanometers of iron oxide nanoparticles (with 5% NaOH) inside chitosan spheres) with three cell lines. (b) The MTT assay data of various iron oxide nanoparticles loaded chitosan spheres (MCF-7 cells). Sample A: 5.4±1.7 nanometers of iron oxide nanoparticles (with 5% NaOH) inside chitosan spheres. Sample B: 4.4±0.9 nanometers of iron oxide nanoparticles (with 20% NaOH) inside chitosan spheres. Sample C: 3.8±0.9 nanometers of iron oxide nanoparticles (with 30% NaOH) inside chitosan spheres. Sample D: 2.5±0.2 nanometers of iron oxide nanoparticles (with 50% NaOH) inside chitosan spheres.
(TIF)