Abstract
Background
A major hurdle in the use of exogenous stems cells for therapeutic regeneration of injured myocardium remains the poor survival of implanted cells. To date, the delivery of stem cells into myocardium has largely focused on implantation of cell suspensions.
Methodology and Principal Findings
We hypothesize that delivering progenitor cells in an aggregate form would serve to mimic the endogenous state with proper cell-cell contact, and may aid the survival of implanted cells. Microwell methodologies allow for the culture of homogenous 3D cell aggregates, thereby allowing cell-cell contact. In this study, we find that the culture of cardiac progenitor cells in a 3D cell aggregate augments cell survival and protects against cellular toxins and stressors, including hydrogen peroxide and anoxia/reoxygenation induced cell death. Moreover, using a murine model of cardiac ischemia-reperfusion injury, we find that delivery of cardiac progenitor cells in the form of 3D aggregates improved in vivo survival of implanted cells.
Conclusion
Collectively, our data support the notion that growth in 3D cellular systems and maintenance of cell-cell contact improves exogenous cell survival following delivery into myocardium. These approaches may serve as a strategy to improve cardiovascular cell-based therapies.
Introduction
Heart failure is a clinical condition arising from the progressive loss of functional muscle cells following cardiac injury [1]. While it has recently been established that the adult heart possess a progenitor cell population capable of differentiation into functional cardiomyocytes, this capacity for regeneration is severely limited and is unable to adequately account for the lost tissue [2]. The degree of cardiomyocyte loss is closely related to subsequent cardiac dysfunction, as well as cardiovascular morbidity and mortality [1], [3]. Currently, while pharmacologic therapies represent the standard treatment approach for heart failure, these treatment options fail to address the loss of functional cardiomyocytes. Recently, stem-cell based therapies have been explored as a means for regeneration of heart tissue [4], with functional differentiation of implanted stem cells into mature cardiomyocytes [5], [6], [7], [8]. Thus far, different populations of adult stem cells have been examined following implantation in animal models of and humans with heart disease [9], [10], [11], [12], [13]. While the reported therapeutic benefits have been inconsistent, autologous cell implantation has generally been deemed safe. A major obstacle to realizing therapeutic regeneration is the very poor survival of implanted cells [14], [15], [16], [17]. Following implantation, it has been reported that greater than 95% cells die within hours [18], leaving only a small percentage of viable cells. Augmenting implanted cell survival would allow for greater new cardiomyocyte formation and presumably, greater improvement in cardiac performance. The low survival of implanted cells has been attributed to cell death induced by the hostile environment within injured myocardium [15], [19]. Thus far, attempts to overcome this survival obstacle have focused on biochemical means, including but not limited to, preconditioning of cells prior to implantation, exposure to pro-survival factors, and genetic modification of cells [20], [21]. Few reports, however, have examined the importance of biophysical properties, such as cell-cell contact, in modulating implanted stem cell survival.
Similar to the resident stem cells found in other organs, adult cardiac stem cells reside in a microenvironment, or cellular niche, in the myocardium [22], [23], [24], [25]. The cells within the niche are in close contact with each other, and include progenitor cells, cardiomyocytes and surrounding matrix proteins [23], [25]. It has been shown that the establishment of cell-cell contact is beneficial for promoting cell survival [26], [27]. Thus, we hypothesized that delivering cells in 3D aggregates to maintain cell-cell contact may promote implanted cell survival. In this study, utilizing our recently established microwell array methodology, we demonstrated that cardiac side population (CSP) progenitor cells [28], [29], when delivered in 3D cell aggregates exhibit enhanced survival against stressors and toxins in vitro, as well as improved survival following implantation in vivo in a murine model of cardiac injury.
Materials and Methods
Animals
For in vitro studies, CSP cells were isolated from eight-week-old male C57/BL6 mice purchased from Jackson Laboratories. Eight-week-old female Friend Virus B-type (FVB) mice were obtained from Charles River Laboratories for in vivo ischemia reperfusion and cell implantation experiments. Dual luciferase and GFP transgenic (L2G) mice were kindly provided by Dr. Joseph Wu (Stanford University School of Medicine) [30]. All animal studies strictly adhered to the guidelines of the Harvard Medical School Institutional Animal Care and Use Committee (IACUC) and the National Society for Medical Research. All animal studies were conducted according to guidelines provided by National Research Council, National Institutes of Health and Institute of Laboratory Animal Resources. The protocols were reviewed and approved by the IACUC of Harvard Medical School (protocol number: 04745) or Massachusetts General Hospital (Protocol number: 2011N000009).
Cardiac Side Population Cell Isolation and Culture
CSP cells were isolated as described previously [28], [29], [31], [32]. Briefly, hearts from adult mice as described above were digested using dispase and collagenase B (Roche, Indianapolis, IN). The mononuclear cell fraction was isolated and stained using 5 µg/ml Hoechst 33342 (Sigma-Aldrich, St. Louis, MO) at 37°C for 90 minutes and further stained for CD31 and Sca-1 (BD, Franklin Lakes, NJ). CD31− Sca-1+ CSP cells were isolated using fluorescence activated cell sorting (FACS Aria, BD, Franklin Lakes, NJ) and cultured in our established proliferation media as described before [28], [29], [31], [32]. The CSP cells used in this study were passage 4–6 cells.
Microwell Assembly
Microwells at a diameter of 200 µm were generated using micromolding on UV-photocrosslinkable polyethylene glycol dimethacrylate (PEGDM, MW = 1000, 10% in PBS) (Monomer-Polymer & Dajac Labs, Trevose, PA) with 0.5% photoinitiator 2-hydroxy-1-[4-(hydroxyethoxy)phenyl]-2-methyl-L-propanone (Irgacure D2959, Ciba Specialty Chemicals Inc., Florham Park, NJ). To create surfaces capable of PEG attachment, glass slides were treated with 3-(trimethoxysilyl) propylmethacrylate (TMSPMA) (Sigma-Aldrich Co., St. Louis, MO) at 80°C for 12 hours. A patterned PDMS stamp was placed on an evenly distributed film of PEGDM macromer solution on the TMSPMA coated glass slide and photo-crosslinked (λ = 350–500 nm, 180 sec, 10 mW/cm2; OmniCure Series 2000 curing station, EXFO, Mississauga, Canada). For adhesive (glass bottom) microwells, the patterned PDMS stamp was first placed on the glass slide and then PEG monomer solution was added at the edge of PDMS stamp. After polymerization the PDMS stamp was peeled from the substrate.
Generation of CSP Aggregates
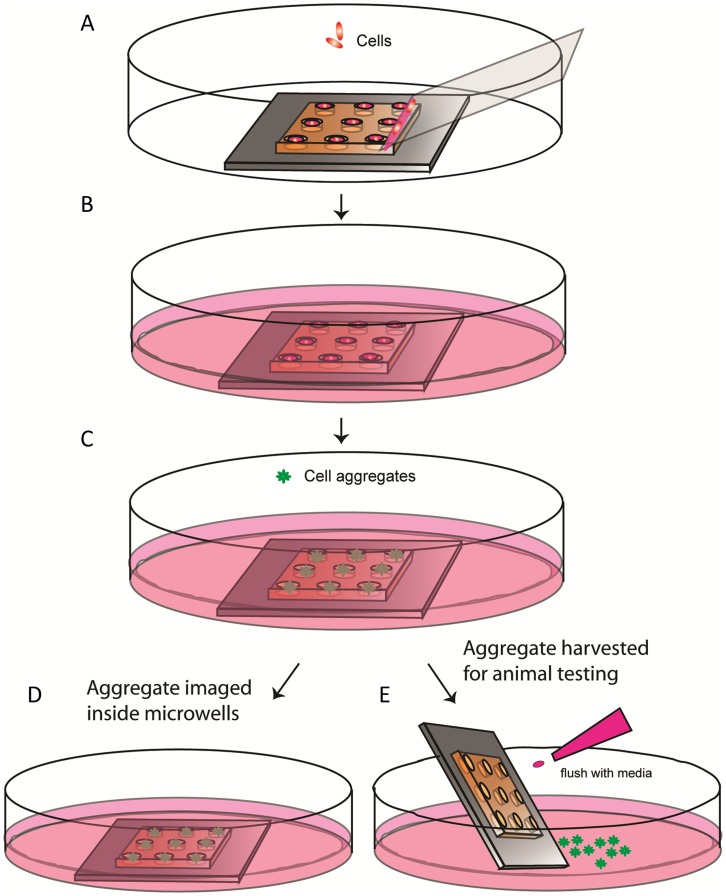
For controlled cell aggregation, CSP cells were seeded into microwells by using a previously developed method [33]. A schematic summarizing the protocol is shown in Figure 1 . Briefly, 15 µl of cell suspension (4−16×106 cells/mL) was pipetted along the edge of a cover glass, which was then slowly (∼1 mm/s) wiped across a microwell array. Once the array was traversed, the excess fluid outside the array was removed and the array was immersed in media in a culture dish. After 3D cellular aggregates formed overnight, they were imaged directly inside microwells or harvested by flushing with a stream of culture media.
Figure 1. Cell patterning and aggregate formation inside microwells.
A) Cell patterning. Cells were localized inside the microwells. B) After cell seeding, the cells in the microwell array were cultured in a petri dish and aggregates formed within 24 h. C) Once the aggregate formation is complete inside the microwells, they can be stained. D) Aggregates can be imaged inside microwells. E) Aggregates can be easily released from the microwells by gentle flushing with media for other applications.
As a comparison, cell aggregates were also generated by plating cells (2000 cells/well) in 96 well plates (2000 cells/well) with ultra low attachment (ULA) surface (Corning life sciences, Lowell, MA). Twenty-four hours post-seeding, the aggregated size was determined by imaging cells using phase contrast microscopy.
Conventional 2D CSP cell culture was achieved by seeding cells with 5.9×103 cells/mm2 using the method similar to the 3D cell aggregate but with glass-bottom microwells. Cells were cultured overnight in their respective conditions for all in vitro and in vitro experiments.
In vitro Viability Assays
Cell viability in vitro was determined using direct fluorescence microscopy imaging methods and FACS-based analysis. For imaging, CSP cells in either 3D aggregates or 2D culture were stained using a combination of ethidium homodimer (EthD) (Invitrogen, Carlsbad, CA) and DAPI (Invitrogen, Carlsbad, CA). The microwells were washed with PBS and incubated with EthD (4 µM) for 10 minutes at 37°C to label dead cells. Cells were washed using PBS, fixed with 4% paraformaldehyde and counter-stained with DAPI (5 µg/ml) for 10 minutes at 37°C and washed with PBS. To quantify cell viability, microarrays were imaged using a fluorescence microscope equipped with a motorized stage (Axiovert 200M, Zeiss, Thornwood, NY). Fluorescence intensities for both DAPI and EthD were measured using ImageJ (NIH, Bethesda, MD). Briefly a grid of ROIs was produced to match the microarray and then fluorescence values in both the DAPI and the EthD channels were measured. Minimal values in each ROI were taken as the local background and subtracted from the fluorescence reading. The cell death was calculated as the ratio between EthD and DAPI readings.
For FACS-based cell death analysis, cells in microwells were incubated with EthD as described above. Following staining, the cell aggregates or 2D cultures were washed out and dissociated into single cell suspensions with 0.05% trypsin (Gibco, Invitrogen, Carlsbad, CA). Cell death analysis was performed using flow cytometry (Accuri C6, Accuri Flow Cytometry, MI).
Hydrogen Peroxide Treatment and Anoxia/Reoxygenation
To determine the susceptibility to stress, CSP cells in cell aggregates or 2D cultures were treated with 200 µM H2O2 or anoxia followed by reoxygenation. Briefly, CSP cells were incubated with culture medium containing 200 µM H2O2 or vehicle for 2 hours at 37°C. For the anoxia and reoxygenation, cells were subjected to 24 hours anoxia, 5% CO2 balanced medical grade 99.9999% N2 (Airgas, Salem, NH), using a standard hypoxia chamber followed by 2 hours reoxygenation. Cells cultured under regular culture conditions for the same period of time were used as control. Viability of cells was determined as described above.
Mouse Model of Ischemia Reperfusion and Cell Implantation
A murine model of ischemia reperfusion was generated by ligating the left coronary artery of FVB mice for 45 minutes, followed by release, as described previously [34]. Two weeks following ischemia reperfusion, 1×105 CSP cells in 12.5 µl total volume in either 3D aggregate or single cell suspensions were delivered via direct myocardial injection into the border zone of the infarct [34].
In vivo Cell Survival Assay
Implanted cell survival was determined by bioluminescence imaging in vivo and immunohistochemtry ex vivo. For in vivo imaging, the mice were anesthetized with isoflurane and an aqueous solution of the substrate D-Luciferin (Molecular Imaging Products, Bend OR) was injected intraperitoneally (200 mg/kg body weight at 15 mg/ml). The animals were then allowed to recover before being re-anesthetized and placed in the Xenogen IVIS Spectrum imaging system (Caliper Life Sciences, Hopkinton, MA) with the platform preheated to 38°C. The CCD (Charge-coupled device) camera was cooled to −90°C and the field of view (FOV) set to 22.4×22.4 cm2. Luminescent images were acquired with a 300 s exposure time, 8 binning, 1 f/stop, open filter, and with a spatial resolution of 270 µm. Photographic images of the subjects were acquired as well.
Quantification of the luminescence signal was performed using the Living Image software (Caliper) by applying a region of interest (ROI) over the heart and measuring the photon intensity in average radiance (photons·sec−1·cm−2·sr−1). Measurements were taken at 0, 4, and 6 days post cell implantation, background subtracted, normalized to the corresponding day 0 signal, and expressed as percent survival. Each data point represents n = 6.
Data Analysis and Statistics
Data were collected using a spreadsheet program and further analyzed using the R statistical framework 11. Student’s t-tests were used to compare two groups, while analysis of variance (ANOVA) to compare three or more groups. A p<0.05 was considered statistically significant. Data are presented as mean ± S.D.
Results
Size of CSP Cell Aggregates is Regulated by Controlling Cell Seeding Density in Microwells
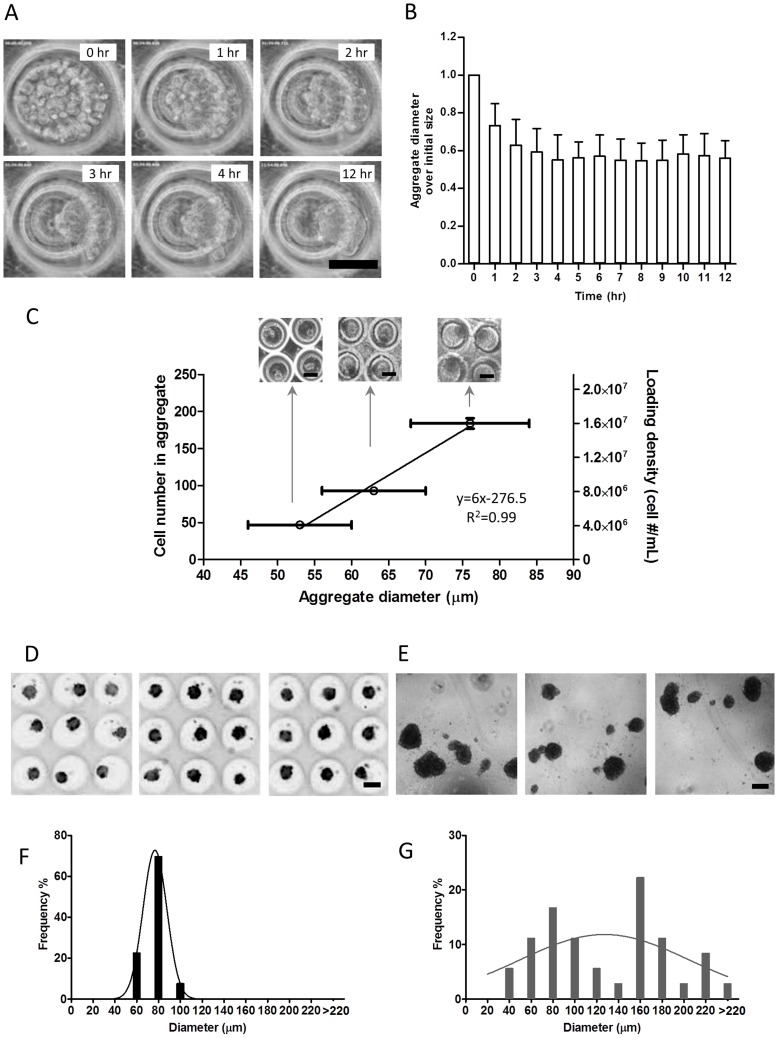
The formation of CSP cell aggregates took place in culture within a short period of time. CSP cells seeded in microwells and imaged using a time-lapse mode in phase-contrast showed initial formation of aggregates within 1 hour post seeding ( Figures 2A ). Aggregate size reduced to the final size in approximately 3 hours ( Figures 2A ). Thereafter, over a subsequent 9 hours, aggregate size did not significantly increase but a further smoothening of the surface was observed (Video S1).
Figure 2. Characterization of aggregate formation and uniformity.
A) Time course of aggregate formation in the microwell at 0,1,2,3,4, and 12 h post seeding. B) Quantification of aggregates size over time (n = 2). C) Size of the aggregates can be controlled by seeding microwell arrays for CSP aggregation with different cell numbers in microwells. The cell suspension densities used for seeding were 4×106/mL, 8×106/mL, and 16×106/mL, resulting in aggregates with controllable sizes defined hereinafter as small aggregate (S), medium aggregate (M), and large aggregates (L), respectively. D) Subsets of a microwell array with CSP cell aggregates after 24 h showing high uniformity. E) CSP cells cultured in an ultra low adhesive (ULA) 96-well plate for 24 h. Cells form aggregates of various sizes. F) Frequency distribution of diameter of aggregates formed in microwells shows narrow distribution. The standard deviation (S.D.) of Gaussian distribution fitting is 10.9 µm. G) Frequency distribution of diameter of aggregates formed in ULA 96-well plates shows wide distribution. The S.D. of Gaussian distribution fitting is 77.7 µm. (All bars represent 100 µm).
To determine whether the cell aggregate size can be regulated, we seeded cells in three concentrations ranging from 4×106, 8×106, to 16×106 CSP cells per mL in microwell arrays. We found that the number of cells in each aggregate increased with greater seeding density as measured 24 hours following seeding ( Figure 2B ). Moreover, the diameter of cell aggregate correlated (R2 = 0.99) to the cell number in each aggregate ( Figure 2C ). We next compared the uniformity of size of each aggregate between cell seeding in microwells and conventional 96 well non-adherent plates with ULA surface. Twenty-four hours following seeding, CSP cells cultured in suspension formed aggregates in both microwells ( Figure 2D ) and ULA 96 well plates ( Figure 2E ). As shown in Figure 2F , the size of CSP cells was homogenous with low variation with standard deviation of 10.9 µm. In contrast, the aggregate size of cells cultured in ULA 96 well plates varied largely with broader range of distribution as evidenced by a 77.7 µm standard deviation ( Figure 2G ). These data demonstrated that microwell methodology can be used to generate desired aggregate size for biological applications.
CSP Cell Aggregates are Less Susceptible to Oxidative and Hypoxic Stress
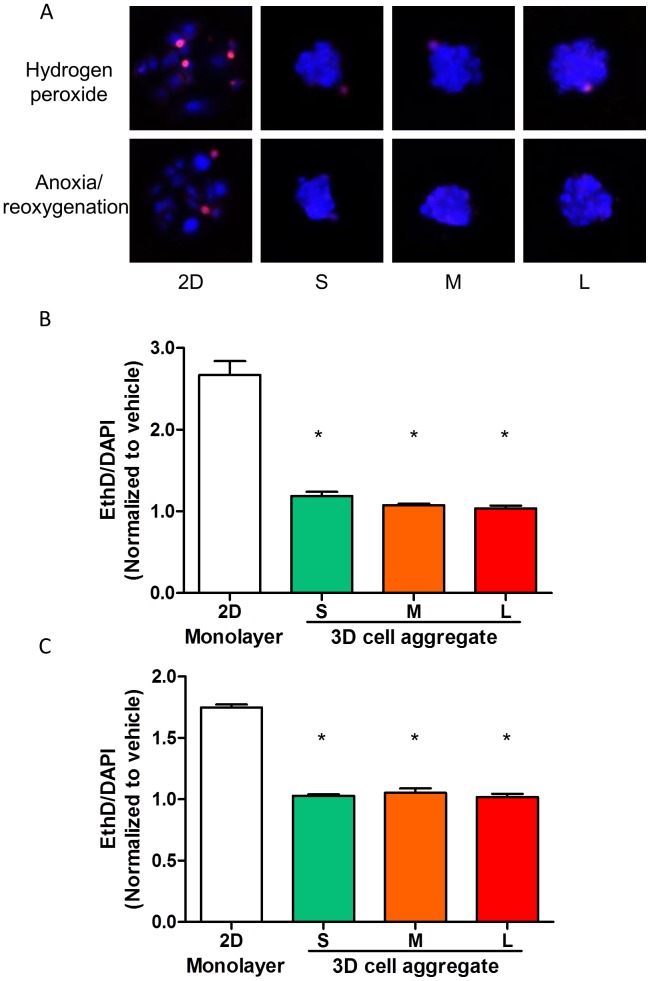
To test the hypothesis that cells in 3D aggregates enhance protection against stressors and toxins, CSP cells in either 2D monolayer culture or 3D cell aggregate with aggregates of three different sizes, S: 53±7 µm; M: 63±7 µm; L: 76±8 µm, were subjected to 200 µM H2O2 for 2 hours to mimic the increased oxidative stress present in injured myocardium. Cells grown in 3D cell aggregates were less susceptible to increased oxidative stress compared to those cultured in 2D monolayer as determined by EthD/DAPI ratio. As demonstrated in Figure 3A and 3B , cells cultured as 3D aggregates showed a lower EthD/DAPI ratio as compared to cells in single layer. Interestingly, this survival benefit was independent of the cell number per aggregate within the range tested in this study (47±1 to 184±7 cells). In addition, we examined the response of CSP cells to 24 hours of anoxia followed by 2 hours reoxygenation. Similar to results obtained following H2O2 exposure, reduced cell death, as determined by EthD/DAPI, was observed in 3D cell aggregates ( Figure 3C and 3D , p<0.05, n = 3). These observations were confirmed using additional measures of cell death, including by FACS analysis, in a subset of experiments (Figure S1A and S1B).
Figure 3. Aggregate survival tests in vitro.
A) Subsets of microwell arrays with 2D monolayer of cell culture (2D) and aggregates of three sizes (S, M, and L). Hydrogen peroxide and anoxia/reoxygenation treatments were employed to induce cell death. EthD (red) and DAPI (blue) staining were performed for the determination of cell death. B) Quantification of dead CSP cells in 2D single layer culture and aggregates with variable diameters subjected to 200 µM-hydrogen peroxide treatment using EthD/DAPI fluorescent intensity ratio. Data were normalized to the vehicle groups of 2D monolayer culture and aggregates in three sizes. C) Quantification of dead CSP cells in 2D single layer culture and aggregates with variable diameters subjected to anoxia/reoxygenation using EthD/DAPI fluorescent intensity ratio. Data were normalized to the vehicle groups of 2D monolayer culture and aggregates in three sizes.
To verify that protection from cell death observed in cell aggregates was not due to the in-accessibility of dye staining in 3D aggregate format, we subjected CSP cell aggregates to supraphysiologic concentrations of H2O2 and were indeed able to detect dead cells using the fluorometric assay (Figure S1C).
CSP Cell Aggregates Show Improved Survival in vivo
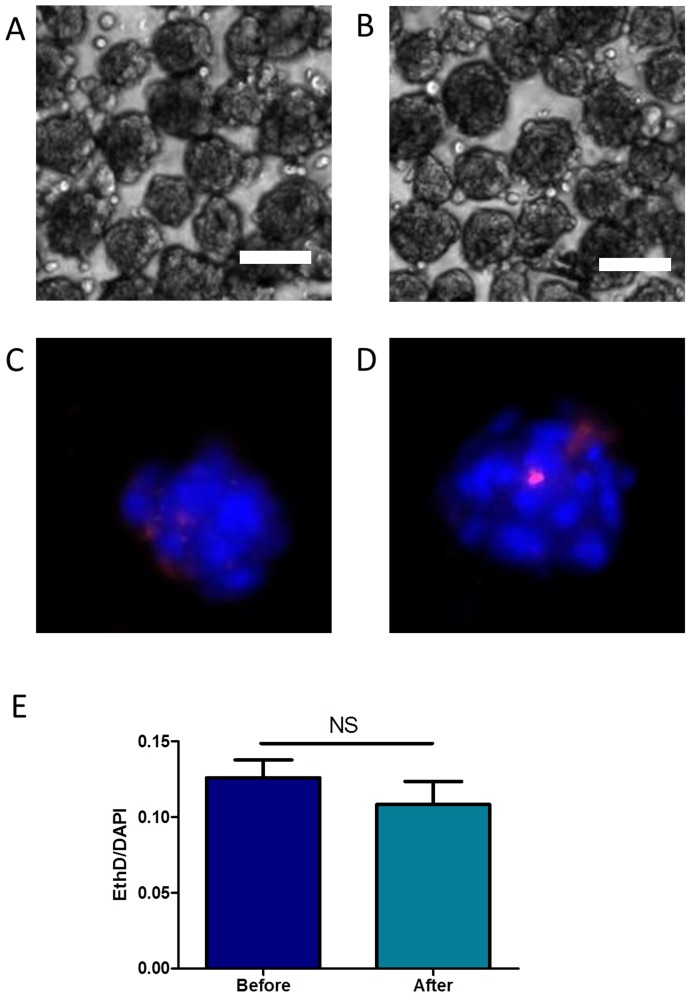
Prior to testing the survival of cell aggregates in vivo, we sought to determine whether CSP cell aggregates were able to withstand the shear stress of passing though a 30G needle, which is required for intra-myocardial implantation. As shown in Figures 4A & 4B , the gross morphology of CSP cell aggregates was unchanged following passage through a small caliber needle. Moreover, cell viability, as determined by cell death assay, was unchanged with passage ( Figures 4C – 4E ).
Figure 4. Aggregate integrity and survival in fluidic manipulations.
A) Aggregates formed in microwells can be easily flushed out from the microwell and centrifuged while remaining intact. B) Aggregate can be easily passed through a 30G needle without loosing integrity. C) A representative DAPI/EthD fluorescent image of aggregates before injection. D) A representative DAPI/EthD fluorescent image of aggregates after injection. E) Quantification of dead CSP cells in aggregates passing a 30G needle using EthD/DAPI fluorescent intensity ratio. (All bars represent 100 µm).
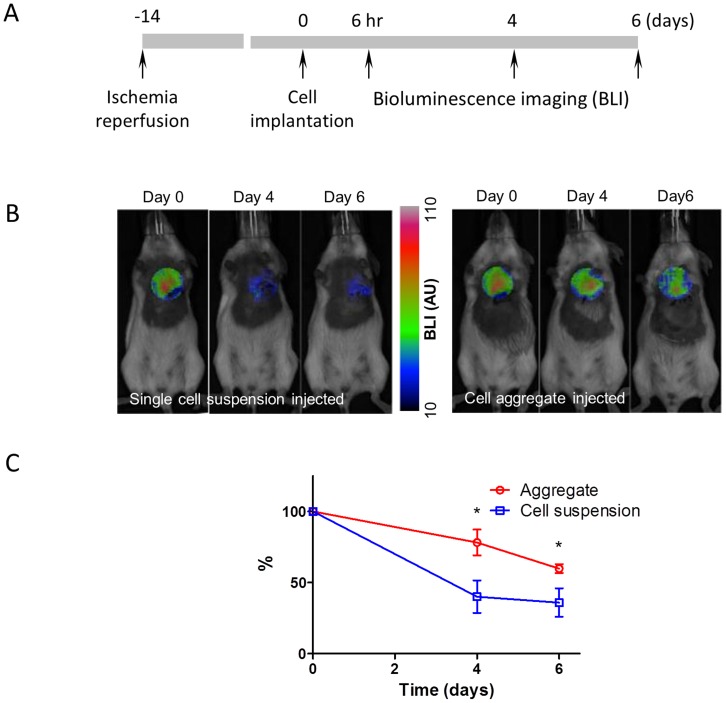
A myocardial ischemia-reperfusion (post-IR) model was used to assess the survival benefit of CSP cell aggregates in vivo. CSP cells were isolated and cultured from mice over expressing luciferase/eGFP (L2G mice). Two weeks post-IR, 100,000 L2G CSP cells in either single cell suspension or 3D aggregates (76±8 µm) were injected into the border zone. The initial graft survival was determined 6 hours post injection as shown in the Figures 5A & 5B , with a marked initial cell engraftment. Only 40% of implanted cells remained alive at day 4 post implantation when cells were delivered in single cell suspension, whereas approximately 80% of implanted cells survived when cells were delivered as cell aggregates ( Figure 5C ). CSP cells in either aggregates or suspension showed clear bioluminescence signals 6 hours post injection ( Figure 5B ), and the survival benefit of 3D cell aggregates persisted at 6 days post-cell implantation ( Figure 5C ). Taken together, these data support the hypothesis that cells delivered in the form of 3D aggregates exhibit greater survival when implanted in vivo following cardiac injury.
Figure 5. CSP cell survival in vivo following cardiac injury.
A) Protocol to measure the in vivo survival of CSP aggregates and suspensions. B) Representative serial bioluminescence images (BLI) of mice injected with CSP cell aggregates and CSP single cell suspensions. C) Percentage of CSP cell survival measured with BLI.
Discussion
In this report, we utilized a microwell array system to study the effect of 3D cell culture on cardiac side population (CSP) cells, a proven adult resident cardiac progenitor cell population [24], [28]. Using this newly developed system, CSP cell survival both in vitro and in vivo was remarkably improved in 3D aggregates. This work suggests that manipulation of cellular aggregates and growth of cells in 3D structures may facilitate cell-cell interaction and provide strategies for enhancing therapeutic tissue regeneration.
Microwell Approach is Feasible for Regulating Size of Cell Aggregate
Similar to other type of cells, CSP cells form aggregates when cultured in suspension. However these aggregates vary in size and the numbers of aggregates are not easily controlled. Results from embryonic stem cells have suggested an influence of aggregate size on biologic properties [35]. Furthermore, the ability to control the size of cell aggregates allows for closer replication of conditions believed to exist in vivo in the cellular niche. There are several methods that have been previously used to control aggregate size when forming aggregates. Most common among these is the hanging drop method [36]. However this method does not allow for the production of large numbers of aggregates for mass production. Microwells can be used to control aggregate size and contrary to hanging drops, they facilitate the production of a large number of aggregates [37]. When seeded into microwells, CSP cells formed homogenous aggregates. Importantly, the size of the aggregate was tightly dictated by the number of cells seeded into each well. The benefits of microwells also include direct in vitro testing within wells without having to transfer cells. Furthermore, harvesting aggregates from microwells is simple and straightforward. This application may be adapted for seeding stem or progenitor cells from different sources for experimental or therapeutic applications.
Survival Benefit of 3D Cell Aggregate to Stress or Injury in vitro and in vivo
Cell therapy aims at regenerating lost myocardium by delivering primitive cells into the injured hearts. While some functional benefits have been observed in most prior studies of cell implantation, to date these improvements have been relatively modest, and ascribed to the paracrine effects of implanted cells rather the direct differentiation of engrafted cells. One major reason remaining for the lack of significant tissue regeneration is the poor survival of implanted cells. Toward this end, several strategies have been explored to augment graft survival [15] in the past. Most of the efforts have been focused on supplementing cells with soluble survival or anti-apoptotic factors [38], overexpression of proteins involved in survival pathways [39], as well as delivery of a cocktail of miRNA species [40]. Limited efforts, however, have been put forth to exploit the intrinsic properties of 3D environment of cells within tissue. Using microwell arrays as an in vitro test platform we compared the viability of CSP cells cultured in aggregates to CSP cells cultured as 2D monolayer under oxidative stress. To account for possible effects of cell density or cell number, 2D monolayer controls were performed in microwells with adhesive bottom. It is noteworthy that while the combination of Calcein AM and EthD is a well known cell viability stain, this method is not suitable to determine the cell survival under the condition of oxidative stress due to the fact that Calcein itself is a sensor for oxidative activity [41]. Therefore, we modified the method, combining EthD for staining nuclei of necrotic cells with a counterstain of DAPI to stain for total nuclei. This method delivered reliable results when used in fluorometric measurements verified by the FACS analysis.
Our observations are consistent with reports suggesting that 3D cell aggregates exhibit better survival compared to cells cultured in monolayer conditions in vitro [27]. In our study, however, the observed effects were not dependent on aggregate size, at least among the three aggregate sizes tested. In contrast, a size dependent effect has been reported in studies of embryonic stem cells aggregates [42]. The differences in cell types and aggregate sizes studied, as well as the specific end points tested in the two studies may underscore the varying results between studies. We extended these observations of survival benefits with 3D cell aggregates, and have now provided evidence suggesting that the benefits of 3D cell aggregates persist following implantation into mouse hearts with ischemia perfusion injury in vivo. It is important to note that given aggregate size, the suitability of delivering stem cells via intra-coronary methods needs to be carefully evaluated, particularly as delivery of aggregates has the potential for micro-embolization. Furthermore, future studies will be required to subsequently determine the in vivo functional benefits of 3D cell aggregates on progenitor cell differentiation, tissue regeneration, cardiac function and cardiovascular outcomes.
In summary, in this study we provide the first proof of concept of (1) the feasibility to generate 3D cell aggregates with control dimension; (2) that adult cardiac progenitor cells when cultured in 3D aggregates format tolerate oxidative stress and anoxia reoxygenation better in vitro; and finally, (3) 3D cell aggregates possess survival benefits when implanted into mouse hearts following ischemia reperfusion, in vivo. Collectively, our data provides evidence that these methods may be utilized to advance the efficacy of cell-based therapy in the cardiovascular system.
Supporting Information
Quantification method of dead cells and validation using FACS. Cells subjected to 200 µM hydrogen peroxide for 2 hr. A) Representative EthD/DAPI fluorescent microscopic images and quantification of dead cells using EthD/DAPI fluorescent intensity ratio. B) Representative FACS profiles and quantification of dead cells in FACS. The X-axis represents forward scatter (FSC) and y-axis EthD fluorescence in channel 3 (fl-3). C) CSP cell aggregates subject to supra-physiologic concentrations of hydrogen peroxide.
(TIF)
A video clip shows the time course of CSP cell aggregation in a microwell.
(MP4)
Acknowledgments
The authors would like to thank Dr. Grigoriy Losyev at Brigham and Women’s Hospital Flow Cytometry Core for his expertise with CSP cell sorting and analysis. Mr. Soeun Ngoy is also acknowledged for performing animal surgery.
Funding Statement
This work was supported, in part, by National Institutes of Health research grants HL099073 (RL, AK, DES), HL086967 and HL093147 (RL), EB012597, and DE019024 (AK) and HL093038 (DES) grants. AK was supported, in part, by the National Science Foundation CAREER award. MB was supported by a postdoctoral fellowship from American Heart Association. LK was supported by a NUS overseas postdoctoral fellowship. The funders played no role in study design, data collection, analysis, preparation and publication of manuscript.
References
- 1. Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, et al. (1996) Apoptosis in myocytes in end-stage heart failure. N Engl J Med 335: 1182–1189. [DOI] [PubMed] [Google Scholar]
- 2. Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, et al. (2005) Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A 102: 8692–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, et al. (1997) Apoptosis in the failing human heart. N Engl J Med 336: 1131–1141. [DOI] [PubMed] [Google Scholar]
- 4. Ye Z, Zhou Y, Cai H, Tan W (2011) Myocardial regeneration: Roles of stem cells and hydrogels. Adv Drug Deliv Rev 63: 688–697. [DOI] [PubMed] [Google Scholar]
- 5. Ptaszek LM, Mansour M, Ruskin JN, Chien KR (2012) Towards regenerative therapy for cardiac disease. Lancet 379: 933–942. [DOI] [PubMed] [Google Scholar]
- 6. Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, et al. (2011) Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378: 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7. Passier R, van Laake LW, Mummery CL (2008) Stem-cell-based therapy and lessons from the heart. Nature 453: 322–329. [DOI] [PubMed] [Google Scholar]
- 8. Bernstein HS, Srivastava D (2012) Stem cell therapy for cardiac disease. Pediatr Res 71: 491–499. [DOI] [PubMed] [Google Scholar]
- 9. Gnecchi M, Danieli P, Cervio E (2012) Mesenchymal stem cell therapy for heart disease. Vascul Pharmacol 57: 48–55. [DOI] [PubMed] [Google Scholar]
- 10. Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, et al. (2012) Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379: 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanz-Ruiz R, Santos ME, Munoa MD, Martin IL, Parma R, et al. (2008) Adipose tissue-derived stem cells: the friendly side of a classic cardiovascular foe. J Cardiovasc Transl Res 1: 55–63. [DOI] [PubMed] [Google Scholar]
- 12. Chiu RC (2003) Bone-marrow stem cells as a source for cell therapy. Heart Fail Rev 8: 247–251. [DOI] [PubMed] [Google Scholar]
- 13. Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, et al. (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114: 763–776. [DOI] [PubMed] [Google Scholar]
- 14. Haider H, Ashraf M (2008) Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol 45: 554–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu KH, Mo XM, Han ZC, Zhou B (2011) Stem cell engraftment and survival in the ischemic heart. Ann Thorac Surg 92: 1917–1925. [DOI] [PubMed] [Google Scholar]
- 16. Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, et al. (2006) Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med 355: 1873–1884. [DOI] [PubMed] [Google Scholar]
- 17. Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, et al. (2001) Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol 33: 907–921. [DOI] [PubMed] [Google Scholar]
- 18. Penicka M, Widimsky P, Kobylka P, Kozak T, Lang O (2005) Images in cardiovascular medicine. Early tissue distribution of bone marrow mononuclear cells after transcoronary transplantation in a patient with acute myocardial infarction. Circulation 112: e63–65. [DOI] [PubMed] [Google Scholar]
- 19. Zhang H, Chen H, Wang W, Wei Y, Hu S (2010) Cell survival and redistribution after transplantation into damaged myocardium. J Cell Mol Med 14: 1078–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang YL, Tang Y, Zhang YC, Qian K, Shen L, et al. (2005) Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol 46: 1339–1350. [DOI] [PubMed] [Google Scholar]
- 21. Lu G, Haider HK, Jiang S, Ashraf M (2009) Sca-1+ stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexin-43. Circulation 119: 2587–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, et al. (2007) Human cardiac stem cells. Proc Natl Acad Sci U S A 104: 14068–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, et al. (2006) Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A 103: 9226–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, et al. (2007) Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol 176: 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, et al. (2006) The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells 24: 1779–1788. [DOI] [PubMed] [Google Scholar]
- 26. Akins RE, Boyce RA, Madonna ML, Schroedl NA, Gonda SR, et al. (1999) Cardiac organogenesis in vitro: reestablishment of three-dimensional tissue architecture by dissociated neonatal rat ventricular cells. Tissue Eng 5: 103–118. [DOI] [PubMed] [Google Scholar]
- 27. Bartosh TJ, Wang Z, Rosales AA, Dimitrijevich SD, Roque RS (2008) 3D-model of adult cardiac stem cells promotes cardiac differentiation and resistance to oxidative stress. J Cell Biochem 105: 612–623. [DOI] [PubMed] [Google Scholar]
- 28. Pfister O, Mouquet F, Jain M, Summer R, Helmes M, et al. (2005) CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 97: 52–61. [DOI] [PubMed] [Google Scholar]
- 29. Pfister O, Oikonomopoulos A, Sereti KI, Liao R (2010) Isolation of resident cardiac progenitor cells by Hoechst 33342 staining. Methods Mol Biol 660: 53–63. [DOI] [PubMed] [Google Scholar]
- 30. Swijnenburg RJ, Schrepfer S, Cao F, Pearl JI, Xie X, et al. (2008) In vivo imaging of embryonic stem cells reveals patterns of survival and immune rejection following transplantation. Stem Cells Dev 17: 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pfister O, Oikonomopoulos A, Sereti KI, Sohn RL, Cullen D, et al. (2008) Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circ Res 103: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oikonomopoulos A, Sereti KI, Conyers F, Bauer M, Liao A, et al. (2011) Wnt signaling exerts an antiproliferative effect on adult cardiac progenitor cells through IGFBP3. Circ Res 109: 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kang L, Hancock MJ, Brigham MD, Khademhosseini A (2010) Cell confinement in patterned nanoliter droplets in a microwell array by wiping. J Biomed Mater Res A 93: 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jain M, DerSimonian H, Brenner DA, Ngoy S, Teller P, et al. (2001) Cell therapy attenuates deleterious ventricular remodeling and improves cardiac performance after myocardial infarction. Circulation 103: 1920–1927. [DOI] [PubMed] [Google Scholar]
- 35. Bauwens CL, Peerani R, Niebruegge S, Woodhouse KA, Kumacheva E, et al. (2008) Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells 26: 2300–2310. [DOI] [PubMed] [Google Scholar]
- 36. Chen CS, Pegan J, Luna J, Xia B, McCloskey K, et al. (2008) Shrinky-dink hanging drops: a simple way to form and culture embryoid bodies. J Vis Exp 13: 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moeller HC, Mian MK, Shrivastava S, Chung BG, Khademhosseini A (2008) A microwell array system for stem cell culture. Biomaterials 29: 752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kofidis T, de Bruin JL, Yamane T, Tanaka M, Lebl DR, et al. (2005) Stimulation of paracrine pathways with growth factors enhances embryonic stem cell engraftment and host-specific differentiation in the heart after ischemic myocardial injury. Circulation 111: 2486–2493. [DOI] [PubMed] [Google Scholar]
- 39. Penn MS, Mangi AA (2008) Genetic enhancement of stem cell engraftment, survival, and efficacy. Circ Res 102: 1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu S, Huang M, Nguyen PK, Gong Y, Li Z, et al. (2011) Novel microRNA prosurvival cocktail for improving engraftment and function of cardiac progenitor cell transplantation. Circulation 124: S27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uggeri J, Gatti R, Belletti S, Scandroglio R, Corradini R, et al. (2004) Calcein-AM is a detector of intracellular oxidative activity. Histochem Cell Biol 122: 499–505. [DOI] [PubMed] [Google Scholar]
- 42. Hwang YS, Chung BG, Ortmann D, Hattori N, Moeller HC, et al. (2009) Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A 106: 16978–16983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantification method of dead cells and validation using FACS. Cells subjected to 200 µM hydrogen peroxide for 2 hr. A) Representative EthD/DAPI fluorescent microscopic images and quantification of dead cells using EthD/DAPI fluorescent intensity ratio. B) Representative FACS profiles and quantification of dead cells in FACS. The X-axis represents forward scatter (FSC) and y-axis EthD fluorescence in channel 3 (fl-3). C) CSP cell aggregates subject to supra-physiologic concentrations of hydrogen peroxide.
(TIF)
A video clip shows the time course of CSP cell aggregation in a microwell.
(MP4)