Abstract
According to conventional understanding regarding dependence of cell behavior on substrate stiffness, tissue cells typically remain round on soft substrates but spread on stiff substrates. The current studies were carried out to learn if the growth factor environment influenced the foregoing relationship. Using standard methods, we prepared planar (2D) polyacrylamide (PA) gels ranging from 0.5 to 40 kPa and covalently crosslinked with fibronectin and collagen at concentrations ranging from 2.5 to 50 μg/ml. We carried out experiments with fibroblasts varying in their ability to form actin stress fibers and focal adhesions. In fetal bovine serum (FBS) containing medium -- the growth factor environment in which most studies on cell spreading and substrate stiffness have been carried out -- cell spreading increased with increasing substrate stiffness and adhesion ligand density. However, in platelet-derived growth factor (PDGF) containing medium, cell spreading was relatively independent of substrate stiffness and adhesion ligand density except little cell attachment occurred in the complete absence of crosslinked adhesion ligands. If cell contraction was blocked with blebbistatin, then cell spreading in FBS-containing medium became independent of substrate stiffness. The findings suggest that under growth factor conditions that stimulate global cell contraction (FBS), cell spreading cannot occur unless adhesion ligand density and substrate stiffness result in cell-substrate interactions strong enough to resist and overcome the inward tractional force. Under growth factor conditions that stimulate global cell protrusion (PDGF), such resistance is not required.
Keywords: Cell spreading, Collagen, Compliance, Fibronectin, Growth factors, Mechanical properties
INTRODUCTION
Mechanical interactions between cells and their extracellular environment play key roles in diverse aspects of normal cell physiology including cell migration and proliferation [1–4]. Changes in cell-matrix interactions contribute to the pathological features associated with tumor progression [5–8], scarring [9–11] and aging [12]. One of the key goals of studying biomechanical interactions between cells and matrix has been development of materials for tissue engineering [13–17].
Substrate stiffness is one of the biomechanical properties of particular interest with regard to its influence on tissue cell behavior. On soft 2D substrates (e.g., ~1 kPa polyacrylamide gels), cells spread poorly even at an optimal concentration of covalently crosslinked adhesion ligand. As stiffness increases to 50 kPa, cell spreading increases [18–20]. Also, cells migrating on soft substrates exhibit a property called durotaxis meaning that they move preferentially up stiffness gradients and not the reverse [21–23].
Research in our laboratory focuses on the interactions between fibroblasts and three dimensional collagen matrices with a particular interest in motile and mechanical interactions involved in matrix remodeling and cell migration [24]. Important differences exist between the biomechanical behavior of fibroblasts interacting with 3D matrices compared to those described above with soft 2D substrates. For instance, fibroblasts can spread well on soft (5–65 Pa) collagen matrices, much softer than the softest PA gels. The shape of cell spreading varies from dendritic extensions lacking stress fibers to more stellate extensions containing prominent stress fibers depending on collagen matrix density. However, the difference reflects a change in spacing between collagen fibrils rather than a difference in matrix stiffness [25]. Also, fibroblasts migrating in 3D collagen matrices are not limited by durotaxis. In nested collagen matrices, cells can migrate from inner ~15 mg/ml collagen matrices to outer 1.5 mg/ml collagen matrices [26], which means crossing a stiffness gradient from ~600 Pa to ~6 Pa [25]. Keratocytes can cross an even higher stiffness gradient from inner ~133 mg/ml inner collagen matrices to outer 2.5 mg/ml matrices [Kim, 2010 #40].
The experiments described above with soft 2D substrates were carried out in serum-containing medium, whereas those with 3D collagen matrices were carried out in platelet-derived growth factor (PDGF)-containing medium. Since differences in the growth factor environment profoundly alter cell contractile and migratory behavior in 3D collagen matrices [28], we reasoned that a similar effect might occur with soft 2D substrates. To test this possibility, we carried out the current experiments to compare fibroblast spreading on soft polyacrylamide substrates in FBS vs. PDGF medium.
MATERIALS AND METHODS
Materials
Type I collagen (Rat tail) was purchased from BD Bioscience (Bedford, MA). Human plasma fibronectin was obtained from New York blood center (New York, NY). Dulbecco's modified Eagle medium (DMEM), 0.25% trypsin/EDTA solution and Alexa Fluor 568 goat anti-mouse IgG (H/L) were purchased from Invitrogen (Grand Island, NY). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, Georgia). PDGF (BB isotype) was purchased from Upstate Biotechnology (Lake Placid, NY). TGFβ1 (human platelet) was purchased from Calbiochem (La Jolla, CA) and Cell Signaling Technology (Beverly, MA). Fluoromount G was obtained from Southern Biotechnology Associates (Birmingham, AL). Alexa Fluor 488 phalloidin was purchased from Molecular Probes (Eugene, OR). 3-Aminopropyltriethoxy-silane (APES), fatty acid-free bovine serum albumin (BSA), lysophosphatidic acid (LPA), monoclonal anti-vinculin, and monoclonal anti-α smooth muscle actin were obtained from Aldrich-Sigma (St. Louis, Mo). HRP-conjugated goat anti-mouse and RBS35 detergent concentrate were from Pierce (Rockford, IL). Bovine serum albumin (BSA fraction V) was obtained from Equitech-Bio, Inc (Derrville, TX). Blebbistatin was obtained from TRC (North York, Ontario, Canada). Pierce ECL Western blotting substrate and Sulfo-SANPAH were purchased from Thermo Scientific (Rockford, IL). SurfaSil Siliconizing Fluid was obtained from Thermo Hypersil-Keystone (Bellefonte, Pa). 40% acrylamide solution, 2% bis-acrylamide solution and ammonium persulfate were purchased from Bio-Rad (Hercules, CA USA). Microscope coverslips, 12 mm diameter (#1) and 22×22mm (#1½), were purchased from Fisher scientific and VWR respectively.
Cell culture
Use of de-identified human foreskin fibroblasts was approved by the University Institutional Review Board (Exemption #4). Early passage, hTERT-immortalized cells (BR5) and oncogenic Ras-transformed BR5 cells (BR5-RAS) [29] were cultured in DMEM supplemented with 10% FBS at 37° C, in a 5% CO2 humidified incubator. For preparing myofibroblasts (BR5-MF), BR5 cells were incubated 5 days in 3% FBS/DMEM containing 5 ng/ml TGFβ1 and subsequently maintained in 10% FBS/DMEM containing 5 ng/ml TGFβ1.
Collagen matrices and PA gels
Collagen matrices (1–4 mg/ml), 50 μg/ml collagen-coated glass coverslips, and 20 μg/ml fibronectin-coated glass coverslips were prepared as described previously [25]. PA gels varying in stiffness from ~0.5 to 40 kPa were prepared following the protocol of Tse and Engler [30]. Briefly, 12 mm dia. glass coverslips were treated sequentially with RBS-35 cleaning solution, ddH2O, 95% alcohol, and air dried. The cleaned coverslips were immersed in a freshly prepared 2% APTES in dry acetone for 5 min, rinsed with ddH2O, dried overnight at 42° C, and stored at 22° C. 22×22mm glass coverslips were treated similarly as above except that 100 μl SurfaSil Siliconizing Fluid was used to coat the coverslips instead of 3-APTS. To obtain PA gels varying in stiffness from ~0.5 to 40 kPa, 40% acrylamide and 2% bis-acrylamide were mixed at the recommended ratios [30] to which was added 0.1% TEMED and 1% ammonium persulfate (100 mg/ml). 35 μl aliquots of the PA solution were placed on 3-APTS coverslips (treated side up) and covered with siliconized coverslips (treated side down) to establish a uniform gel layer that was allowed to polymerize 20–30 min at 22° C. After polymerization, the gel “sandwich” was covered with ddH2O and the upper coverslip removed. Polymerized PA gels were placed in 50 mm Hepes buffer (pH=8.5) and “activated” by addition of freshly prepared 350 μl Sulfo-SANPAH in 0.5 mg/ml in Hepes buffer and explosure to UV light (365nm) for 10 min at room temperature. Activated gels were rinsed twice with DMEM and then incubated 1 h at 37° C in a 5% CO2 incubator with DMEM containing collagen, fibronectin, or BSA at the concentrations indicated in the figure legends. Subsequently, the ligand-coated PA gels were rinsed with DMEM and used for experiments.
Immunofluorescence microscopy
Fibroblasts (104/sample) in DMEM containing PDGF (50 ng/ml), BSA (5 mg/ml) and 10% FBS as indicated were incubated on collagen-coated coverslips, collagen matrices and PA gels as described for the time periods shown in the figure legends. At the end of incubations, cells were fixed with 3% formaldehyde in phosphate-buffered saline, permeabilized and processed for immunofluorescence staining as previously [25, 26] using mouse anti-α smooth muscle actin IgG (1:50), mouse anti-vinculin IgG (1:100), Alexa fluor 568 goat anti-mouse IgG (1:300) and Alexa fluor 488 phalloidin (1:500) as indicated. After staining, samples were mounted on glass slides with Fluoromount G. Microscopic images were collected with a Nikon Eclipse E600 fluorescence microscope and 10×/0.45 and 20×/0.75 Nikon Plan Apo infinity corrected objectives using a Photometrics SenSys CCD camera and MetaVue imaging software (Molecular Devices). Data shown are representative images. All experiments were carried out multiple times.
RESULTS
Effect of growth factor environment on fibroblast spreading
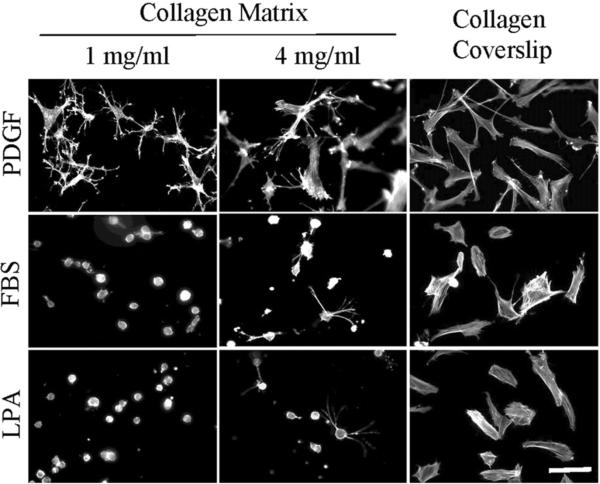
Figure 1 shows the morphology of early passage human fibroblasts (BR5 cells) incubated 4 hr on 1 and 4 mg/ml collagen matrices and collagen-coated coverslips in medium containing PDGF, FBS and lysophosphatidic acid (LPA). LPA is a Rho kinase-activating growth factor found in FBS that exhibits a procontractile influence on fibroblasts in 3D collagen matrices similar to FBS [28]. Consistent with previous findings, fibroblasts on collagen matrices in PDGF-containing medium spread with dendritic extensions on 1 mg/ml collagen matrices. Cells on 4 mg/ml matrices formed more stellate extensions. In marked contrast, fibroblasts in FBS or LPA-containing medium were mostly round on 1 and 4 mg/ml collagen matrices. Cells incubated on collagen-coated glass coverslips spread in flattened morphology under all growth factors conditions.
Figure 1.
Fibroblast spreading on collagen matrices and collagen-coated coverslips.
BR5 fibroblasts were cultured 4 hr in PDGF, FBS and LPA-containing medium on 1 mg/ml and 4 mg/ml collagen matrices and collagen-coated coverslips as indicated. At the end of incubations, samples were fixed and stained for actin. Scale bar, 100 μm.
The stiffness of 1 mg/ml collagen matrices is ~ 5 Pa and of 4 mg/ml matrices is ~65 Pa [25]. Subsequent experiments were carried out with polyacrylamide (PA) substrates ranging in stiffness from 0.5 to 40 kPa and covalently crosslinked with fibronectin and collagen at concentrations from 2.5 to 50 μ/ml.
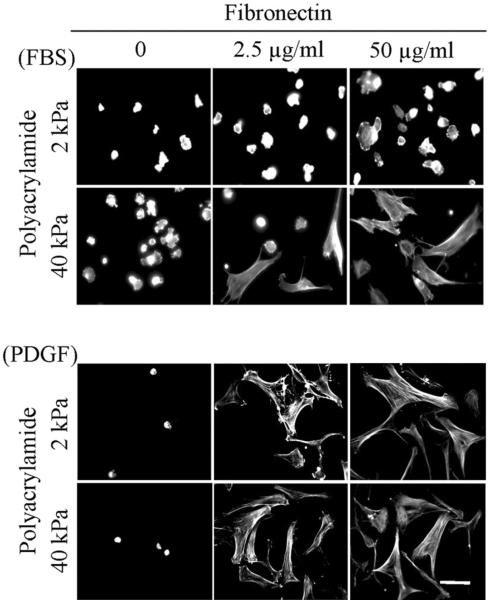
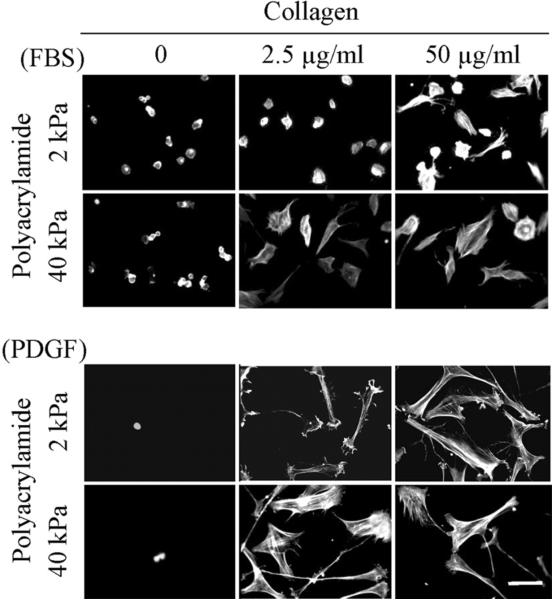
Figure 2 (PA-fibronectin) and Figure 3 (PA-collagen) compare fibroblast spreading on 2 and 40 kPa polyacrylamide gels. In FBS-containing medium, some cell attachment to PA gels occurred in the absence of adhesion ligand but no cell spreading. With 2 kPa polyacrylamide gels, fibroblasts spread poorly even if the substrates were crosslinked 50 μg/ml adhesion ligands. On the other hand, with 40 kPa polyacrylamide gels, some cell spreading occurred even with 2.5 μg/ml adhesion ligands.
Figure 2.
Fibroblast spreading on fibronectin-crosslinked PA gels.
BR5 fibroblasts were cultured 4 hr in FBS and PDGF-containing medium on 2 and 40 kPa polyacrylamide gels with fibronectin crosslinked at the concentrations indicated. At the end of incubations, samples were fixed and stained for actin. Scale bar, 100 μm.
Figure 3.
Fibroblast spreading on collagen-crosslinked PA gels.
BR5 fibroblasts were cultured 4 hr in FBS and PDGF-containing medium on 2 and 40 kPa polyacrylamide gels with collagen crosslinked at the concentrations indicated. At the end of incubations, samples were fixed and stained for actin. Scale bar, 100 μm.
In PDGF-containing medium, few cells attached to the PA gels in the absence of adhesion ligand. However, with adhesions ligands crosslinked at 2.5 μg/ml, attachment and spreading occurred on both 2 and 40 kPa polyacrylamide gels. Cell morphology was elongated and stellate with actin stress fibers.
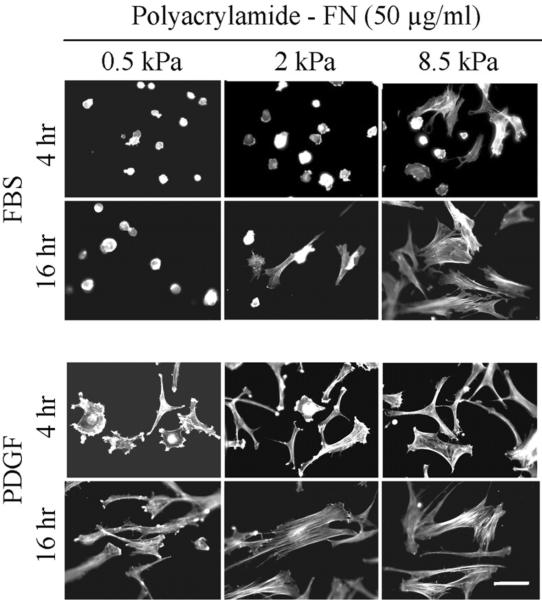
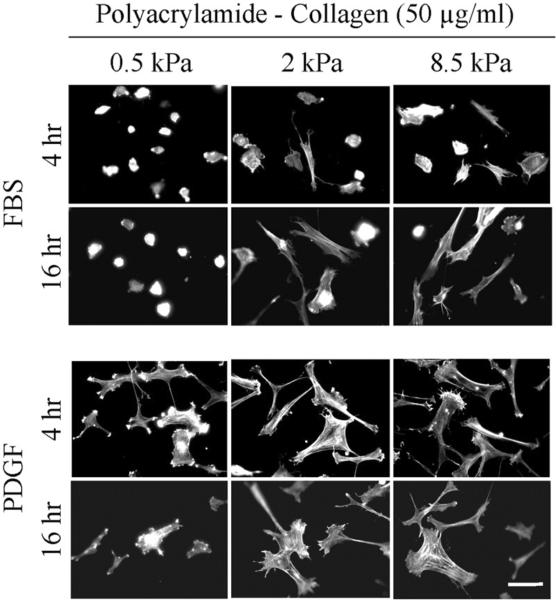
Figure 4 (PA-fibronectin) and Figure 5 (PA-collagen) show cell spreading after 4 and 16 hr using substrates at the softer end of the range (0.5 to 8.5 kPa) and crosslinked with the highest concentration of adhesion ligands (50 μg/ml adhesion ligands). Regardless whether measured after 4 or 16 hr, spreading of cells in FBS-containing medium depended on polyacrylamide stiffness; whereas cells in PDGF-containing medium were able to spread even on the softest substrates (0.5 kPa).
Figure 4.
Fibroblast spreading on 0.5 – 8.5 kPa polyacrylamide gels crosslinked with 50 μg/ml fibronectin.
BR5 fibroblasts were cultured 4 and 16 hr in FBS and PDGF-containing medium on 0.5 – 8.5 kPa polyacrylamide gels as indicated with fibronectin crosslinked at 50 μg/ml. At the end of incubations, samples were fixed and stained for actin. Scale bar, 100 μm.
Figure 5.
Fibroblast spreading on 0.5 – 8.5 kPa polyacrylamide gels crosslinked with 50 μg/ml collagen.
BR5 fibroblasts were cultured 4 and 16 hr in FBS and PDGF-containing medium on 0.5 – 8.5 kPa polyacrylamide gels as indicated with collagen crosslinked at 50 μg/ml. At the end of incubations, samples were fixed and stained for actin. Scale bar, 100 μm.
Cell contraction and growth factor dependence of substrate stiffness
The results in Figures 2–5 showed that fibroblasts were able to attach and spread on soft PA gels as long as the growth factor environment contained PDGF. If the growth factor environment contained FBS then cell spreading became substrate stiffness dependent. Similar to PDGF, little cell spreading was observed on soft substrates if LPA was substituted for FBS (not shown).
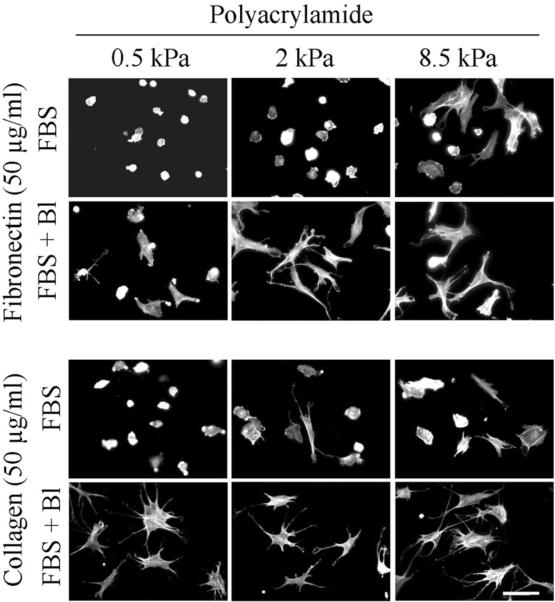
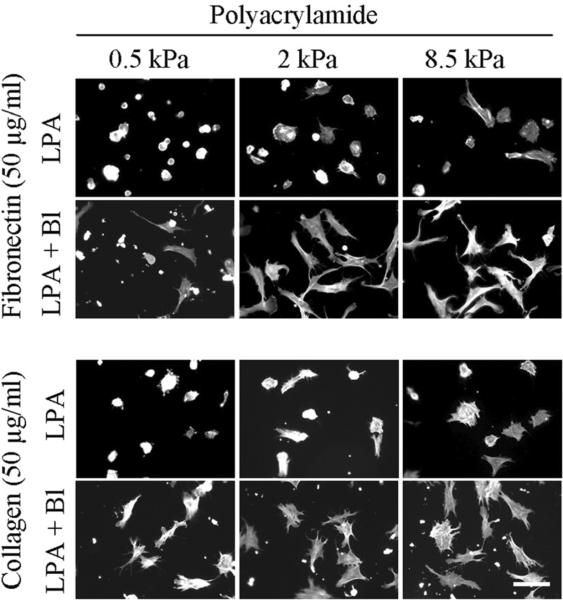
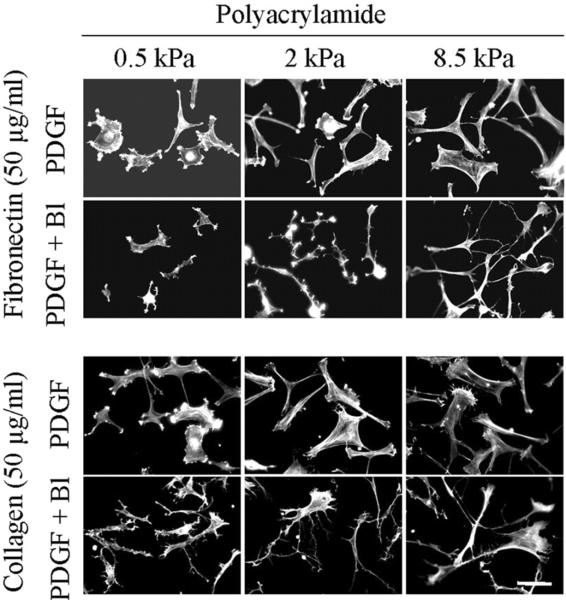
Previous studies on fibroblasts interacting with 3D collagen matrices suggested that the round cell morphology exhibited in FBS and LPA (Figure 1) resulted from the inability of the collagen matrix to resist the force of cell contraction resulting from Rho activation [28]. If a similar effect accounted for the inability of fibroblasts of spread on soft PA gels, then if cell contraction was blocked, cell spreading would be predicted to occur even in the presence of FBS. Figure 6 (FBS medium) and Figure 7 (LPA medium) show the results of experiments to test this possibility using 0.5 to 8.5 kPa polyacrylamide gels crosslinked with 50 μg/ml fibronectin and collagen. Addition of blebbistatin (Bl) permitted fibroblasts in FBS or LPA-containing medium to spread on the soft substrates. Figure 8 shows parallel experiments with fibroblasts in PDGF-containing medium. In this case, blocking cell contraction did not prevent cell spreading. However, cell extensions tended to remain dendritic on all of the substrates rather than becoming stellate with actin stress fibers.
Figure 6.
Effect of blebbistatin on fibroblast spreading in FBS-containing medium.
BR5 fibroblasts were cultured 4 hr in FBS-containing medium with and without 20 μM blebbistatin (Bl) on 0.5 – 8.5 kPa polyacrylamide gels as indicated with fibronectin and collagen crosslinked at 50 μg/ml. At the end of incubations, samples were fixed and stained for actin. Scale bar, 100 μm.
Figure 7.
Effect of blebbistatin on fibroblast spreading in LPA-containing medium.
BR5 fibroblasts were cultured 4 hr in LPA-containing medium with and without 20 μM blebbistatin (Bl) on 0.5 – 8.5 kPa polyacrylamide gels as indicated with fibronectin and collagen crosslinked at 50 μg/ml. At the end of incubations, samples were fixed and stained for actin. Scale bar, 100 μm.
Figure 8.
Effect of blebbistatin on fibroblast spreading in PDGF-containing medium.
BR5 fibroblasts were cultured 4 in LPA-containing medium with and without 20 μM blebbistatin (Bl) on 0.5 – 8.5 kPa polyacrylamide gels as indicated with fibronectin and collagen crosslinked at 50 μg/ml. At the end of incubations, samples were fixed and stained for actin. Scale bar, 100 μm.
Spreading of myofibroblasts and oncogenic Ras transformed fibroblasts
Cells vary greatly in their ability to exert mechanical force. To test if the differential effect of growth factor environment on BR5 fibroblasts occurred over a broad range of cell mechanical force exertion, additional experiments were carried out with BR5 cells that were treated with TGBβ to become myofibroblasts (BRF-MF) or were transformed with oncogenic Ras (BR5-RAS).
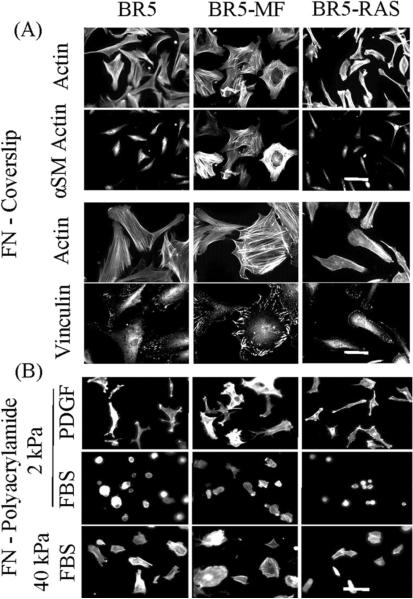
Figure 9A shows key morphological features of the three cell types after spreading on FN-coated coverslips. BR5-MF but not BR5 and BR5-Ras exhibited increased staining for α-smooth muscle (αSM) actin and co-localization of αSM-actin with stress fibers. Compared to BR5 or BR5-Ras, BR5-MF had more prominent stress fibers and vinculin-staining focal adhesions. Conversely, BR5-Ras had less well developed stress fibers and smaller focal adhesions.
Figure 9.
Spreading of BR5, BR5 myofibroblasts, and oncogenic Ras-transformed BR5 cells on fibronectin-coated coverslips.
(A) BR5, BR5-MF and BR5-Ras fibroblasts as indicated were cultured 4 hr in PDGF-containing medium on fibronectin-coated coverslips. At the end of incubations, samples were fixed and stained for actin and α-smooth muscle actin and actin and vinculin. Scale bar, 100 μm (α-smooth muscle actin) and 50 μm (vinculin). (B) BR5, BR5-MF and BR5-Ras fibroblasts were cultured 4 hr in PDGF and FBS-containing medium as indicated on 2 and 40 kPa acrylamide gels with 50 μg/ml covalently crosslinked fibronectin. At the end of incubations, samples were fixed and stained for actin. Scale bar, 100 μm.
Figure 9B shows that on 2 kPa polyacrylamide gels, all three cell types were round in FBS-containing medium but spread in PDGF-containing medium. However, if the substrate stiffness was increased to 40 kPa, then all three cell types also were able to spread in FBS-containing medium.
DISCUSSION
In what has become the conventional understanding regarding dependence of cell behavior on substrate stiffness, tissue cells typically remain round on soft substrates but spread on stiff substrates. Growth factors have been viewed as playing an inductive role in cell proliferation and differentiation [15, 31] but not as a determining factor in the cellular morphological response to substrate stiffness. However, our findings suggest that the growth factor environment has a major influence on the cell morphological response to substrate stiffness
Using standard methods [18, 30], we prepared PA gels ranging from 0.5 to 40 kPa and covalently crosslinked with fibronectin and collagen at concentrations ranging from 2.5 to 50 μg/ml. We tested both fibronectin and collagen because some research has demonstrated differences in cellular mechanical responses to these adhesion ligands [32, 33]. We carried out experiments with cells that varied in their ability to form actin stress fibers and focal adhesions, i.e., early passage human fibroblasts, fibroblasts transformed by oncogenic Ras, and human fibroblasts stimulated by TGFβ to become myofibroblasts. In general, our findings were similar with fibronectin and collagen and with all three cell types.
In FBS-containing medium -- the growth factor environment in which most studies on cell spreading and substrate stiffness have been carried out -- cell spreading increased with increasing substrate stiffness and adhesion ligand density. On the softest PA gels, cells remained round even at the highest concentration of adhesion ligand. However, in PDGF-containing medium, cell spreading was relatively independent of substrate stiffness and adhesion ligand density. On the softest PA gels, cells underwent spreading even at the lowest concentration of adhesion ligand tested, except cells were unable to attach to the PA substrate in the complete absence of added adhesion ligand. That cell attachment occurred to PA gels in the absence of added covalently crosslinked fibronectin or collagen in FBS but not PDGF-containing or LPA-containing (not shown) medium may be a result of passive adsorption to the polyacrylamide of fibronectin and other serum cell adhesion factors found in serum [34].
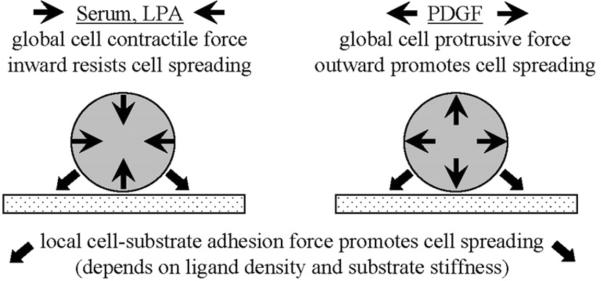
Figure 10 offers a simplified schematic to help explain the results. In FBS-containing medium, the global effect of Rho activation leads to myosin II-dependent contraction of the cellular cortical actin network and actin cytoskeleton [35–37]. The resulting inward tractional force works against cell spreading, which cannot occur unless the opposing force of cell-substrate interactions is strong enough to resist and overcome the inward force. Consistent with this idea, similar results were observed if the Rho-activating lipid growth factor LPA replaced FBS. Moreover, with FBS or LPA-containing medium, blocking myosin II-dependent cell contraction with blebbistatin permitted fibroblast spreading even on the softest PA gels.
Figure 10.
Schematic showing the interrelationship of global and local forces.
Under growth factor conditions that stimulate global cell contraction (FBS/LPA), cell spreading cannot occur unless adhesion ligand density and substrate stiffness result in cell-substrate interactions strong enough to resist and overcome the inward tractional force. Under growth factor conditions that stimulate global cell protrusion (PDGF), such resistance is not required.
In contrast to FBS/LPA activation of Rho, PDGF-stimulates global Rac activation and leads to cell protrusion rather than contraction [38]. In this case, a stiff substrate is not required to counter cell contractile force. Cell spreading can occur even on very soft substrates. Formation of initial cell adhesions at the tips of cell protrusions does not require cell contractile force, but these adhesions can undergo local myosin II-dependent maturation to form focal adhesions and stress fibers [39–41]. Consistent with the latter interpretation, we found that blocking myosin II-dependent cell contraction did not prevent PDGF-stimulated dendritic cell spreading, but did prevent cells from forming stress fibers.
The above findings overlap with our previous understanding of fibroblasts in 3D collagen matrices. In 3D collagen matrices, cell behavior differs markedly in procontractile (FBS, LPA) vs. promigratory (PDGF) growth factors environments [24, 28]. These differences tends to be masked when cells interact with stiff substrates such as glass and tissue culture plastic. The current findings indicate that for soft 2D substrates as well as for 3D matrices, to understand cell morphological response requires making a distinction between procontractile and promigratory growth factor environments. This recognition should be of particular significance for those designing and testing materials in the field of tissue engineering.
CONCLUSIONS
In the current studies, we characterize cell spreading on polyacrylamide matrices varying in stiffness and adhesion ligand density. Our findings suggest that the growth factor environment has a large influence on the cell morphological response. Unlike FBS/LPA conditions, in PDGF-containing medium cell spreading becomes relatively independent of substrate stiffness and adhesion ligand density. For soft 2D substrates as well as for 3D matrices, to understand cell morphological response requires making a distinction between procontractile and promigratory growth factor environments.
ACKNOWLEDGMENTS
We are grateful to Drs. Bruno da Rocha-Azevedo and Zhenan Liu for their advice. This research was supported by a grant from the National Institutes of Health, GM31321.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- [2].Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol. 2011;3:a005033. doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].DuFort CC, Paszek MJ. Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–19. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- [7].Cukierman E, Bassi DE. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin Cancer Biol. 2010;20:139–45. doi: 10.1016/j.semcancer.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007257:143–79. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- [10].Ogawa R. Mechanobiology of scarring. Wound Repair Regen. 2011;19(Suppl 1):s2–9. doi: 10.1111/j.1524-475X.2011.00707.x. [DOI] [PubMed] [Google Scholar]
- [11].Sarrazy V, Billet F, Micallef L, Coulomb B, Desmouliere A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen. 2011;19(Suppl 1):s10–5. doi: 10.1111/j.1524-475X.2011.00708.x. [DOI] [PubMed] [Google Scholar]
- [12].Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–72. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- [14].Brown RA, Phillips JB. Cell responses to biomimetic protein scaffolds used in tissue repair and engineering. Int Rev Cytol. 2007;262:75–150. doi: 10.1016/S0074-7696(07)62002-6. [DOI] [PubMed] [Google Scholar]
- [15].Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–26. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–9. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- [18].Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–28. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- [20].Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–61. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Isenberg BC, Dimilla PA, Walker M, Kim S, Wong JY. Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys J. 2009;97:1313–22. doi: 10.1016/j.bpj.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Krzyszczyk P, Wolgemuth CW. Mechanosensing can result from adhesion molecule dynamics. Biophys J. 2011;101:L53–5. doi: 10.1016/j.bpj.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol. 2010;26:335–61. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- [25].Miron-Mendoza M, Seemann J, Grinnell F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials. 2010;31:6425–35. doi: 10.1016/j.biomaterials.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Grinnell F, Rocha LB, Iucu C, Rhee S, Jiang H. Nested collagen matrices: a new model to study migration of human fibroblast populations in three dimensions. Exp Cell Res. 2006;312:86–94. doi: 10.1016/j.yexcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- [27].Kim A, Lakshman N, Karamichos D, Petroll WM. Growth factor regulation of corneal keratocyte differentiation and migration in compressed collagen matrices. Invest Ophthalmol Vis Sci. 2010;51:864–875. doi: 10.1167/iovs.09-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rhee S, Grinnell F. Fibroblast mechanics in 3D collagen matrices. Adv Drug Deliv Rev. 2007;59:1299–1305. doi: 10.1016/j.addr.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Menezes GC, Miron-Mendoza M, Ho CH, Jiang H, Grinnell F. Oncogenic Ras-transformed human fibroblasts exhibit differential changes in contraction and migration in 3D collagen matrices. Exp Cell Res. 2008;314:3081–91. doi: 10.1016/j.yexcr.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tse JR, Engler AJ. Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol. 2010;10.16 doi: 10.1002/0471143030.cb1016s47. [DOI] [PubMed] [Google Scholar]
- [31].Assoian RK, Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008;18:347–52. doi: 10.1016/j.tcb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol. 2008;295:C1037–44. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- [33].Byfield FJ, Wen Q, Levental I, Nordstrom K, Arratia PE, Miller RT, et al. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys J. 2009;96:5095–102. doi: 10.1016/j.bpj.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grinnell F. Cellular adhesiveness and extracellular substrata. Int Rev Cytol. 1978;53:65–144. doi: 10.1016/s0074-7696(08)62241-x. [DOI] [PubMed] [Google Scholar]
- [35].Ridley AJ, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- [36].Sheetz MP, Dai J. Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol. 1996;6:85–9. doi: 10.1016/0962-8924(96)80993-7. [DOI] [PubMed] [Google Scholar]
- [37].Ingber DE, Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116:1157–73. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- [38].Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- [39].Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–13. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- [40].Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:1039–50. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annual Rev Cell Dev Biol. 2010;26:315–33. doi: 10.1146/annurev.cellbio.011209.122036. [DOI] [PMC free article] [PubMed] [Google Scholar]