Abstract
PURPOSE
To compare the validity and effectiveness of 2 methods for expanding depth of focus to correct for presbyopia; that is, induction of spherical aberration and small pupil apertures.
SETTING
University of California, Berkeley, California, USA.
DESIGN
Comparative case series.
METHODS
A random 4-alternative forced-choice acuity task was performed. Visual performance and depth of focus was compared using adaptive optics–corrected distance visual acuity (CDVA) values and mean visual acuity over a 3.0 diopter (D) range of defocus using the following 3 adaptive optics–corrected profiles: 2.0 mm pupil, 5.0 mm pupil, and 5.0 mm pupil with −0.274 µm of spherical aberration.
RESULTS
The study enrolled 13 subjects. The 5.0 mm pupil profile had a CDVA of −0.218 logMAR and a mean visual acuity through focus of 0.156 logMAR. The 2.0 mm pupil profile had a worse CDVA (0.012 logMAR) but an improved mean visual acuity (0.061 logMAR). The 5.0 mm pupil profile with −0.274 µm of spherical aberration measured a CDVA of −0.082 logMAR and a mean visual acuity 0.103 logMAR.
CONCLUSIONS
The spherical aberration and small-pupil profiles improved the mean visual acuity across a 3.0 D range of defocus but resulted in decreased CDVA at the plane of best focus in comparison to an adaptive optics–corrected 5.0 mm pupil. Small-pupil profiles are a better choice than spherical aberration profiles for presbyopic corrections due to expected accuracy, predictability, and patient satisfaction.
With more than 1 billion people worldwide requiring near-vision correction for their age-related loss of focusing ability, the field of presbyopia research is an area of significant interest in vision care.1 Bifocal and multifocal contact lenses can help alleviate the dependence on spectacles but are not without disadvantages in comfort and convenience, and many people would prefer a 1-time surgical procedure that would lead to a permanent solution to near-vision blur. Multifocal contact lenses have been found to increase depth of focus, thereby aiding in near vision tasks; however, this improvement in near vision has been found to cause a reduction in peak visual performance at distance.2,3
Laser vision correction and cataract intraocular surgery can also improve near vision by using multifocality. It has been proposed that laser vision correction could achieve this by inducing spherical aberration through an ablation pattern using an excimer laser that increases the prolateness of the cornea, thereby inducing negative spherical aberration.4 Femtosecond treatments, such as lamellar keratoplasty or intrastromal cylindrical rings, could conceivably create similar conditions of multifocality. In addition, intraocular lenses (IOLs) allow for an even wider variety of multifocal solutions to loss of near vision.
Monovision is another method that has been used successfully with contact lenses as well as with refractive surgery. The success of monovision would depend on the ability and willingness of a patient to adapt to and accept using 1 eye for near vision and the other eye for distance vision to achieve a wider range of good vision. The resultant loss of good binocular vision, however, has remained a significant concern for many patients.
Perhaps as early as 1677, Descartes realized that the human eye had spherical aberration. In 1801, Thomas Young5 described an instrument that could demonstrate its existence, and now spherical aberration in the eye has been successfully measured and described in significant detail.6–9 It is known that the natural human lens typically exhibits negative spherical aberration,9 which could cause reduced vision quality if not counteracted by the positive spherical aberration generally measured in the cornea.10,11 On average, the human population exhibits an overall small amount of positive spherical aberration.12 There is some suggestion that the spherical aberration that is present could benefit our natural vision through improved depth of focus.13 Gou et al.14 suggest that spherical aberration would protect against the worsening of contrast sensitivity that occurs in the presence of defocus.
Attempts have been made to apply this understanding of spherical aberration to surgical corrections for improving near vision. Multifocal laser in situ keratomileusis (LASIK) ablation profiles create varying curvatures across the surface of the cornea to provide focused light at the retinal plane for distant objects and near objects. This lack of a singular focus, however, would cause the image quality to be compromised at all distances but operates on the assumption that it is possible to expand the depth of focus using multifocality with only minor loss in visual performance at distance and near.15 There is some evidence that these multifocal surface ablations delay the near-vision loss from presbyopia, although there might be some loss of peak performance vision.16,17
Multifocal IOLs function on a similar principle, with multiple zones on the artificial implanted lens being designed to focus incoming light on the retina for distant objects and near objects. There has been some discussion about whether there is certain amount of spherical aberration could be placed in an IOL to optimize visual performance.18,19 Most studies, however, concluded that although multifocal IOLs can increase depth of focus, they also reduce contrast sensitivity as well as peak visual performance.20,21
Another area of interest is the effect that small pupil apertures have on increasing depth of focus. It is known that although higher-order aberrations (HOAs) increase with age, pupil size decreases.22,23 Both these age-related changes could result in a wider depth of focus, although any beneficial effect could be minimal if the resulting peak performance decreases as well. Small-aperture corneal implants have been shown to improve near vision in patients by expanding the depth of focus. Some evidence also shows that this method might not produce the same loss in peak performance as measured with induced spherical aberration.24,25
Because both spherical aberration profiles and small-pupil-aperture profiles are designed to improve near vision by expanding the depth of focus, this study was performed to compare, in the same experimental setting, the vision quality and performance that are obtained through these 2 methods.
SUBJECTS AND METHODS
Participants were obtained through local volunteers and an online recruitment web site. Informed consent was obtained from all participants after written and oral explanations were given regarding possible complications. The experiment was approved by the University of California, Berkeley, Committee for the Protection of Human Subjects, and all protocol adhered to the tenets of the Declaration of Helsinki.
Subjects were chosen from a pool of volunteers who reported good ocular health and no significant need for spectacle correction. All subjects had 20/20 or better corrected distance visual acuity (CDVA) and uncorrected distance visual acuity and did not routinely wear spectacle correction. All included subjects had between −0.25 diopter (D) and +1.00 D of spherical correction and between 0.00 D and −0.75 D of cylindrical correction needed for best correction using the Shack-Hartmann wavefront aberrometer.
One eye of each subject was dilated and cyclopleged using 1 drop of tropicamide 1.0% and 1 drop of phenylephrine 2.5% approximately 20 minutes before testing. Additional drops were added to maintain cycloplegia if testing lasted more than 1 hour. An eye patch was placed to cover the nontested eye. Testing occurred over 90 minutes or less for each subject.
After dilation, the pupil size was selected using an adjustable artificial aperture conjugate to the participant’s pupil plane; the aperture was calibrated during each session to ensure accuracy. An adaptive optics scanning laser ophthalmoscope was used to project a high-contrast stimulus on the retina using an 840 nm infrared low-coherence light source.26,27 Before testing, the field size was calibrated using a calibration grid to ensure precise settings for image size. Optimization was performed on the central portion of the grid to prevent distortion of the aspect ratio for horizontal and vertical dimensions that could provide cues for determining letter orientation. Further calibration was performed as described by Rossi et al.27 to overcome errors caused by the nonlinear scanning velocity of the resonant scanner.
Aberrations were measured using a Hartmann-Shack wavefront sensor, and best spectacle correction was obtained using spherical and cylindrical trial lenses based on Hartman-Shack wavefront sensor measurements.28 A MEMS deformable mirror (140 actuator with 3.5 µm stroke, Boston Micromachines) was used to correct or control remaining lower-order aberrations and HOAs, and adaptive-optics performance was gauged by computing the aberrations of the eye using Zernike terms up to the 10th order. Adaptive-optics control of the optics was set before testing and then fixed. It was reset when the subject sat out of the instrument or any other time it was deemed necessary. Custom aberration profiles were generated through a graphical user interface menu system that allowed the operator to type in Zernike coefficients for the desired wave aberration. The reference offsets for the Hartmann-Shack spots were set accordingly, and the adaptive-optics system drove the mirror to the reference in closed-loop operation. As such, the desired aberration replaced, and was not added to, each subject’s aberrations. To correct all aberrations, the reference was to a flat-plane wavefront. The Hartmann-Shack wavefront sensor displayed and logged the actual coefficients to allow the user to easily monitor adaptive-optics performance.
For each trial, participants were presented with a random 4-alternative forced-choice test using a tumbling E Snellen letter at a letter size initially determined to be slightly larger than the predicted threshold. Letters were adjusted in 5-pixel fixed-step increments (1 pixel/letter line) in a 1-up, 2-down procedure with a value of 62.5% correct used to calculate threshold values. All visual acuity values were reported in logMAR units.
Participants were shown 40 trials for each defocus value to establish threshold values. Between each series of 40 trials, defocus was added or subtracted in 0.50 D increments using trial lenses. Before each series of trials, a best adaptive-optics correction was acquired before inserting trial lenses for providing defocus.
Participants were tested for visual acuity over a range of 3.00 D of defocus under the following 3 conditions: adaptive optics–corrected 5.0 mm pupil, adaptive optics–corrected 5.0 mm pupil with −0.274 µm of Z(4,0) spherical aberration, and adaptive optics–corrected 2.0 mm pupil. Under the 2.0 mm pupil condition, an optical system is near the diffraction limit. Adaptive-optics correction was obtained at 5.0 mm and was used to ensure that no residual defocus or astigmatism remained to interfere with testing. An artificial aperture conjugate to the participant’s pupil was then reduced to 2.0 mm and checked for accuracy. Visual performance and depth of focus were evaluated using the best adaptive optics–corrected visual acuity as well as the mean visual acuity across the entire 3.00 diopter range of defocus values, which was measured in 0.50 diopter increments (a mean of the means).
RESULTS
Thirteen eyes of 13 subjects were included in the study. The mean age of the 7 men and 6 women was 28.1 years ± 7.3 (SD). The mean total root mean square (RMS) for 5.0 mm pupil was 1.08 ± 0.66 µm. The mean HOA RMS for 5.0 mm pupil was 0.55 ± 0.28 µm. One subject previously had LASIK but reported good results and no complications. Testing results of the subject who had LASIK were very similar to the other subjects and did not differ significantly in any category.
Good optical correction was obtained under the initial condition of adaptive optics–corrected 5.0 mm pupil with mean RMS of 0.061 ± 0.012 µm. Higher-order aberrations accounted for 85% of residual aberrations with a mean RMS of 0.052 ± 0.010 µm. The mean RMS was calculated to be 0.0002 ± 0.0002 µm for the 2.0 mm pupil condition.
Under the Z(4,0) spherical aberration condition with a 5.0 mm pupil, the mean RMS was 0.302 ± 0.019 µm. For all participants, the Hartman-Shack wavefront sensor measured the mean spherical aberration applied using the deformable mirror to be −0.274 ± 0.013 µm. This value was consistent across the 13 participants with only slight variability.
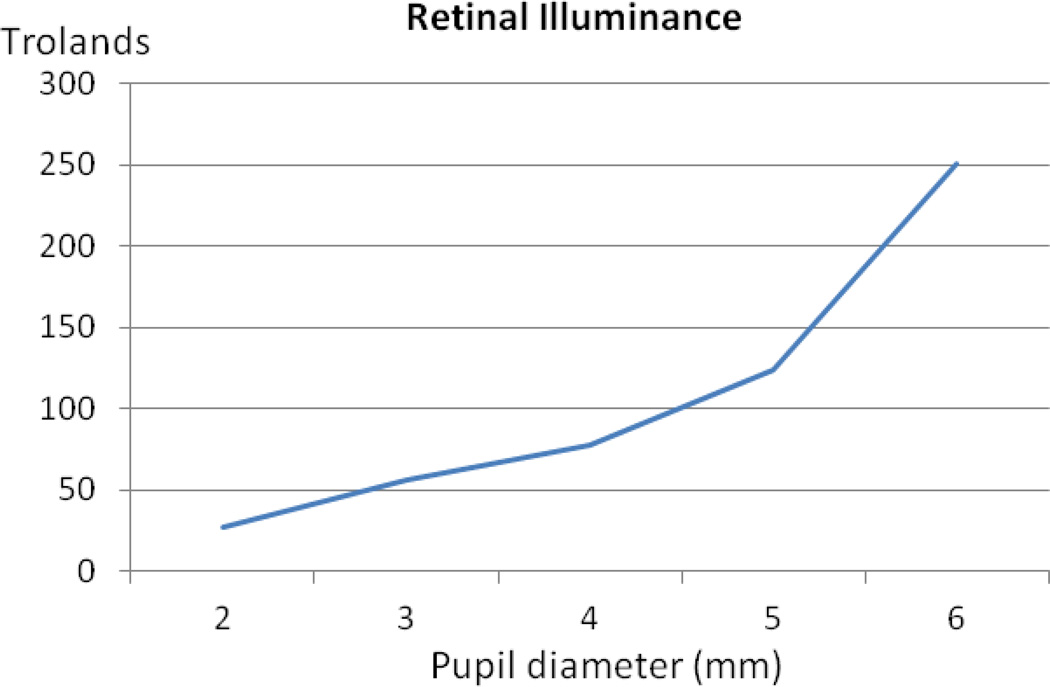
Light-source levels were held constant regardless of pupil size to realistically compare visual performance for each condition. A constant light source level, however, would result in decreased illumination on the retina for smaller pupil sizes. A change from a 5.0 mm pupil to a 2.0 mm pupil results in a 6-fold decrease in pupil area and, because of the slightly Gaussian profile of the entrance beam, a 5-fold decrease in the retinal illuminance was measured by a power meter at the level of the pupil plane (Figure 1).
Figure 1.
Measured retinal illuminance with a change in pupil diameter (mm, x-axis)
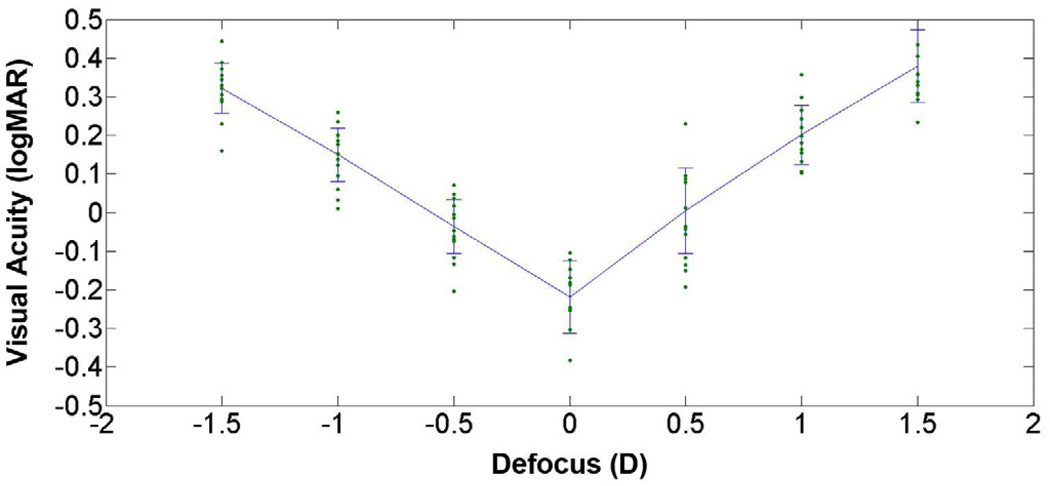
Through-focus visual acuities of subjects under the adaptive optics–corrected 5.0 mm pupil condition had a V pattern, with the best visual acuity obtained at 0.00 D defocus (Figure 2). The mean visual acuity at best focus was −0.218 ± 0.095 logMAR. The mean visual acuity across the entire 3.00 D range of defocus values was 0.156 ± 0.052 logMAR.
Figure 2.
Mean visual acuity scores with standard deviations across 3.00 D of defocus along with data points for 13 subjects with an adaptive optics–corrected 5.0 mm pupil.
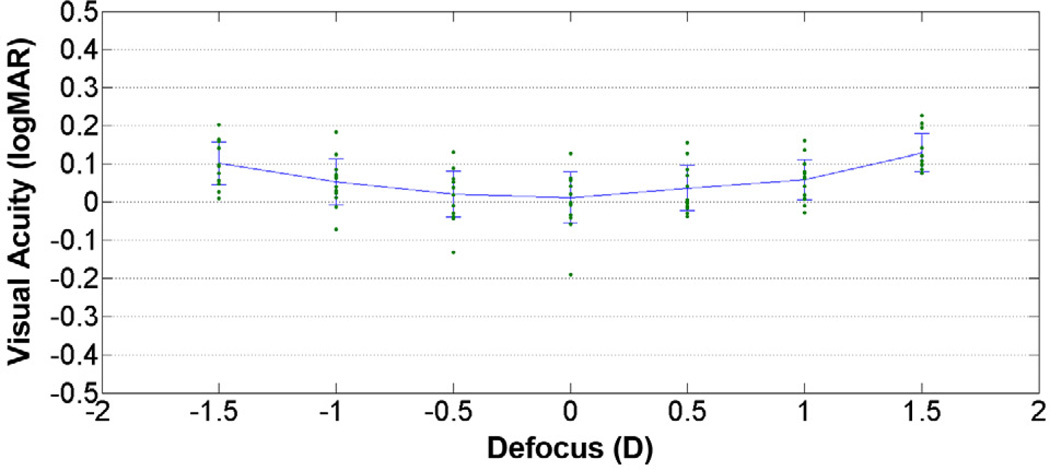
When the pupil size was decreased to 2.0 mm, the through-focus curve flattened considerably (Figure 3). The mean visual acuity at the plane of best focus was still obtained at 0.00 D defocus but worsened to 0.012 ± 0.067 logMAR, a 2-line drop in Snellen visual acuity compared with the 5.0 mm adaptive optics–corrected condition. The visual performance remained a much more stable through focus, and the mean visual acuity across the entire 3.00 D range of defocus values improved to 0.061 ± 0.041 logMAR, averaging 1 line of Snellen acuity better through the entire range of defocus compared with the 5.0 mm adaptive optics–corrected condition.
Figure 3.
Mean visual acuity scores with standard deviations across 3.0 D of defocus along with data points for 13 participants with adaptive optics–corrected 2.0 mm pupil.
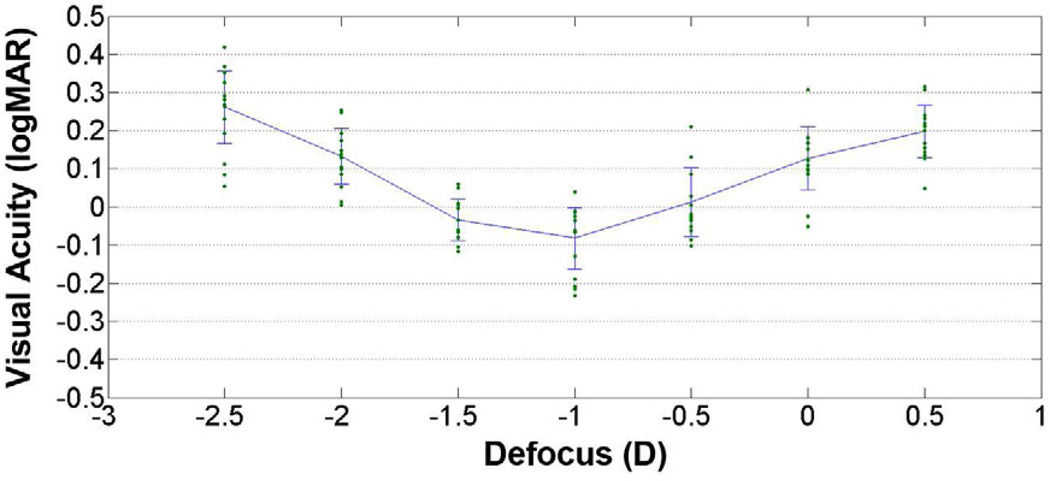
For the adaptive optics–corrected pupil with added negative spherical aberration Z(4,0) = −0.274, the plane for the best mean visual acuity shifted to −1.00 D of defocus (Figure 4). The best visual acuity was almost 1 line of Snellen acuity better than the 2.0 mm pupil adaptive optics–corrected condition but at −0.082 ± 0.080 logMAR, it was more than 1 line of Snellen visual acuity worse than without the induced spherical aberration. The mean visual acuity across the entire 3.00 D range of defocus values improved by a half line of Snellen visual acuity over the adaptive optics–corrected 5.0 mm condition but a half line of Snellen visual acuity worse than the 2.0 mm pupil condition at 0.103 ± 0.040 logMAR.
Figure 4.
Mean visual acuity scores with standard deviations across 3.0 D of defocus along with data points for 13 participants with adaptive optics–corrected 5.0 mm pupil with Z(4,0) spherical aberration equaling −0.274.
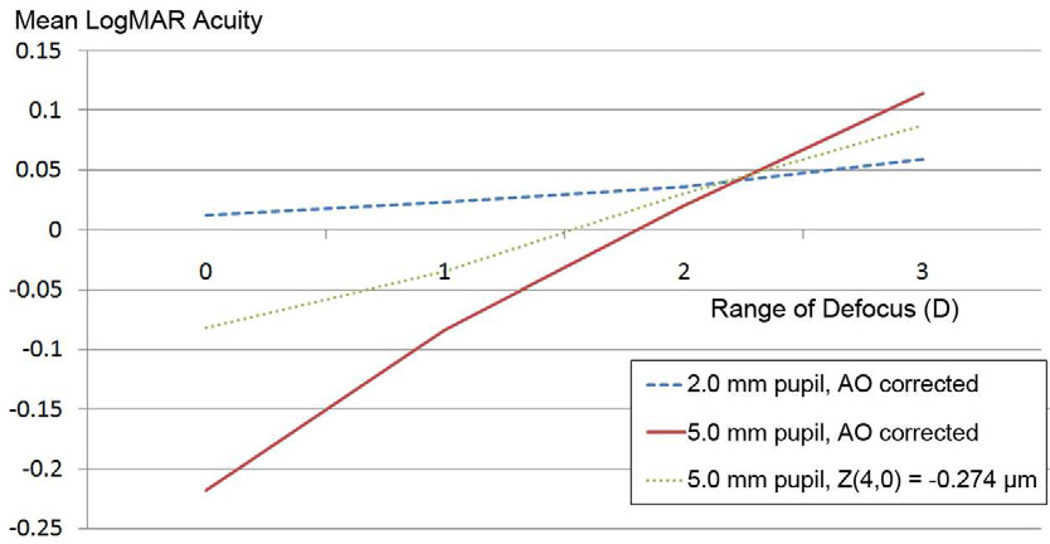
Although a 3.00 D range of defocus values was chosen to represent a satisfactory depth of focus for an individual wanting to see both distance and near, a smaller or larger range of defocus values could be selected arbitrarily as well. As Figure 5 shows, an adaptive optics–corrected 5.0 mm pupil (solid line) provided the best mean visual acuity for all ranges of defocus values until approximately 2.25 D range. At that point, a 2.0 mm pupil (dashed line) began to provide a better mean visual acuity value. At no point did the spherical aberration profile (dotted line) provide the best mean visual acuity for any range of defocus values.
Figure 5.
Comparison of mean visual acuity with 3 optical profiles and varying ranges of defocus (AO = adaptive optics).
The t tests showed that the difference in the best adaptive optics–corrected visual acuity scores were significantly different for all 3 groups at the plane of best focus. The adaptive optics–corrected 5.0 mm pupil (−0.218 logMAR) was better than the adaptive optics–corrected 5.0 mm pupil with Z(4,0) equaling −0.274 (−0.082 logMAR) (P = .0014) and better than the adaptive optics–corrected 2.0 mm pupil (0.012 logMAR) (P < .00001). The adaptive optics–corrected 5.0 mm pupil with Z(4,0) equaling −0.274 (−0.082 logMAR) was better than adaptive optics–corrected 2.0 mm pupil (0.012 logMAR) (P = .006).
The t tests also showed that the difference in mean visual acuity scores across 3.00 D of defocus were significantly different in all 3 groups. The adaptive optics–corrected 2.0 mm pupil (logMAR = 0.061) was better than the adaptive optics–corrected 5.0 mm pupil with Z(4,0) equaling −0.274 (0.103 logMAR) (P = .018) and better than the adaptive optics–corrected 5.0 mm pupil (0.156 logMAR) (P < .001). The adaptive optics–corrected 5.0 mm pupil with Z(4,0) equaling −0.274 (0.103 logMAR) was better than adaptive optics–corrected 5.0 mm pupil (0.156 logMAR) (P = .014).
DISCUSSION
We found that the 2.0 mm pupil profile provided a significantly better mean visual acuity than the 5.0 mm adaptive optics–corrected pupil profile or the 5.0 mm pupil profile with −0.274 µm spherical aberration. The 5.0 mm spherical aberration profile did not offer an improvement in mean visual acuity over the adaptive optics–corrected 5.0 mm pupil until the range of defocus was greater than 2.25 D. At no range of defocus was the mean logMAR acuity for spherical aberration profile better than the 2.0 mm adaptive optics–corrected pupil or the 5.0 mm adaptive optics–corrected pupil value.
Although objective image-quality metrics have shown accuracy in predicting subjective image quality, variability in individual subjects and the added variability of changing pupil sizes and resulting light levels can decrease their predictive validity. Because of this, subjective testing remains an important step in comparing the effects of spherical aberration and small pupil apertures on vision across a range of defocus values.29–31
When comparing different pupil sizes, it should be remembered that smaller pupils reduce the amount of light that enters the aperture and cause the stimulus to have reduced signal-to-noise compared with larger pupils. A similar study could be performed in which perceived stimulus brightness was kept constant by increasing the laser light source. In this study, the 840 nm light source was already at maximum brightness to provide maximum contrast in the 5.0 mm pupil conditions. It was, therefore, not possible to increase illumination in the 2.0 mm pupil condition to match the perceived brightness of the 5.0 mm pupil conditions. It is expected that increasing the illumination for the 2.0 mm pupil condition would increase visual performance for all defocus values by increasing contrast levels. Visual performance at the plane of best focus would still be expected to be worse than the adaptive optics–corrected 5.0 mm pupil because the 2.0 mm pupil condition is more limited by diffraction. Clinically, with good illumination and a high-contrast visual acuity chart, it is possible to achieve Snellen visual acuities of 20/20 or even 20/16 when viewing through a 2.0 mm artificial aperture. Thus, it would be expected that a 2.0 mm pupil could achieve improved logMAR acuity at the plane of best focus with increased luminance.
Surgical or optical corrections that rely on small pupils to provide improved depth of focus could cause a worsening of visual function in areas of low lighting because of the significant decrease in retinal illuminance. This would be particularly debilitating for individuals who have cataracts or other optical media opacities. Because the onset of presbyopia is an early precursor to cataract formation, a surgical or pharmaceutical treatment that induces small pupils would require a careful ocular examination to determine whether the patient would be a good candidate for such a procedure. Although the contrast sensitivity loss that occurs with age worsens with higher spatial frequencies, there is evidence that the gradual miosis that occurs with age has a measurable positive effect on contrast sensitivity.32
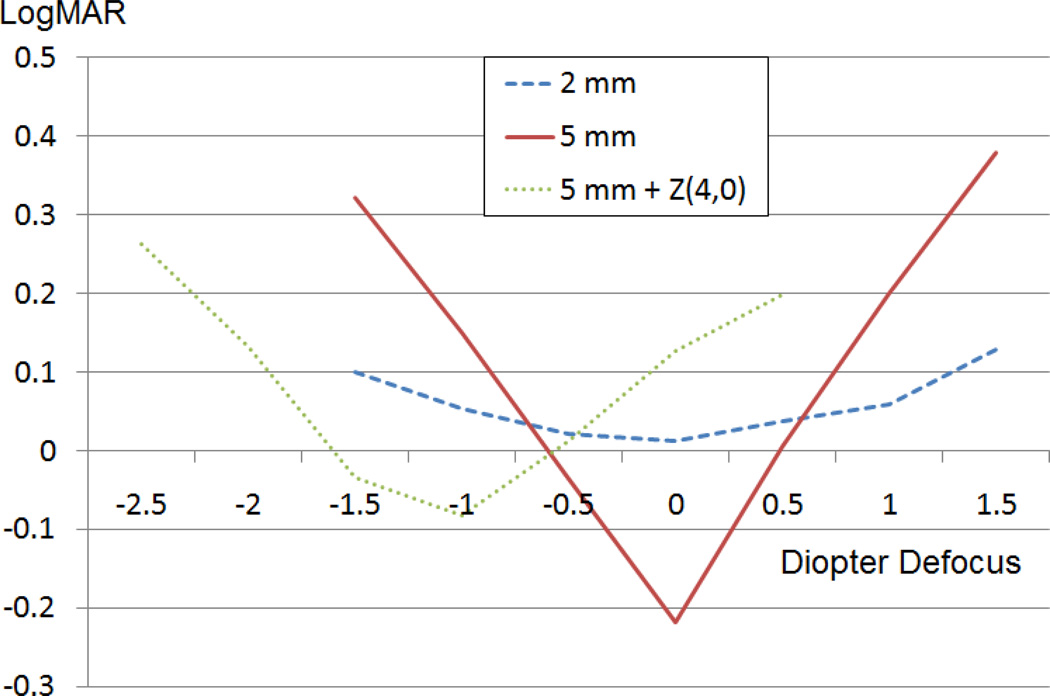
Although spherical aberration was shown to increase depth of focus, the amount of improvement may not be considered significant enough to justify the loss in CDVA (Figure 6). Measures of depth of focus (eg, width at 50% threshold) often rely on the peak value, which is the maximum visual acuity level in this case. Spherical aberration lowers the peak value and would therefore artificially expand the depth of focus by changing the point at which the width is measured. Spherical aberration also causes a shift in the plane of best focus, as seen in Figure 6. In this case, negative spherical aberration can be partially corrected with negative defocus.
Figure 6.
Comparison of mean visual acuities of 13 participants for all 3 tested conditions for 3.00 D range of defocus.
A small pupil (2.0 mm in this case) would be the easier solution to implement and would be more predictable and uniform across a target population. A small (2.0 mm) pupil will approach the diffraction limit and would virtually neutralize all inherent HOAs except in extreme circumstances, such as keratoconus or other conditions causing high levels of wavefront distortions. In contrast, HOAs have been extremely difficult to correct using wavefront-guided surgical corrections with laser vision correction procedures (eg, LASIK) or intraocular implants. Despite the advancements in wavefront-guided technologies over the past decades, wavefront surgical corrections have failed to show evidence of a predictable and uniform reduction in HOAs.33,34 It follows that the ability to generate a specific level of aberration in the eye would be equally difficult. Spherical aberration would be less effective in increasing depth of focus in the presence of other HOAs and therefore requires the capacity to minimize other existing aberrations to be effective. Current surgical wavefront corrections continue to measure similar or greater levels of postoperative aberrations.
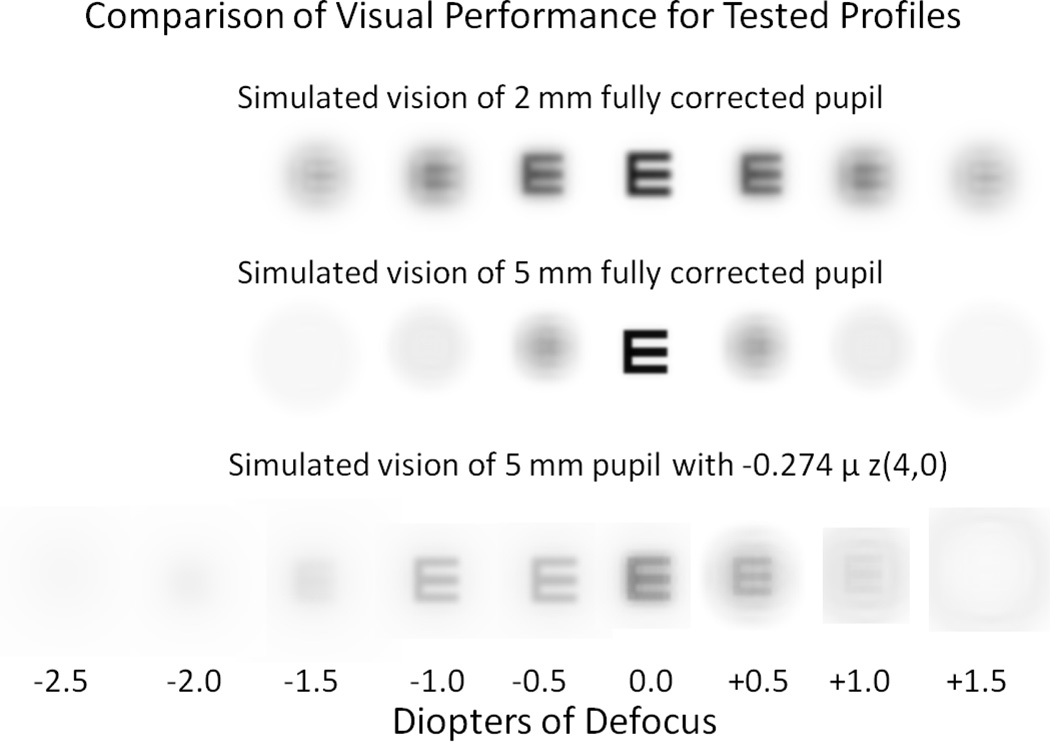
Even if spherical aberration could be successfully applied to the human visual system in isolation, the blur from this specific aberration is asymmetrical, varying with positive and negative defocus. By comparison, the blur for a small pupil is symmetrical, identical with positive and negative defocus and involves a simple reduction in contrast. Figure 7 shows the difference in legibility created by spherical aberration versus a small pupil for a 20/20 Snellen letter.
Figure 7.
Simulated vision through 3 tested profiles. 20/20 Snellen E letter (0 logMAR) was convolved with point spread function of each condition through focus.
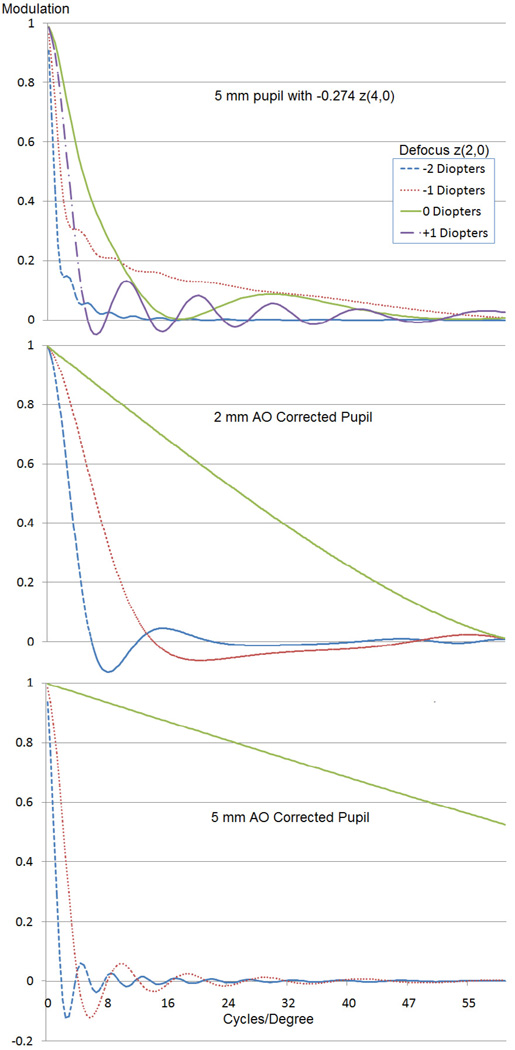
In our study, the majority of participants reported a subjective visual preference for the small pupil profile over the profile with induced spherical aberration, although the reduction in illumination for the 2.0 mm pupil profile was described as problematic. Some subjects reported a subjective visual preference for the brighter, more distorted stimulus produced by the spherical aberration profile over the dimmer, but more uniform stimulus of the 2.0 mm pupil. The uniformity of the stimulus across defocus levels and across spatial frequencies for the 2.0 mm pupil profile can be shown by examining the modulation transfer function (MTF). As shown in Figure 8, the MTF fluctuates much more significantly over the range of defocus values for the profile with spherical aberration than with the 2.0 mm pupil profile. As seen in the MTF graphs, the 2.0 mm profile shows very few contrast reversals across 1.00 D or 2.00 D of defocus. In contrast, the 5.0 mm profile with negative spherical aberration shows frequent contrast reversals across spatial frequencies in the presence of defocus, exhibiting a lack of uniformity in vision across the range of defocus values that may not be satisfactory.
Figure 8.
Simulated MTFs for 5.0 mm pupil, 5.0 mm pupil with negative spherical aberration (−0.274 µm), and 2.0 mm pupil (AO = adaptive optics). The x-axis represents spatial frequencies. The y-axis represents modulation for given spatial frequencies.
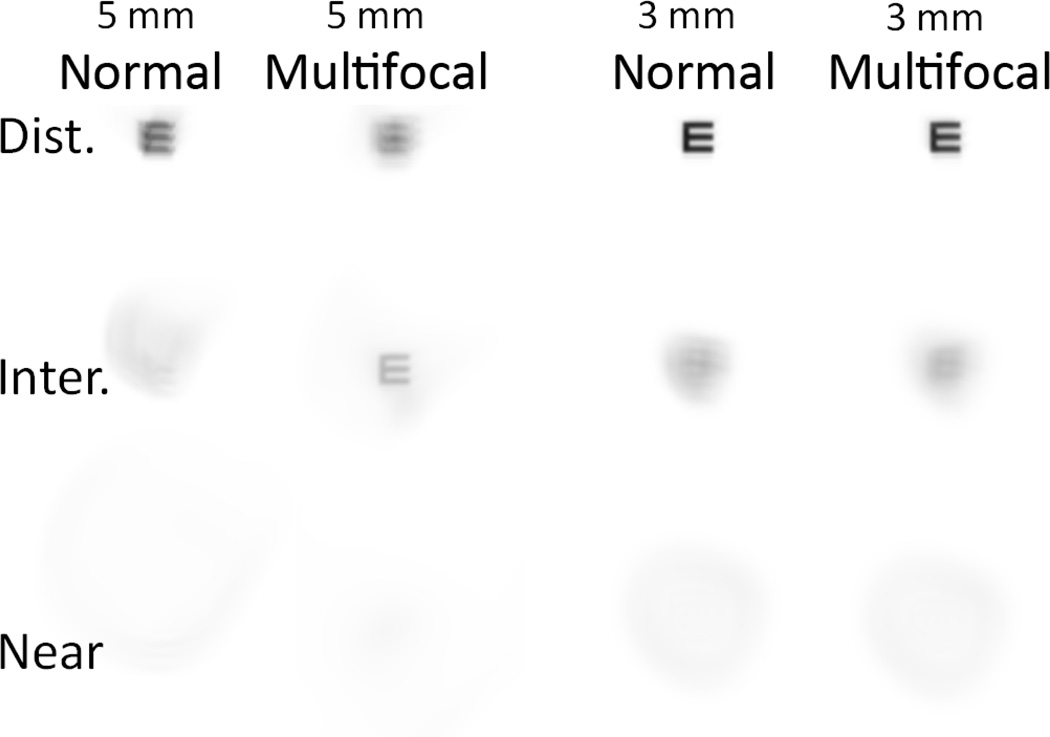
Although a 2.0 mm pupil provided inferior visual quality at the best focal plane when compared with an adaptive optics–corrected 5.0 mm pupil (a 2-line difference in Snellen acuity), this comparison would be unlikely to occur in a clinical setting because the average eye has significant aberrations that can reduce vision quality compared with the more ideal adaptive optics–corrected condition. As Figure 9 shows, for individuals with normal wavefront aberration profiles, there is an improvement in distance vision (0.00 D defocus) when the pupil decreases from 5.0 mm to 3.0 mm. Also, when negative spherical aberration is added to a normal 5.0 mm wavefront profile, there is an improvement at the intermediate range of vision (−1.00 D defocus). This improvement is negated, however, if the pupil size is reduced to 3.0 mm. This leads to 2 conclusions. First, individuals with large pupils and/or greater than normal levels of HOAs could realistically expect to observe a significant improvement in CDVA with a 2.0 mm pupil. Second, multifocal wavefront profiles would not add significant value to intermediate or near vision for patients with smaller pupils (<4.0 mm). Because pupil size decreases with age,35 this would hinder efforts to correct presbyopia with a multifocal solution, particularly since HOAs also tend increase with age.23 Multifocal solutions that use diffractive corrections would be less influenced by pupil size but would not be unaffected.
Figure 9.
Simulated vision for a normal wavefront profile and a normal profile with added spherical aberration (−0.274 µm for 5.0 mm pupil). 20/20 Snellen E letter (0 logMAR) was convolved with point-spread function with 0.00, −1.00 D, and −2.50 D of defocus (Dist. = distance; Inter. = intermediate).
In conclusion, both spherical aberration and small-pupil profiles were shown to be valid methods of increasing depth of focus, although both also resulted in decreased visual acuity at the plane of best focus compared with an adaptive optics–corrected 5.0 mm profile. For presbyopia corrections that rely on an improved depth of focus to improve near vision, one would have to consider whether the loss in corrected visual acuity is an acceptable tradeoff for the improvement in near vision that is produced with the expanded depth of focus. In this study, no tested profile achieved a mean visual acuity of 0.0 logMAR (20/20 equivalent Snellen Acuity) across an entire 3.00 D range of defocus.
The 5.0 mm pupil profile with spherical aberration produced a better peak visual acuity but smaller depth of focus than the 2.0 mm pupil profile. The 2.0 mm profile decreased illumination levels, resulting in decreased contrast, but produced an image that was more uniform across spatial frequencies and less variable through focus, as demonstrated by the MTF. Due to an inability to consistently and accurately change, correct, and/or induce desired levels of HOAs in refractive surgery, we conclude that small-pupil profiles are a better choice than spherical aberration profiles for surgical presbyopic correction. A correction using a small-pupil profile would be more predictable and uniform in its vision results and would therefore provide greater patient satisfaction if expectations were managed appropriately.
In this study, we considered a small pupil and the induction of spherical aberration as separate treatment modalities. A third option would be to combine the benefits of a smaller pupil on depth of focus with added negative spherical aberration. Due to technical issues, we were not able to induce a desired level of spherical aberration and measure its accuracy on a 2.0 mm pupil using a deformable mirror because the level of light was not adequate for accurate reading by the wavefront aberrometer. This could be a consideration for a future study.
WHAT WAS KNOWN
Spherical aberration and small pupils have been shown to increase the depth of focus.
WHAT THIS PAPER ADDS
Small pupils increased depth of focus more than spherical aberration.
Results indicate that small pupil apertures would be more predictable and more effective in presbyopia correction than the application of spherical aberration.
Acknowledgments
Supported by National Institutes of Health (grant K12 EY017269), Bethesda, Maryland, USA (Dr. Hickenbotham).
Biography

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: No author has a financial or proprietary interest in any material or method mentioned.
REFERENCES
- 1.Roorda A. Adaptive optics for studying visual function: a comprehensive review. [Accessed August 3, 2012];J Vis. 2011 11(5):6, 1–21. doi: 10.1167/11.5.6. Available at: http://www.journalofvision.org/content/11/5/6.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legras R, Benard Y, Rouger H. Through focus visual performance measurements and predictions with multifocal contact lenses. Vision Res. 2010;50:1185–1193. doi: 10.1016/j.visres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Roorda A. Predicting and assessing visual performance with multizone bifocal contact lenses. [Accessed August 3, 2012];Optom Vis Sci. 2003 80:812–819. doi: 10.1097/00006324-200312000-00011. Available at: Available at: http://vision.berkeley.edu/roordalab/Pubs/MartinRoordaOVS.pdf. [DOI] [PubMed] [Google Scholar]
- 4.Amigo A, Bonaque S, López-Gil N, Thibos L. Simulated effect of corneal asphericity increase (Q-factor) as a refractive therapy for presbyopia. J Refract Surg. 2012;28:413–418. doi: 10.3928/1081597X-20120518-04. [DOI] [PubMed] [Google Scholar]
- 5.Young T. The Bakerian Lecture. On the mechanism of the eye. [Accessed August 3, 2012];Phil Trans R Soc Lond. 1801 91:23–88. http://www.jstor.org/stable/pdfplus/107085.pdf?acceptTC=true. [Google Scholar]
- 6.Koomen M, Tousey R, Scolnik R. The spherical aberration of the human eye. J Opt Soc Am. 1949;39:370–376. doi: 10.1364/josa.39.000370. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins TCA. Aberrations of the eye and their effects on vision: I. Br J Physiol Opt. 1963;20:59–91. [PubMed] [Google Scholar]
- 8.Ivanoff A. About the spherical aberration of the eye. J Opt Soc Am. 1956;46:901–903. doi: 10.1364/josa.46.0901_1. [DOI] [PubMed] [Google Scholar]
- 9.Roorda A, Glasser A. Wave aberrations of the isolated crystalline lens. [Accessed August 3, 2012];J Vis. 2004 4:250–261. doi: 10.1167/4.4.1. Available at: http://www.journalofvision.org/content/4/4/1.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter J, Guirao A, Cox IG, Williams DR. Monochromatic aberrations of the human eye in a large population. J Opt Soc Am A Opt Image Sci Vis. 2001;18:1793–1803. doi: 10.1364/josaa.18.001793. [DOI] [PubMed] [Google Scholar]
- 11.Artal P, Berrio E, Guirao A, Piers P. Contribution of the cornea and internal surfaces to the change of ocular aberrations with age. J Opt Soc Am A Opt Image Sci Vis. 2002;19:137–143. doi: 10.1364/josaa.19.000137. [DOI] [PubMed] [Google Scholar]
- 12.Artal P, Benito A, Tabernero J. The human eye is an example of robust optical design. [Accessed August 3, 2012];J Vis. 2006 6:1–7. doi: 10.1167/6.1.1. Available at: http://www.journalofvision.org/content/6/1/1.full.pdf. [DOI] [PubMed] [Google Scholar]
- 13.Rocha KM, Vabre L, Chateau N, Krueger RR. Expanding depth of focus by modifying higher-order aberrations induced by an adaptive optics visual simulator. J Cataract Refract Surg. 2009;35:1885–1892. doi: 10.1016/j.jcrs.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 14.Guo H, Atchison DA, Birt BJ. Changes in through-focus spatial visual performance with adaptive optics correction of monochromatic aberrations. Vision Res. 2008;48:1804–1811. doi: 10.1016/j.visres.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Xiong Y, Li J, Wang N, Dai Y, Xue L, Zhao H, Jiang W, Zhang Y, He JC. Effects of monochromatic aberration on visual acuity using adaptive optics. [Accessed August 3, 2012];Optom Vis Sci. 2009 86:868–874. doi: 10.1097/OPX.0b013e3181adfdff. Available at: http://journals.lww.com/optvissci/Abstract/2009/07000/Effects_of_Monochromatic_Aber ration_on_Visual.11.aspx. [DOI] [PubMed] [Google Scholar]
- 16.Cantú R, Rosales MA, Tepichín E, Curioca A, Montes V, Ramirez-Zavaleta JG. Objective quality of vision in presbyopic and non-presbyopic patients after pseudoaccommodative advanced surface ablation. J Refract Surg. 2005;21:S603–S605. doi: 10.3928/1081-597X-20050902-08. [DOI] [PubMed] [Google Scholar]
- 17.Artola A, Patel S, Schimchak P, Ayala MJ, Ruiz-Moreno JM, Alió JL. Evidence for delayed presbyopia after photorefractive keratectomy for myopia. Ophthalmology. 2006;113:735–741. doi: 10.1016/j.ophtha.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 18.Beiko GHH. Personalized correction of spherical aberration in cataract surgery. J Cataract Refract Surg. 2007;33:1455–1460. doi: 10.1016/j.jcrs.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Werner JS, Elliott SL, Choi SS, Doble N. Spherical aberration yielding optimum visual performance: evaluation of intraocular lenses using adaptive optics simulation. J Cataract Refract Surg. 2009;35:1229–1233. doi: 10.1016/j.jcrs.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piers PA, Fernandez EJ, Manzanera S, Norrby S, Artal P. Adaptive optics simulation of intraocular lenses with modified spherical aberration. [Accessed August 3, 2012];Invest Ophthalmol Vis Sci. 2004 45:4601–4610. doi: 10.1167/iovs.04-0234. Available at: http://www.iovs.org/cgi/reprint/45/12/4601. [DOI] [PubMed] [Google Scholar]
- 21.Piers PA, Manzanera S, Prieto PM, Gorceix N, Artal P. Use of adaptive optics to determine the optimal ocular spherical aberration. J Cataract Refract Surg. 2007;33:1721–1726. doi: 10.1016/j.jcrs.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Birren JE, Casperson RC, Botwinick J. Age changes in pupil size. J Gerontol. 1950;5:216–221. doi: 10.1093/geronj/5.3.216. [DOI] [PubMed] [Google Scholar]
- 23.Applegate RA, Donnelly WJ, III, Marsack JD, Koenig DE, Pesudovs K. Three-dimensional relationship between high-order root-mean-square wavefront error, pupil diameter, and aging. [Accessed August 3, 2012];J Opt Soc Am A Opt Image Sci Vis. 2007 24:578–587. doi: 10.1364/josaa.24.000578. Available at: Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2083284/pdf/nihms29165.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dexl AK, Seyeddain O, Riha W, Hohensinn M, Rückl T, Hitzl W, Grabner G. Reading performance after implantation of a small-aperture corneal inlay for the surgical correction of presbyopia: two-year follow-up. J Cataract Refract Surg. 2011;37:525–531. doi: 10.1016/j.jcrs.2010.10.044. [DOI] [PubMed] [Google Scholar]
- 25.Seyeddain O, Riha W, Hohensinn M, Nix G, Dexl AK, Grabner G. Refractive surgical correction of presbyopia with the AcuFocus small aperture corneal inlay: two-year follow-up. [Accessed August 3, 2012];J Refract Surg. 2010 26:707–715. doi: 10.3928/1081597X-20100408-01. Available at: http://kamrainlay.com/sites/kamrainlay.com/files/JRS-Orang-Seyeddain-April-2010.pdf. [DOI] [PubMed] [Google Scholar]
- 26.Roorda A, Romero-Borja F, Donnelly WJ, III, Queener H, Hebert TJ, Campbell MCW. Adaptive optics scanning laser ophthalmoscopy. [Accessed August 3, 2012];Opt Express. 2002 10:405–412. doi: 10.1364/oe.10.000405. Available at: http://secure.breault.com/resources/kbasePDF/wp_osa_011_adaptive_optics.pdf. [DOI] [PubMed] [Google Scholar]
- 27.Rossi EA, Weiser P, Tarrant J, Roorda A. Visual performance in emmetropia and low myopia after correction of high-order aberrations. [Accessed August 3, 2012];J Vis. 2007 7(8):14, 1–14. doi: 10.1167/7.8.14. Available at: http://www.journalofvision.org/content/7/8/14.full.pdf. [DOI] [PubMed] [Google Scholar]
- 28.Liang J, Grimm B, Goelz S, Bille JF. Objective measurement of wave aberrations of the human eye with use of a Hartmann-Shack wave-front sensor. J Opt Soc Am A Opt Image Sci Vis. 1994;11:1949–1957. doi: 10.1364/josaa.11.001949. [DOI] [PubMed] [Google Scholar]
- 29.Cheng X, Bradley A, Thibos LN. Predicting subjective judgment of best focus with objective image quality metrics. [Accessed August 3, 2012];J Vis. 2004 4:310–321. doi: 10.1167/4.4.7. Available at: http://www.journalofvision.org/content/4/4/7.full.pdf. [DOI] [PubMed] [Google Scholar]
- 30.Watson AB, Ahumada AJ., Jr Predicting visual acuity from wavefront aberrations. [Accessed August 3, 2012];J Vis. 2008 8(4):17, 1–19. doi: 10.1167/8.4.17. Available at: http://www.journalofvision.org/content/8/4/17.full.pdf. [DOI] [PubMed] [Google Scholar]
- 31.Marsack JD, Thibos LN, Applegate RA. Metrics of optical quality derived from wave aberrations predict visual performance. [Accessed August 3, 2012];J Vis. 2004 4:322–328. doi: 10.1167/4.4.8. Available at: http://www.journalofvision.org/content/4/4/8.full.pdf. [DOI] [PubMed] [Google Scholar]
- 32.Sloane ME, Owsley C, Alvarez SL. Aging, senile miosis and spatial contrast sensitivity at low luminance. Vision Res. 1988;28:1235–1246. doi: 10.1016/0042-6989(88)90039-9. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Food and Drug Administration. Summary of Safety and Effectiveness Data for a Supplemental Premarket Approval Application (VISX STAR S4 IR™ Excimer Laser System with variable spot Scanning (VSS™) and WaveScan WaveFront® System) [Accessed August 3, 2012];2007 :46. Available at: http://www.accessdata.fda.gov/cdrh_docs/pdf/P930016S025b.pdf.
- 34.U.S. Food and Drug Administration. Summary of Safety and Effectiveness Data for a Supplemental Premarket Approval Application (for the wavefront-optimized correction of myopia with the ALLEGRETTO WAVE) [Accessed August 3, 2012];2006 :35. Available at: http://www.accessdata.fda.gov/cdrh_docs/pdf2/P020050S004b.pdf.
- 35.Winn B, Whitaker D, Elliott DB, Phillips NJ. Factors affecting light-adapted pupil size in normal human subjects. [Accessed August 3, 2012];Invest Ophthalmol Vis Sci. 1994 35:132–1137. Available at: http://www.iovs.org/cgi/reprint/35/3/1132.pdf. [PubMed] [Google Scholar]