Abstract
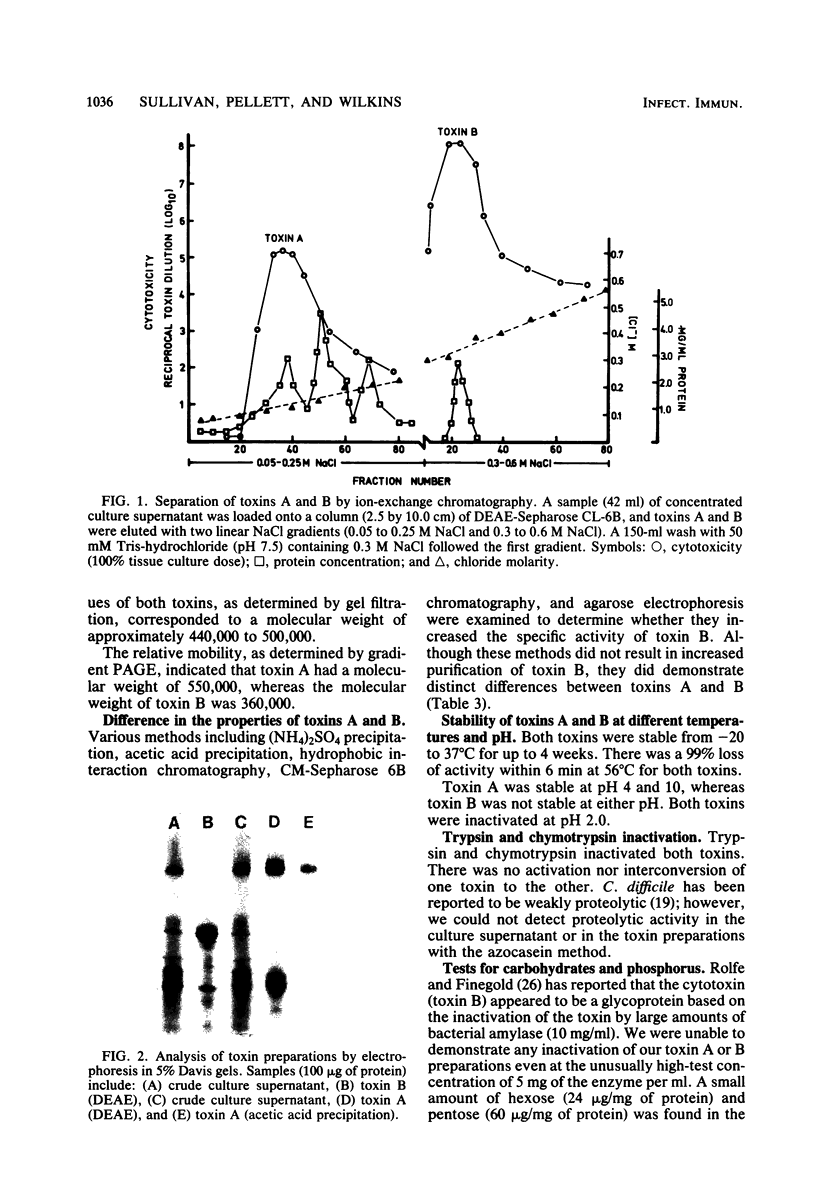
Toxin preparations were obtained by growing Clostridium difficile VPI strain 10463 in 2-liter brain heart infusion dialysis flasks at 37 degrees C for 3 days. The initial step of the purification scheme involved ultrafiltration through an XM-100 membrane filter. Two toxic activities, designated toxins A and B, were separated by ion-exchange chromatography on DEAE-NaCl gradients. Toxin A was purified to homogeneity by an acetic acid precipitation at pH 5.5. Other separation techniques, including CM Sepharose CL-6B, (NH4)2SO4 and acetic acid precipitations, and hydrophobic interaction chromatography, were examined in attempts to further purify toxin B. Although these methods failed to increase the specific activity of toxin B, they provided additional evidence that the two toxins are distinct molecules. The toxins are acid and heat labile and are inactivated by trypsin and chymotrypsin, but not by amylase. The molecular weight of toxin A, as estimated by gel filtration and gradient polyacrylamide electrophoresis, ranged from 440,000 to 500,000. The estimated molecular weight of toxin B was 360,000 to 470,000.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspberg K., Porath J. Group-specific adsorption of glycoproteins. Acta Chem Scand. 1970;24(5):1839–1841. doi: 10.3891/acta.chem.scand.24-1839. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Onderdonk A. B., Cisneros R. L., Kasper D. L. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1977 Nov;136(5):701–705. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Taylor N. S., Chang T., Dzink J. Clinical and laboratory observations in Clostridium difficile colitis. Am J Clin Nutr. 1980 Nov;33(11 Suppl):2521–2526. doi: 10.1093/ajcn/33.11.2521. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Bartlett J. G., Gorbach S. L., Onderdonk A. B. Clindamycin-induced enterocolitis in hamsters as a model of pseudomembranous colitis in patients. Infect Immun. 1978 May;20(2):526–529. doi: 10.1128/iai.20.2.526-529.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., Lauermann M., Bartlett J. G. Cytotoxicity assay in antibiotic-associated colitis. J Infect Dis. 1979 Nov;140(5):765–770. doi: 10.1093/infdis/140.5.765. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Spectrophotometric method for the determination of free pentose and pentose in nucleotides. J Biol Chem. 1949 Nov;181(1):379–392. [PubMed] [Google Scholar]
- De Siervo A. J. Alterations in the phospholipid composition of Escherichia coli B during growth at different temperatures. J Bacteriol. 1969 Dec;100(3):1342–1349. doi: 10.1128/jb.100.3.1342-1349.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich M., Van Tassell R. L., Libby J. M., Wilkins T. D. Production of Clostridium difficile antitoxin. Infect Immun. 1980 Jun;28(3):1041–1043. doi: 10.1128/iai.28.3.1041-1043.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekety R., Silva J., Browne R. A., Rifkin G. D., Ebright J. R. Clindamycin-induced colitis. Am J Clin Nutr. 1979 Jan;32(1):244–250. doi: 10.1093/ajcn/32.1.244. [DOI] [PubMed] [Google Scholar]
- Hauschild A. H., Hilsheimer R. Purification and characteristics of the enterotoxin of Clostridium perfringens type A. Can J Microbiol. 1971 Nov;17(11):1425–1433. doi: 10.1139/m71-227. [DOI] [PubMed] [Google Scholar]
- Humphrey C. D., Condon C. W., Cantey J. R., Pittman F. E. Partial purification of a toxin found in hamsters with antibiotic-associated colitis. Reversible binding of the toxin by cholestyramine. Gastroenterology. 1979 Mar;76(3):468–476. [PubMed] [Google Scholar]
- Kreger A. S., Gray L. D. Purification of Pseudomonas aeruginosa proteases and microscopic characterization of pseudomonal protease-induced rabbit corneal damage. Infect Immun. 1978 Feb;19(2):630–648. doi: 10.1128/iai.19.2.630-648.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby J. M., Wilkins T. D. Production of antitoxins to two toxins of Clostridium difficile and immunological comparison of the toxins by cross-neutralization studies. Infect Immun. 1982 Jan;35(1):374–376. doi: 10.1128/iai.35.1.374-376.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheka H. D., Enzmann P. J., Bachrach H. L., Migl B. The influence of sodium dodecyl sulfate from different sources on the separation of virus proteins in polyacrylamide gel electrophoresis. Anal Biochem. 1977 Jul;81(1):9–17. doi: 10.1016/0003-2697(77)90592-9. [DOI] [PubMed] [Google Scholar]
- Moldow C., Robertson J., Rothfield L. Purification of bacterial membrane proteins. The use of guanidinium thiocyanate and urea. J Membr Biol. 1972;10(2):137–152. doi: 10.1007/BF01867850. [DOI] [PubMed] [Google Scholar]
- Murthy R. J., Hercz A. Purification of human alpha 1-antitrypsin by affinity chromatography on sepharose bound concanavalin A. FEBS Lett. 1973 Jun 1;32(2):243–246. doi: 10.1016/0014-5793(73)80842-7. [DOI] [PubMed] [Google Scholar]
- Ochoa J. L. Hydrophobic (interaction) chromatography. Biochimie. 1978;60(1):1–15. doi: 10.1016/s0300-9084(78)80193-x. [DOI] [PubMed] [Google Scholar]
- Rolfe R. D., Finegold S. M. Purification and characterization of Clostridium difficile toxin. Infect Immun. 1979 Jul;25(1):191–201. doi: 10.1128/iai.25.1.191-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon L. M., Kay E., Lew J. Y. Peroxidase isozymes from horseradish roots. I. Isolation and physical properties. J Biol Chem. 1966 May 10;241(9):2166–2172. [PubMed] [Google Scholar]
- Taylor N. S., Bartlett J. G. Partial purification and characterization of a cytotoxin from Clostridium difficile. Rev Infect Dis. 1979 Mar-Apr;1(2):379–385. doi: 10.1093/clinids/1.2.379. [DOI] [PubMed] [Google Scholar]
- Taylor N. S., Thorne G. M., Bartlett J. G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981 Dec;34(3):1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]