Abstract
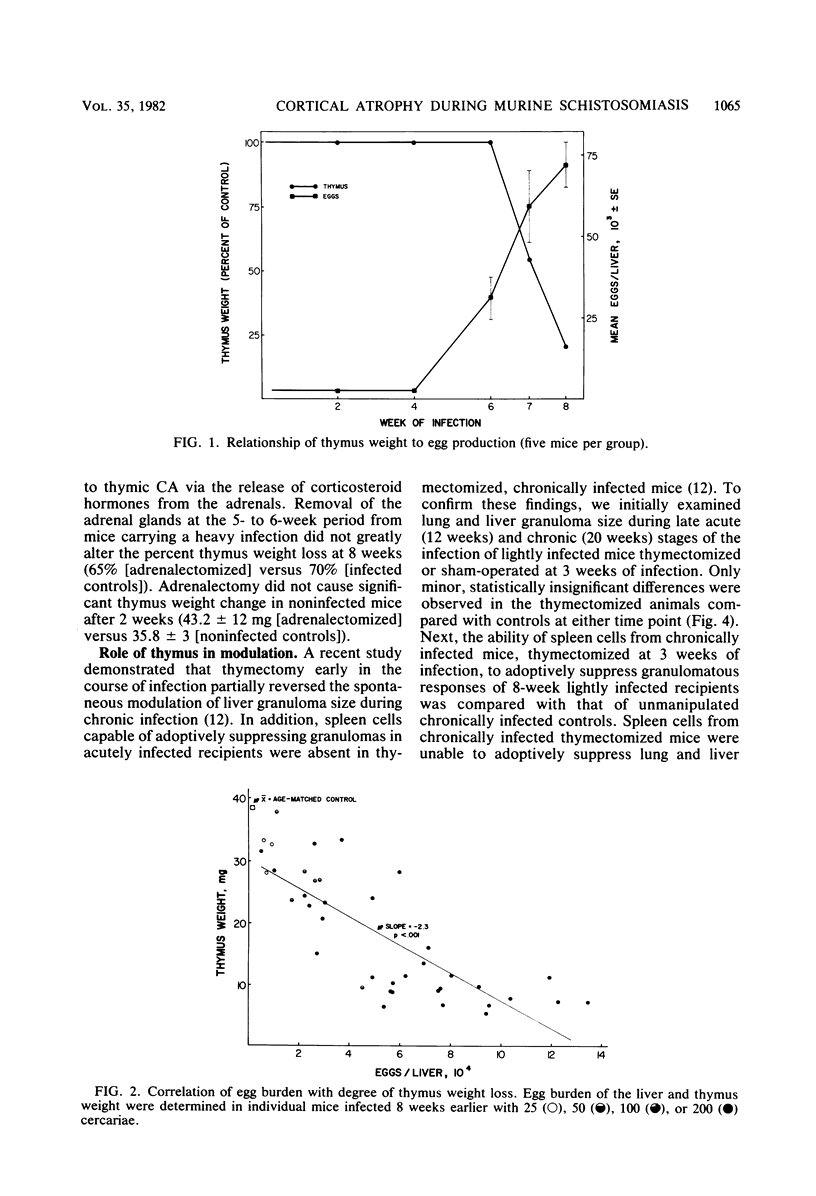
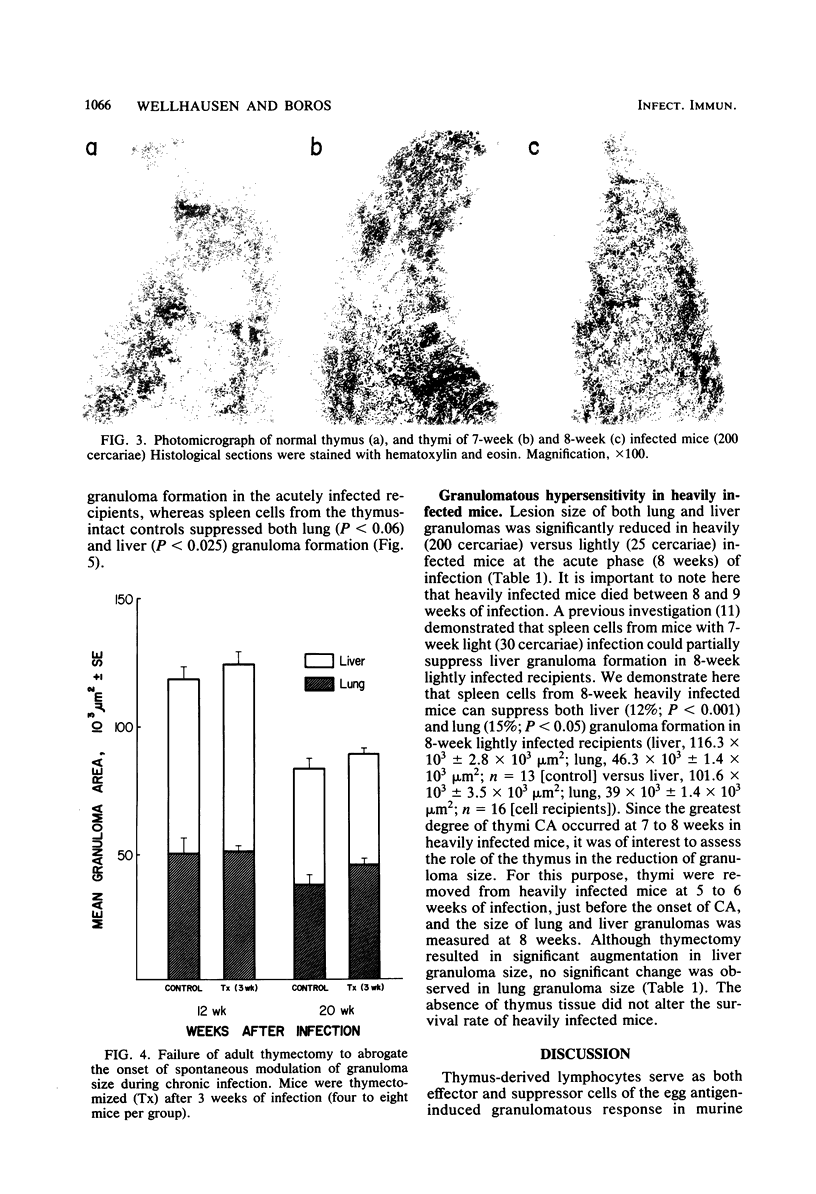
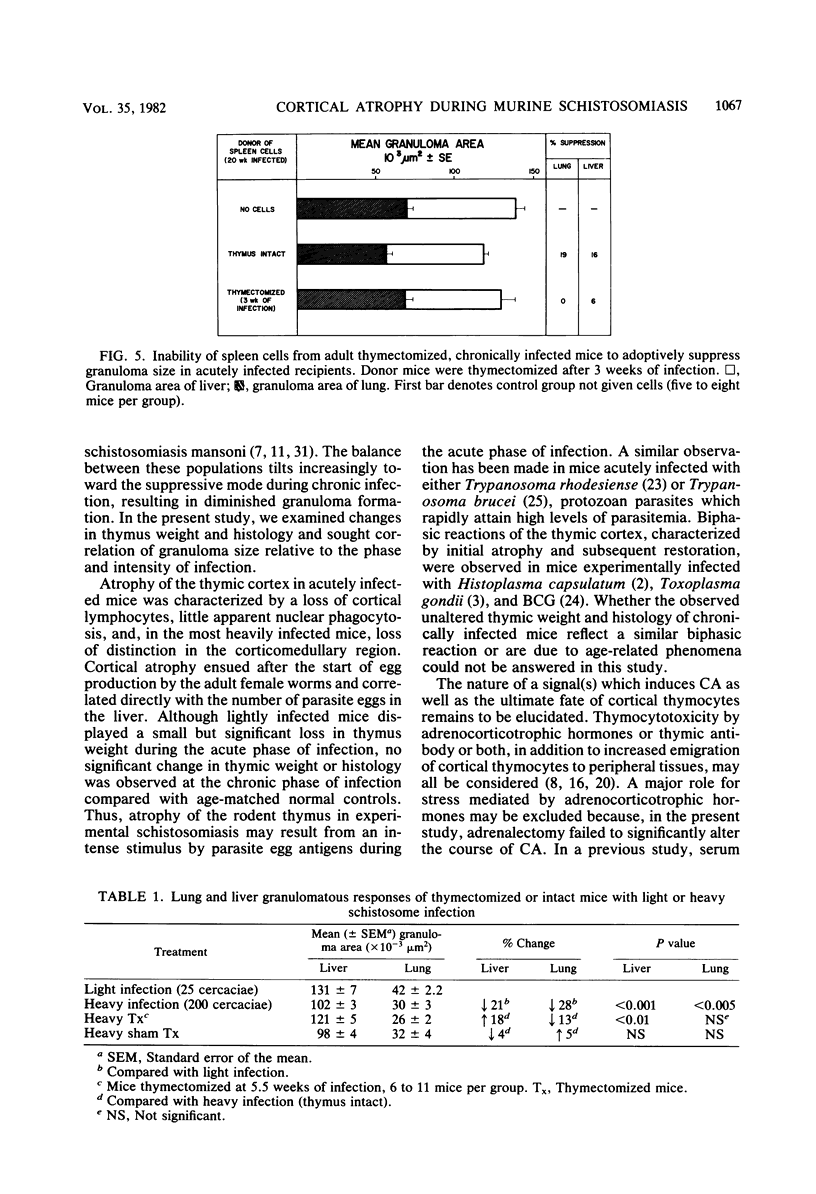
Thymic histopathology and granulomatous hypersensitivity were examined in CBA/J mice infected with varying numbers of Schistosoma mansoni worms. At the acute phase (8 weeks) of infection, the thymi of infected mice showed depletion of cortical lymphocytes that resulted in loss of distinction in the corticomedullary region. The degree of cortical depletion correlated directly with the intensity of infection, as assessed by total egg burden of the liver. Adrenalectomy of heavily infected mice 5 to 6 weeks after infection did not alter the course of cortical lymphocyte depletion. Thymus mass was diminished by as much as 80% in heavily infected mice. However, the thymi of lightly infected mice which survived 20 weeks of infection did not differ in histology or total tissue mass from age-matched uninfected controls. Adult thymectomy at 3 weeks of infection did not abrogate the spontaneous modulation (diminution) of granuloma formation in 20-week infected mice, although spleen cells from these mice failed to adoptively transfer suppression. Heavily infected mice which did not survive beyond the acute phase (8 to 9 weeks) of infection displayed a diminished granulomatous response that was partially restored by thymectomy carried out at 5 to 6 weeks of infection. Spleen cells from heavily infected mice (8 weeks) adoptively suppressed the granulomatous response in lightly infected recipients. It is concluded that histological changes observed in the thymus concurrent with egg dissemination and granulomatous hypersensitivity correlated with T cell-mediated regulatory events.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRADE Z. A. WARREN KS: MILD PROLONGED SCHISTOSOMIASIS IN MICE: ALTERATIONS IN HOST RESPONSE WITH TIME AND THE DEVELOPMENT OF PORTAL FIBROSIS. Trans R Soc Trop Med Hyg. 1964 Jan;58:53–57. doi: 10.1016/0035-9203(64)90068-9. [DOI] [PubMed] [Google Scholar]
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: lymphoid organ histopathology and serological studies. Infect Immun. 1979 Mar;23(3):884–892. doi: 10.1128/iai.23.3.884-892.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley J. K., Henry L. Histopathological changes caused by congenital toxoplasmosis in mice. Lyon Med. 1971 May 9;225(9):883–887. [PubMed] [Google Scholar]
- Borel Y., Kilham L., Kurtz S. E., Reinisch C. L. Dichotomy between the induction of suppressor cells and immunologic tolerance by adult thymectomy. J Exp Med. 1980 Mar 1;151(3):743–748. doi: 10.1084/jem.151.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byram J. E., Doenhoff M. J., Musallam R., Brink L. H., von Lichtenberg F. Schistosoma mansoni infections in T-cell deprived mice, and the ameliorating effect of administering homologous chronic infection serum. II. Pathology. Am J Trop Med Hyg. 1979 Mar;28(2):274–285. doi: 10.4269/ajtmh.1979.28.274. [DOI] [PubMed] [Google Scholar]
- Cheever A. W. Relative resistance of the eggs of human schistosomes to digestion in potassium hydroxide. Bull World Health Organ. 1970;43(4):601–603. [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L., David C. S. Regulation of granulomatous inflammation in murine schistosomiasis. In vitro characterization of T lymphocyte subsets involved in the production and suppression of migration inhibition factor. J Exp Med. 1980 Jun 1;151(6):1398–1412. doi: 10.1084/jem.151.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Modulation of granulomatous hypersensitivity. I. Characterization of T lymphocytes involved in the adoptive suppression of granuloma formation in Schistosoma mansoni-infected mice. J Immunol. 1979 Sep;123(3):1409–1414. [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Population dynamics of T and B lymphocytes in the lymphoid organs, circulation, and granulomas of mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1979 Mar;28(2):291–299. doi: 10.4269/ajtmh.1979.28.291. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Wellhausen S. R., Boros D. L. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981 Jul;127(1):363–367. [PubMed] [Google Scholar]
- Colley D. G. Adoptive suppression of granuloma formation. J Exp Med. 1976 Mar 1;143(3):696–700. doi: 10.1084/jem.143.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley D. G. T lymphocytes that contribute to the immunoregulation of granuloma formation in chronic murine schistosomiasis. J Immunol. 1981 Apr;126(4):1465–1468. [PubMed] [Google Scholar]
- DOUGHERTY T. F., BERLINER M. L., SCHNEEBELI G. L., BERLINER D. L. HORMONAL CONTROL OF LYMPHATIC STRUCTURE AND FUNCTION. Ann N Y Acad Sci. 1964 Feb 28;113:825–843. doi: 10.1111/j.1749-6632.1964.tb40707.x. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY T. F. Effect of hormones on lympatic tissue. Physiol Rev. 1952 Oct;32(4):379–401. doi: 10.1152/physrev.1952.32.4.379. [DOI] [PubMed] [Google Scholar]
- Doenhoff M., Musallam R., Bain J., McGregor A. Schistosoma mansoni infections in T-cell deprived mice, and the ameliorating effect of administering homologous chronic infection serum. I. Pathogenesis. Am J Trop Med Hyg. 1979 Mar;28(2):260–263. doi: 10.4269/ajtmh.1979.28.260. [DOI] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. The inhibition of granuloma formation around Schistosoma mansoni eggs. II. Thymectomy. Am J Pathol. 1967 Nov;51(5):757–767. [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Buchanan R. D., Colley D. G. Schistosoma mansoni infection in mice depleted of thymus-dependent lymphocytes. I. Eosinophilia and immunologic responses to a schistosomal egg preparation. Am J Pathol. 1973 May;71(2):193–206. [PMC free article] [PubMed] [Google Scholar]
- Hang L. M., Boros D. L., Warren K. S. Induction of immunological hyporesponsiveness to granulomatous hypersensitivity in Schistosoma mansoni infection. J Infect Dis. 1974 Nov;130(5):515–522. doi: 10.1093/infdis/130.5.515. [DOI] [PubMed] [Google Scholar]
- Hendry W. S., Tilney N. L., Baldwin W. M., 3rd, Graves M. J., Milford E., Strom T. B., Carpenter C. B. Transfer of specific unresponsiveness to organ allografts by thymocytes. Specific unresponsiveness by thymocyte transfer. J Exp Med. 1979 May 1;149(5):1042–1055. doi: 10.1084/jem.149.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata M., Hosaka Y., Kumada M., Matsui N., Kobayakawa T. Thymocytotoxic autoantibodies found in mice infected with Schistosoma japonicum. Infect Immun. 1981 May;32(2):438–442. doi: 10.1128/iai.32.2.438-442.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A., Rappaport H., Dantchev D., Florentin I., Bourut C. The effects of certain immunity systemic advuvants, PHA, and human gamma globulin on the thymic cortex of mice: a light and electron microscope study. Biomedicine. 1976 Dec 15;24(6):396–404. [PubMed] [Google Scholar]
- Levy J. G., Maier T., Kilburn D. G. Further characterization of thymic suppressor cells and a factor that suppress the generation of cells cytotoxic for a syngeneic tumor in DBA/2 mice. J Immunol. 1979 Mar;122(3):766–771. [PubMed] [Google Scholar]
- Mansfield J. M., Bagasra O. Lymphocyte function in experimental African trypanosomiasis. I. B cell responses to helper T cell-independent and -dependent antigens. J Immunol. 1978 Mar;120(3):759–765. [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. II. The role of the T and B lymphocytes. Immunology. 1974 Nov;27(5):825–840. [PMC free article] [PubMed] [Google Scholar]
- Rao V. S., Bennett J. A., Grodzicki R. L., Mitchell M. S. Suppressor T cells induced by soluble immune complexes can adoptively transfer inhibition of cytophilic antibody receptors on macrophages. Cell Immunol. 1979 Sep 1;46(2):227–238. doi: 10.1016/0008-8749(79)90412-x. [DOI] [PubMed] [Google Scholar]
- Tiboldi T., De Semet M., Colfs B., Van Soom H. Ovaries and adrenals in murine Schistosomiasis mansoni. III. Morphology and function of the adrenals in acute infection. Am J Trop Med Hyg. 1979 Sep;28(5):873–875. [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O., Cowan R. B. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967 Nov;51(5):735–756. [PMC free article] [PubMed] [Google Scholar]
- Weinstock J. V., Boros D. L., Gee J. B. Enhanced granuloma angiotensin I converting enzyme activity associated with modulation in murine schistosomiasis. Gastroenterology. 1981 Jul;81(1):48–53. [PubMed] [Google Scholar]



