Abstract
In most natural environments, association with a surface in a structure known as biofilm is the prevailing microbial life-style of bacteria. Polyphosphate (polyP), an ubiquitous linear polymer of hundreds of orthophosphate residues, has a crucial role in stress responses, stationary-phase survival, and it was associated to bacterial biofilm formation and production of virulence factors. In previous work, we have shown that Escherichia coli cells grown in media containing a critical phosphate concentration >37 mM maintained an unusual high polyP level in stationary phase. The aim of the present work was to analyze if fluctuations in polyP levels in stationary phase affect biofilm formation capacity in E. coli. Polymer levels were modulated by the media phosphate concentration or using mutant strains in polyP metabolism. Cells grown in media containing phosphate concentrations higher than 25 mM were defective in biofilm formation. Besides, there was a disassembly of 24 h preformed biofilm by the addition of high phosphate concentration to the medium. These phenotypes were related to the maintenance or re-synthesis of polyP in stationary phase in static conditions. No biofilm formation was observed in ppk−ppx− or ppk−ppx−/ppk+ strains, deficient in polyP synthesis and hydrolysis, respectively. luxS and lsrK mutants, impaired in autoinducer-2 quorum sensing signal metabolism, were unable to form biofilm unless conditioned media from stationary phase wild type cells grown in low phosphate were used. We conclude that polyP degradation is required for biofilm formation in sufficient phosphate media, activating or triggering the production of autoinducer-2. According to our results, phosphate concentration of the culture media should be carefully considered in bacterial adhesion and virulence studies.
Introduction
In clinical and industrial settings, bacteria are found predominantly forming biofilms and not as planktonic cells, such as those typically studied in the laboratory [1], [2]. Biofilms are defined as a complex cell assemblages enclosed in an adherent matrix which exhibit channels and pillars that are thought to allow the exchange of nutrients and wastes [1], [3], [4]. Biofilm formation is regulated by environmental conditions, such as nutrient availability [1], [5] and ionic strength [6], [7]. A model for biofilm development proposes that this phenomenon is initiated by the attachment of individual cells to a surface, followed by their migration and replication to form microcolonies to eventually produce the mature biofilm [8], [9]. These structures, which are generally hundreds of microns in depth, are difficult to eradicate by conventional techniques, such physical or chemical treatments, and cause problems in many natural, environmental, clinical, and industrial settings [3], [10], [1], [11], [12], [13].
Bacteria regulate gene expression in response to changes in cell population density by a process called quorum sensing (QS) [14]. This bacterial mechanism involves the release and detection of chemical signal molecules called autoinducers. Detection of minimal threshold stimulatory concentration of these molecules enables bacteria to distinguish between low and high cell population density and to control target gene expression in response to fluctuations in cell number [14]–[19]. Among the extracellular signal molecules, autoinducer-2 (AI-2) mediates interspecies communication and facilitates regulation of bacterial behaviors such as biofilm formation and virulence [20]. In Escherichia coli, the above mentioned AI-2, is derived from the precursor (S)-4,5-dihydroxy-2,3-pentanedione (DPD), which is synthesized by LuxS, encoded by the luxS gene [21]. Also, E. coli pathogenesis is regulated in a QS-dependent manner in response to indole production [22]. Indole is synthesized by TnaA, a tryptophanase encoded by tnaA gene [23]. Mtr (encoded by mtr gene), a high-affinity tryptophan permease, is the main conduit for indole import in E. coli [24].
Inorganic polyphosphate (polyP) is a linear chain of tens or many hundreds of phosphate residues linked by high-energy phosphoanhydride bonds, which is found in every cell in nature: bacterial, archaeal, fungal, protozoan, plant, and animal [25], [26]. Some of the polyP functions are the substitution for ATP in kinase reactions; reservoir of phosphate; chelation of divalent metals; capsule production in bacteria; and regulatory roles in growth, development, stress, and nutrients deprivation [25], [26]. In bacteria, polyP is usually accumulated during exponential phase of growth, and degraded at the beginning of stationary phase. The main enzymes associated with polyP metabolism are the polyphosphate kinases (PPK, encoded by ppk gene), responsible for the polyP synthesis from ATP; and the exopolyphosphatase (PPX, encoded by ppx gene), responsible for the polyP hydrolysis [27]–[29]. The different roles of polyP have been inferred from mutant cells lacking polyphosphate kinase (PPK), deficient in polyP synthesis. For instance, using ppk mutants of several microorganisms such as Pseudomonas aeruginosa, E. coli, Salmonella enterica serovar Dublin, Vibrio cholerae, Bacillus cereus and Porphyromonas gingivalis, polyP formation was shown to be critical for attributes such as motility, quorum sensing, biofilm formation, resistance to oxidative, osmotic, heat, nutritional and alkaline stress, and stationary-phase survival [25], [27], [30]–[37]. Although the importance of polyP in various bacterial phenotypes has been reported, the precise molecular mechanisms by which polyP enacts specific functions, as well as the primary and secondary effects of poly-P accumulation, are still not understood in even the best-characterized bacterial species.
We have previously shown that E. coli cells grown in shacked media containing a critical phosphate concentration >37 mM maintained an unusual high polyP level in stationary phase (up to 72 h) [38]. Here, we found that high phosphate media impaired biofilm formation, and this phenotype was related with the maintenance of polyP in stationary phase in static growth. The present study is a first step towards the investigation of how polyP levels fluctuations induce quorum sensing signals involved in biofilm formation.
Materials and Methods
Bacterial strains, growth conditions and media
Bacterial strains used in this study are listed in Table 1 . For inocula preparation, isolated colonies were grown aerobically at 37°C with linear shaking in MT or MT+P medium. MT minimal medium contains per liter of distilled water: 0.272 g KH2PO4 (corresponding to 2 mM), 5.8 g NaCl, 3.7 g KCl, 0.15 g CaCl2.2H2O, 1.1 g NH4Cl, 0.142 g Na2SO4, 12.1 g Tris (Tris [hydroxymethyl] aminomethane), 0.27 mg FeCl3, and 0.2 g MgSO4.7H2O. MT media with 40 mM phosphate buffer pH 7 was designated as MT+P [42]. MT cultures supplemented at 24 or 48 h with 40 mM phosphate buffer pH 7 were named as MT+24P and MT+48P, respectively. Also, 24 h MT+P cells shifted to MT fresh medium were represented as MT+P→24MT. When indicated, minimal medium was prepared with phosphate concentrations other than 2 or 40 mM. Phosphate buffer was prepared with sodium phosphate salts (Sigma). In all experiments, 0.4% glucose and 0.1% tryptone were used as carbon and nitrogen sources, respectively. When required, antibiotics were used: 40 µg mL−1 of ampicillin or 50 µg mL−1 of kanamycin. Viability was determined by counting the CFU on LB-agar plates incubated at 37°C for 24 h.
Table 1. E. coli strains and plasmid used in this work.
| Strains or plasmid | Relevant genotype or description | Source or reference |
| MC4100 | araD, Δlac, rpsL, flbB, deoC, ptsF, rbsR, relA1 | [39] |
| BW25113 | K-12 derivative, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ−, rph-1, Δ(rhaD-rhaB)568, hsdR514 | CGSC |
| MG1655 | F−, λ−, rph-1 | CGSC |
| C | E. coli (Migula) Castellani and Chalmers | ATCC |
| LSB022 | MC4100 (ppkppx::Km) | [38] |
| LSB022/pBC29 | LSB022/pBC29((ppkppx::Km/ppk+, Ap) | This study |
| JW2662-1 | BW25113 (luxS::Km) | CGSC |
| JW3686-7 | BW25113 (tnaA::Km) | CGSC |
| JW3130-1 | BW25113 (mtr::Km) | CGSC |
| MG1655 lsrK− | MG1655 (lsrK−) | [40] |
| pBC29 | (ppk+, Ap) | [41] |
Quantification of biofilm formation
Biofilm formation was assayed on the basis of the ability of cells to adhere and grow on 96-well polystyrene microtiter plates [8]. Overnight stationary phase cultures in MT or MT+P were diluted to an OD560 = 0.1 (corresponding to CFU mL−1 from 5 to 6×107) with fresh medium and grown in statics conditions at 30°C in microtiter plates for 24, 48 or 72 h. After removing the unattached cells and rinsed the plates three times with deionized water, quantification of attached cells was performed as follows. Two hundred microliters of a 0.1% crystal violet solution was added to each well, and the plates were incubated at room temperature for 30 min in dark. Then, the wells were rinsing again three times with water. Crystal violet stained attached cells was extracted with 200 µl of 95% ethanol. Absorbances of the adherent and non-adherent cells were measured at 595 nm and 560 nm, respectively (SpectraMax Plus384 Absorbance Microplate Reader, US). Six replicates were performed for each condition studied in each experiment.
Conditioned media experiments
Conditioned media (CM) are spent media obtained from bacterial cultures by two sequential centrifugation at 15 000 r.p.m. and a 0.2 µm filtration. 24 h MT growing cells were shifted to CM and incubated for further 24 h before biofilm quantification.
Measurements of polyP level
Intracellular polyP was measured in cell suspensions growing in static conditions, using a DAPI (4′,6-diamidino-2-phenylindole) based fluorescence approach [43]. Cells were washed and resuspended in buffer T (100 mM Tris HCl, pH 7.5). 17 µM DAPI (Sigma) was added to cuvettes containing cell suspensions in buffer T, with SDS and chloroform for cell permeabilization, at an OD560 = 0.02. After 5 min of agitation at 37°C, the DAPI fluorescence spectra (excitation, 415 nm; emission, 445 to 650 nm) were recorded using an ISS PCI spectrofluorometer (Champaign, IL). Fluorescence (in arbitrary units) of the DAPI-polyP complex at 550 nm was used as a measure of intracellular polyP since fluorescence emissions from free DAPI and from DAPI-DNA are minimal at this wavelength [43].
Statistical analysis
Data were subjected to analysis of variance (ANOVA) followed by Tukey's test with Statitix 9.0 Analytical Software 2008 for Windows (USA). Differences at p-value of 0.05 were considered significant.
Results
High phosphate concentration impairs biofilm formation
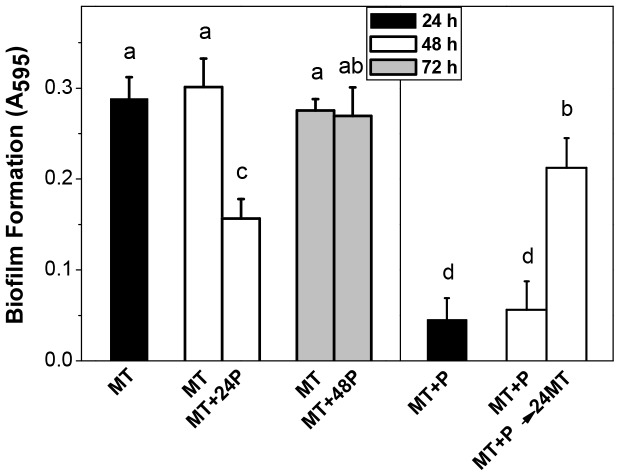
The ability of different E. coli strains to form biofilm was studied in sufficient (MT, 2 mM) or high (MT+P, 40 mM) phosphate media. In all wild type strains tested, the biofilm formed at 48 h by MT+P cells was around 2.5 fold lower than that of cells grown in MT ( Fig. 1 ). Furthermore, when 40 mM phosphate buffer was added to a 24 h MT culture (MT+24P), disassemble of the preformed biofilm was achieved. This effect was not observed when the Pi addition was carried out at 48 h (MT+48P) ( Fig. 2 , left panel). On the other hand, when non-biofilm forming MT+P cells were switched to fresh MT medium at 24 h (MT+P→24MT), the adherence at 48 h increased significantly ( Fig. 2 , right panel). Since similar results were achieved with the other wild type strains (data not shown), following studies were performed using MC4100.
Figure 1. Biofilm formation in MT and MT+P media.
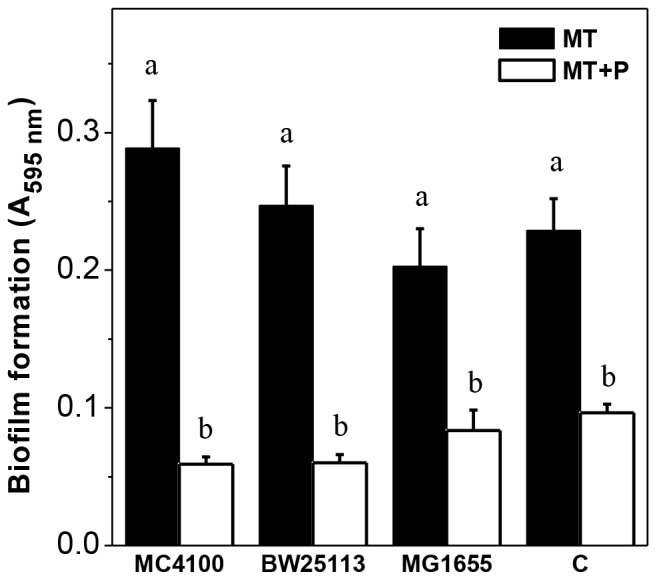
E. coli wild type strains were grown in static conditions during 48 h at 30°C in the indicated media. The biofilm amount was determined by the crystal violet assay. Results represent the mean ± SD of four independent experiments. Different letters indicate significant differences according to Tukey's test with a p-value of 0.05.
Figure 2. Biofilm formation after changes of phosphate concentration in stationary phase.
Biofilm formation by MC4100 was measured at indicated times of growth in MT and in MT with the addition of 40 mM phosphate buffer pH 7 at 24 h (MT+24P) or at 48 h (MT+48P) (left panel), or in MT+P and in MT+P culture switched to fresh MT at 24 h (MT+P→24MT) (right panel). Result represents the mean ± SD of at least three independent experiments performed in triplicate. Different letters indicate significant differences according to Tukey's test with a p-value of 0.05.
Biofilm formation dependency with polyP levels fluctuations
In order to analyze if the impairment of biofilm formation in high phosphate medium was related to intracellular polyP, the polymer levels were measured in cells grown in static conditions at 30°C. Fig. 3 shows the values obtained at different times of growth in MT and MT+P media. In exponential phase, polyP levels increased independently of the growth media. In stationary phase, a high polymer level (AU = 256000±1960) was maintained in MT+P, whereas in MT medium an abrupt decrease was observed, reaching to minimum detectable values at 10 h ( Fig. 3A ). Under our growth conditions, the OD560 nm at 48 h in MT+P was higher than in MT ( Fig. 3B ). It should be noted that biofilm formation in MT started at 12 h of growth, after the drop of polyP levels, reaching the maximum value from 16 h ( Fig. 3A and C ).
Figure 3. PolyP levels and growth in static cultures.
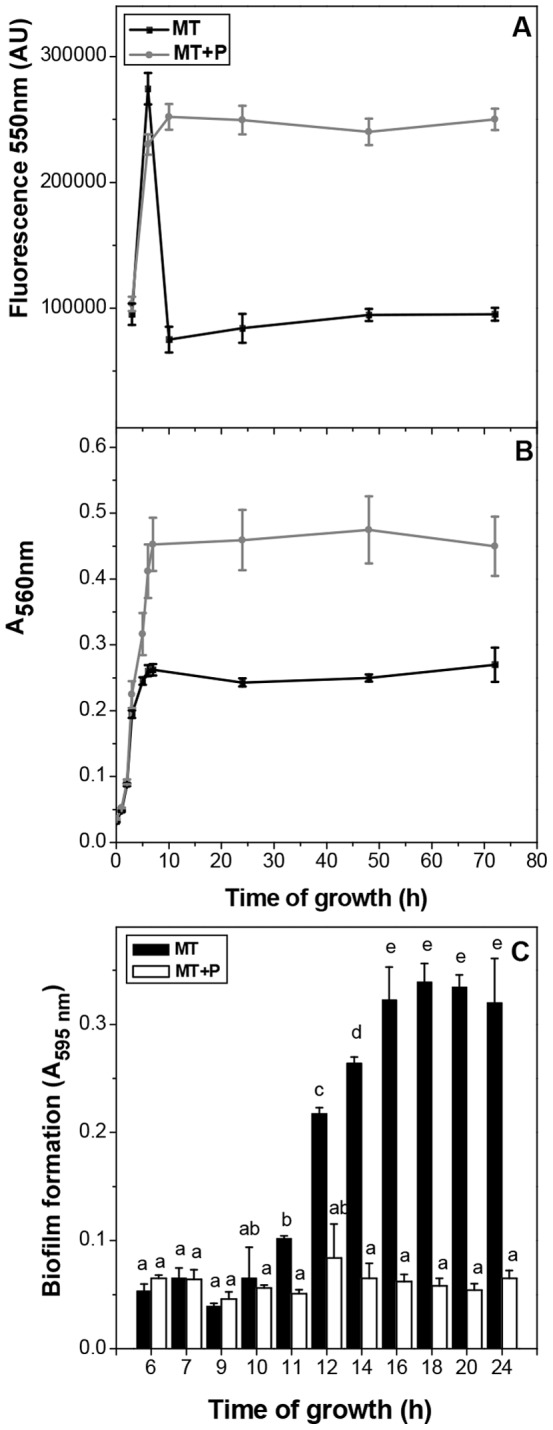
The fluorescence intensity in arbitrary units (AU) of DAPI-polyP complex (excitation λ = 415 nm, emission λ = 550 nm) (A) and A560 nm (B) were measured during the growth curve of MC4100 in static conditions in the indicated media. Biofilm formation was followed at the indicated times and media (C). Data represent the mean ± SD of at least four independent experiments. Different letters indicate significant differences according to Tukey's test with a p-value of 0.05.
In cells from experimental approach shown in Fig. 2 , polyP levels were measured 24 h after the media modification. In MT+24P, a recovery of polyP level was observed (AU = 245000±1840), while in MT+48P polyP remained low (AU = 94562±3215) as in MT stationary cells. In MT+P→24MT, there was a drop in polyP levels (AU = 96562±4515).
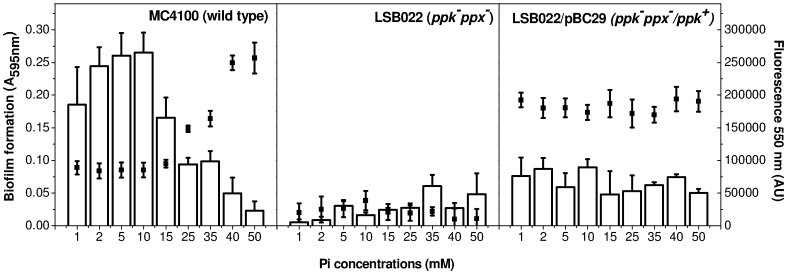
PolyP levels and biofilm formation capacity were tested at 48 h in MC4100, LSB022 (ppk−ppx−) and LSB022/pBC29 (ppk−ppx−/ppk+) cells grown in media supplemented with a wide range of phosphate concentrations. Fig. 4 shows that in MC4100, both biofilm formation and intracellular polyP levels were dependent on phosphate concentration. In a range between 1 and 10 mM phosphate, both low polyP levels and high biofilm formation were observed. On the contrary, when phosphate was above 25 mM, high intracellular polyP levels were detected and biofilm formation was impaired. In an additional experiment, mixing 24 h cultures from MT and MT+P media (Fig. S1), biofilm formation was modified by the media proportion used, similarly to data of MC4100 in Fig. 4 . In any of the phosphate concentrations tested, biofilm formation was observed in LSB022 and LSB022/pBC29 strains, being the polyP level undetectable in ppk−ppx− deficient strain and high in the complemented strain ( Fig. 4 ). For each mutant strain, similar polymer levels to that of Fig. 4 were obtained during the whole growth curve (not shown).
Figure 4. PolyP levels and biofilm formation in different Pi concentrations media.
The biofilm amount (white bars) and DAPI-polyP fluorescence (black dots) were determined at 48 h in the indicated E. coli strains grown in static conditions in MT medium modified with the indicated Pi concentrations. Data represent the mean ± SD of at least three independent experiments.
AI-2 trigger biofilm formation in MT cells
Biofilm formation capacity was analyzed in QS deficient strains, lacking luxS (gene encoding for AI-2 synthesis), lsrK (gene encoding for AI-2 phosphorylation), tnaA (gene encoding for indol synthase) or mtr (gene encoding for indol receptor). As shown in Fig. 5 , indol mutants show similar biofilm phenotypes than that of the wild type in MT and MT+P. However, luxS and lsrK mutants were unable to form biofilm in MT medium, contrarily to the wild type. Despite this difference, it is noted that the polyP profiles for luxS− and wild type cells were similar ( Table 2 and Fig. 3 , respectively).
Figure 5. Biofilm formation by quorum sensing mutants.
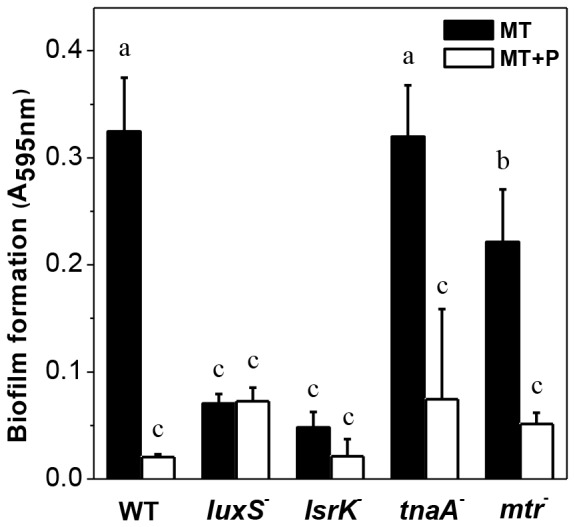
The biofilm amount was determined at 48 h in the indicated strains grown in static conditions in MT or MT+P media. Different letters indicate significant differences according to Tukey's test with a p-value of 0.05.
Table 2. PolyP levels in luxS− strain in static conditions.
| PolyP level (AU)* | ||
| MT | MT+P | |
| 6 h | 258500±10540 a | 245861±10120 a |
| 24 h | 75000±11500 b | 235468±9245 a |
| 48 h | 84652±6856 b | 254896±11486 a |
Fluorescence 550 nm. Different letters indicate significant differences according to Tukey's test with a p-value of 0.05.
Extracellular AI-2 is associated to polyP degradation
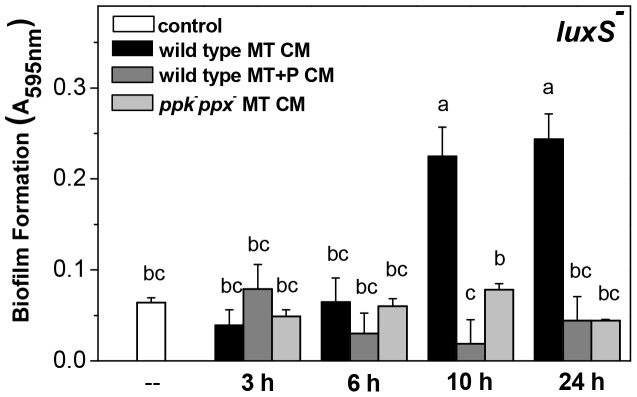
Biofilm formation was quantified in luxS mutant shifted to conditioned media (CM). CM were obtained from MT and MT+P wild type and MT ppk−ppx− cultures at different times of growth (3, 6, 10 and 24 h). The deficiency in biofilm formation by luxS− strain was recovered only when 10 and 24 h wild type MT CM were used ( Fig. 6 ). Besides, only 24 h wild type MT CM but not luxS− MT CM, induced biofilm formation by ppk−ppx− or ppk−ppx−/ppk+ cells ( Table 3 ), indicating that extracellular AI-2 is present only in MT CM from stationary phase wild type cultures.
Figure 6. Biofilm formation by luxS mutant in conditioned media.
luxS− cells growing in MT medium during 24 h were reinoculated in wild type MT CM or MT+P CM and in ppk−ppx− MT CM from different growth times, as indicated. Biofilm formation was determined 24 h after the shift to CM. 48 h MT cultures were used as control (–). Different letters indicate significant differences according to Tukey's test with a p-value of 0.05.
Table 3. Biofilm formation after reinoculation in CM.
| Biofilm formation (A595 nm)* | |||
| Strains | Control | wild type MT CM | luxS− MT CM |
| LSB022 ( ppk−ppx− ) | 0.06223±0.024 a | 0.154±0.0124 b | 0.0525±0.0235 a |
| LSB022/pBC29 ( ppk−ppx−/ppk+ ) | 0.0845±0.0254 a | 0.3015±0.0712 c | 0.0456±0.0123 a |
Biofilm formation was determined 24 h after the cells transference to CM. 48 h MT cultures were used as control. Different letters indicate significant differences according to Tukey's test with a p-value of 0.05.
Discussion
In many bacterial species, biofilm formation seems to be promoted by non-optimal growth conditions or even by cellular stress [44]. In our experimental conditions, biofilm formation by E. coli occurred in MT medium, where stationary phase cells were particularly vulnerable to stress conditions as described by Schurig-Briccio et al. [45]. In sufficient phosphate media, biofilm formation from 12 h of growth was associated to the decrease of polyP levels at early stationary phase, while in high phosphate media, the impairment in biofilm formation was correlated to the maintenance of high polyP levels. Variations of intracellular polyP levels, induced by changes of Pi concentration, were feasible in cultures up to 24 h. These phosphate dependent modulations of polyP levels were responsible for differences in biofilm amount.
PolyP has been described to be critical for mechanisms such as motility, quorum sensing and biofilm formation [33]–[35]. Kim et al. [46] proposed that the presence of polyP in P. aeruginosa exponential phase is important for regulatory purposes, for instance, participating in the production of exopolysaccharides such as alginate. Our data obtained with LSB022 (ppk−ppx−) support that polyP synthesis is necessary for cell adherence. In addition, impairment in biofilm formation was observed in LSB022/pBC29 (ppk−ppx−/ppk+) strain. In the Gram positive bacterium Bacillus cereus, Shi et al. [47] observed that both ppk and ppx mutants were defective in motility and biofilm formation. They considered that maintenance of intracellular concentration of polyP within a narrow range, as that of wild type, might be essential for biofilm formation effectiveness [47]. However, we propose that polyP degradation carried out at the beginning of stationary phase, rather than its presence or maintenance, is relevant to trigger adherence process in E. coli. This affirmation is based on the different biofilm phenotypes observed in wild type cells, promoted by fluctuations of intracellular polyP levels modulated by media phosphate concentration.
We consider that autoinducer-2 is involved in biofilm formation in MT, since luxS mutant was unable to form biofilm in this medium. However, biofilm formation in luxS mutant was induced by CM obtained from MT stationary phase wild type cultures (polyP degrading cells), but not using CM obtained from non-degrading polyP cells. These data suggest that polyP degradation and/or the released Pi or energy may be involved in the regulation of AI-2 synthesis. Eisenbach [48] has described that polyP might be a substitute for ATP in the phosphorilation of chemotaxis systems proteins or that phospho-PPK might directly transfer phosphate (phosphorylation by crosstalk). Further studies are necessary to elucidate the signal produced after polyP degradation responsible for AI-2 synthesis.
Taken together, we conclude that polyP degradation is required for biofilm formation in sufficient phosphate media, activating or triggering the production of autoinducer-2. PPX could be provided as an additional target to discover antibiotics against pathogenic E. coli cells. Our findings on the relationship between exogenous phosphate concentration, biofilm formation and polyP level may help to elucidate the importance of phosphate and/or its metabolites as signals for virulence responses in pathogens.
Supporting Information
Biofilm formation in mixed cultures. Biofilm formation by MC4100 was measured 24 h after mixing 24 h MT and MT+P cultures in the indicated proportions. Result represents the mean +/− SD of at least two independent experiments performed in duplicate. Different letters indicate significant differences according to Tukey's test with a p-value of 0.05.
(TIF)
Acknowledgments
We gratefully acknowledge Dr Ross Carlson for providing the strain MG1655 (lsrK−) and Dr Lici A. Schurig-Briccio for helpful contributions in the subject.
Funding Statement
This research was supported by grants 26/D329 from Consejo de Investigaciones de la Univeridad Nacional de Tucumán (CIUNT), PIP6399 from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49: 711–745. [DOI] [PubMed] [Google Scholar]
- 2. Danese P, Pratt LA, Kolter R (2000) Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J Bacteriol 182: 3593–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284: 1319–1322. [DOI] [PubMed] [Google Scholar]
- 4. Davey ME, O'Toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol Biol Rev 64: 847–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terada A, Hibiya K, Nagai J, Tsuneda S, Hirata A (2003) Nitrogen removal characteristics and biofilm analysis of a membrane-aerated biofilm reactor applicable to high-strength nitrogenous wastewater treatment. J Biosci Bioeng 95: 170–178. [DOI] [PubMed] [Google Scholar]
- 6. Busalmen JP, de Sanchez SR (2001) Influence of pH and ionic strength on adhesion of a wild strain of Pseudomonas sp. to titanium. J Ind Microbiol Biotech 26: 303–308. [DOI] [PubMed] [Google Scholar]
- 7. Gomez MA, Galvez JM, Hontoria E, Gonzalez-Lopez J (2003) Influence of ethanol concentration on biofilm bacterial composition from a denitrifying submerged filter used for contaminated groundwater. J Biosci Bioeng 95: 245–251. [PubMed] [Google Scholar]
- 8. O'Toole GA, Kolter R (1998) Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol Microbiol 42: 449–461. [DOI] [PubMed] [Google Scholar]
- 9. Pratt LA, Kolter R (1998) Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol 30: 285–293. [DOI] [PubMed] [Google Scholar]
- 10. Sung BH, Lee CH, Yu BJ, Lee JH, Lee JY, et al. (2006) Development of a biofilm production-deficient Escherichia coli strain as a host for biotechnological applications. Appl Environ Microbiol 72: 3336–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar CG, Anand SK (1998) Significance of microbial biofilms in food industry: a review. Int J Food Microbiol 42: 9–27. [DOI] [PubMed] [Google Scholar]
- 12. Zottola EA, Sasahara KC (1994) Microbial biofilms in the food processing industry-should they be a concern? Int J Food Microbiol 23: 125–148. [DOI] [PubMed] [Google Scholar]
- 13. Kubota H, Senda S, Nomura N, Tokuda H, Uchiyama H (2008) Biofilm formation by lactic acid bacteria and resistance to environmental stress. J Biosci Bioeng 106: 381–386. [DOI] [PubMed] [Google Scholar]
- 14. Bassler BL (1999) How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol 2: 582–587 Review. [DOI] [PubMed] [Google Scholar]
- 15. Fuqua C, Winans SC, Greenberg EP (1996) Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum sensing transcriptional regulators. Annu Rev Microbiol 50: 727–751 Review. [DOI] [PubMed] [Google Scholar]
- 16. Kleerebezem M, Quadri LE, Kuipers OP, de Vos WM (1997) Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol Microbiol 24: 895–904 Review. [DOI] [PubMed] [Google Scholar]
- 17. Lazazzera BA, Grossman AD (1998) The ins and outs of peptide signaling. Trends in Microbiol 6: 288–294. [DOI] [PubMed] [Google Scholar]
- 18. de Kievit TR, Iglewski B (2000) Bacterial quorum sensing in pathogenic relationships. H Infect Immun 68: 4839–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55: 165–199 Review. [DOI] [PubMed] [Google Scholar]
- 20. Marques JC, Lamosa P, Russell C, Ventura R, Maycock C, et al. (2011) Processing the interspecies quorum sensing signal autoinducer-2 (AI-2): characterization of phospho-(S)-4,5-dihydroxy-2,3-pentanedione isomerization by LsrG protein. J Biol Chem 286: 18331–18343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xavier KB, Bassler BL (2003) LuxS quorum sensing: more than just a numbers game. Curr Opin Microbiol 6: 191–197. [DOI] [PubMed] [Google Scholar]
- 22. Wang D, Ding X, Rather PN (2001) Indole can act as an extracellular signal in Escherichia coli . J Bacteriol 183: 4210–4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han TH, Lee JH, Cho MH, Wood TK, Lee J (2011) Environmental factors affecting indole production in Escherichia coli . Res Microbiol 162: 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yanofsky C, Horn V, Gollnick P (1991) Physiological studies of tryptophan transport and tryptophanase operon induction in Escherichia coli . J Bacteriol 173: 6009–6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulaev IS (1979) The biochemistry of inorganic polyphosphates. New York: Wiley. [DOI] [PubMed]
- 26. Kornberg A, Rao NN, Ault-Riche D (1999) Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem 68: 89–125. [DOI] [PubMed] [Google Scholar]
- 27. Rao NN, Kornberg A (1996) Inorganic polyphosphate supports resistance and survival of stationary-phase Escherichia coli . J Bacteriol 178: 1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahn K, Kornberg A (1990) Polyphosphate kinase from Escherichia coli. Purification and demonstration of a phosphoenzyme intermediate. J Biol Chem 265: 11734–11739. [PubMed] [Google Scholar]
- 29. Akiyama M, Crooke E, Kornberg A (1993) An exopolyphosphatase of Escherichia coli. The enzyme and its ppx gene in a polyphosphate operon. J Biol Chem 268: 633–639. [PubMed] [Google Scholar]
- 30. Rashid MH, Kornberg A (2000) Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa . Proc Natl Acad Sci 97: 4885–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rashid MH, Rao NN, Kornberg A (2000) Inorganic polyphosphate is required for motility of bacterial pathogens. J Bacteriol 182: 225–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rashid MH, Rumbaugh K, Passador L, Davies DG, Hamood AN, et al. (2000) Polyphosphate kinase is essential for biofilm development, quorum sensing, and virulence of Pseudomonas aeruginosa . Proc Natl Acad Sci 97: 9636–9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jahid IK, Silva AJ, Benitez JA (2006) Polyphosphate stores enhance the ability of Vibrio cholerae to overcome environmental stresses in a low-phosphate environment. Appl Environ Microbiol 72: 7043–7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim KS, Rao NN, Fraley CD, Kornberg A (2002) Inorganic polyphosphate is essential for long-term survival and virulence factors in Shigella and Salmonella spp. Proc Natl Acad Sci USA 99: 7675–7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ogawa N, Tzeng CM, Fraley CD, Kornberg A (2000) Inorganic polyphosphate in Vibrio cholerae: genetic, biochemical, and physiologic features. J Bacteriol 182: 6687–6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Price-Carter M, Fazzio TG, Vallbona EI, Roth JR (2005) Polyphosphate kinase protects Salmonella enterica from weak organic acid stress. J Bacteriol 187: 3088–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan S, Fraley CD, Zhang M, Dailidiene D, Kornberg A, et al. (2005) Diverse phenotypes resulting from polyphosphate kinase gene (ppk1) inactivation in different strains of Helicobacter pylori . J Bacteriol 187: 7687–7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schurig-Briccio LA, Farías RN, Rintoul MR, Rapisarda VA (2009) Phosphate-enhanced stationary-phase fitness of Escherichia coli is related to inorganic polyphosphate level. J Bacteriol 191: 4478–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Casadaban MJ, Cohen SN (1979) Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A 76: 4530–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zuroff TR, Bernstein H, Lloyd-Randolfi J, Jimenez-Taracido L, Stewart PS, et al. (2010) Robustness analysis of culturing perturbations on Escherichia coli colony biofilm beta-lactam and aminoglycoside antibiotic tolerance. BMC Microbiol 10: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crooke E, Akiyama M, Rao NN, Kornberg A (1994) Genetically altered levels of inorganic polyphosphate in Escherichia coli . J Biol Chemr 4: 6290–6295. [PubMed] [Google Scholar]
- 42. Schurig-Briccio LA, Rintoul MR, Volentini SI, Farías RN, Baldoma L, et al. (2008) A critical phosphate concentration in the stationary phase maintains ndh gene expression and aerobic respiratory chain activity in Escherichia coli . FEMS Microbiol Lett 284: 76–83. [DOI] [PubMed] [Google Scholar]
- 43. Aschar-Sobbi R, Abramov AY, Diao C, Kargacin ME, Kargacin GJ, et al. (2008) High sensitivity, quantitative measurements of polyphosphate using a new DAPI-based approach. J Fluoresc 18: 859–866. [DOI] [PubMed] [Google Scholar]
- 44. Castonguay MH, van der Schaaf S, Koester W, Krooneman J, van der Meer W, et al. (2006) Biofilm formation by Escherichia coli is stimulated by synergistic interactions and co-adhesion mechanisms with adherence-proficient bacteria. Res Microbiol 157: 471–478. [DOI] [PubMed] [Google Scholar]
- 45. Schurig-Briccio LA, Farías RN, Rodríguez-Montelongo L, Rintoul MR, Rapisarda VA (2009) Protection against oxidative stress in Escherichia coli stationary phase by a phosphate concentration-dependent genes expression. Arch Biochem Biophys 483: 106–110. [DOI] [PubMed] [Google Scholar]
- 46. Kim HY, Schlictman D, Shankar S, Xie Z, Chakrabarty AM, et al. (1998) Alginate, inorganic polyphosphate, GTP and ppGpp synthesis co-regulated in Pseudomonas aeruginosa: implications for stationary phase survival and synthesis of RNA/DNA precursors. Mol Microbiol 27: 717–725. [DOI] [PubMed] [Google Scholar]
- 47. Shi X, Rao NN, Kornberg A (2004) Inorganic polyphosphate in Bacillus cereus: motility, biofilm formation, and sporulation. Proc Natl Acad Sci 101: 17061–17065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eisenbach M (1996) Control of bacterial chemotaxis. Mol Microbiol 20: 903–910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Biofilm formation in mixed cultures. Biofilm formation by MC4100 was measured 24 h after mixing 24 h MT and MT+P cultures in the indicated proportions. Result represents the mean +/− SD of at least two independent experiments performed in duplicate. Different letters indicate significant differences according to Tukey's test with a p-value of 0.05.
(TIF)