Abstract
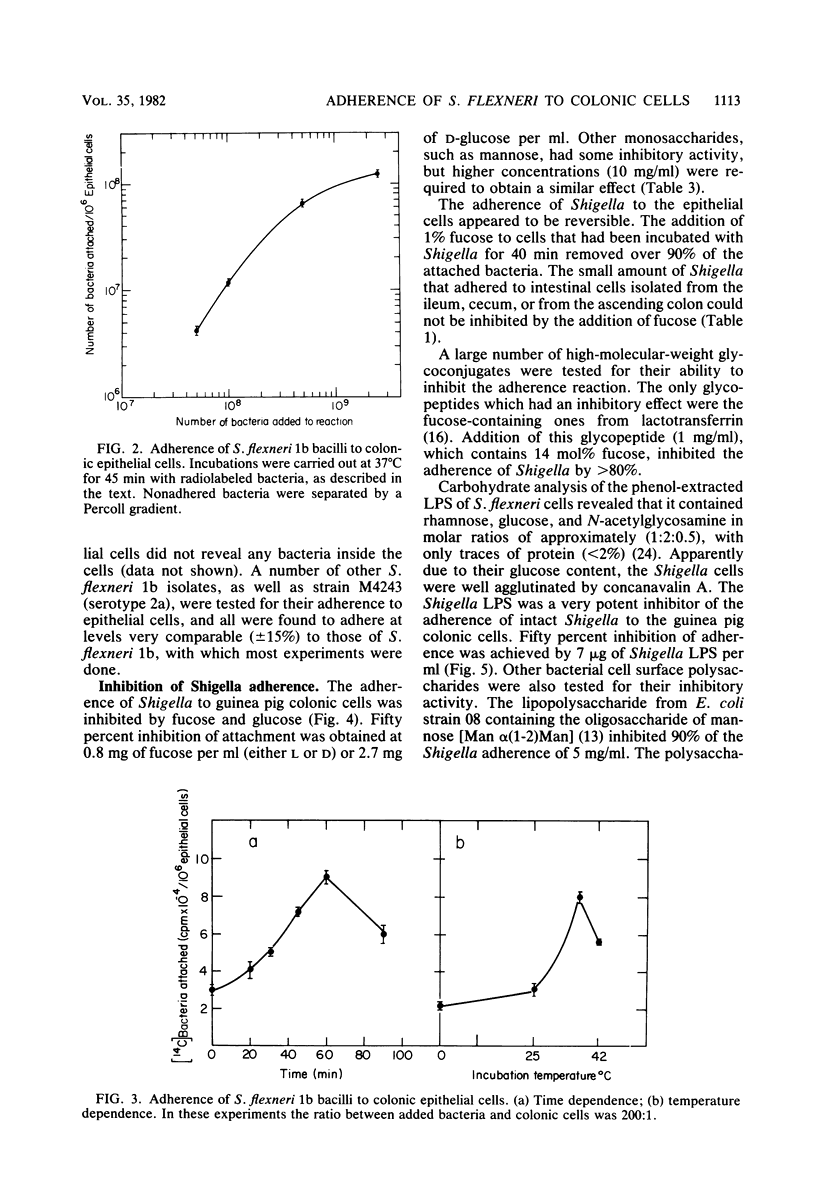
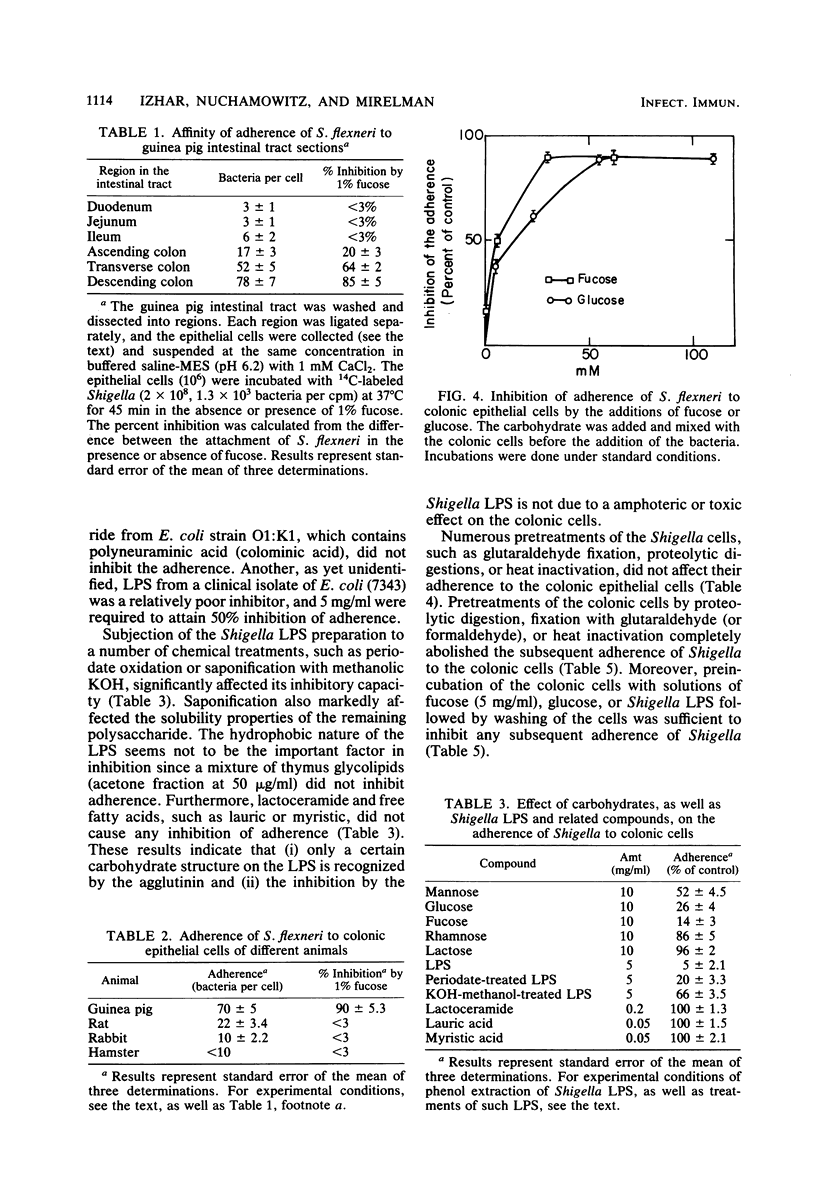
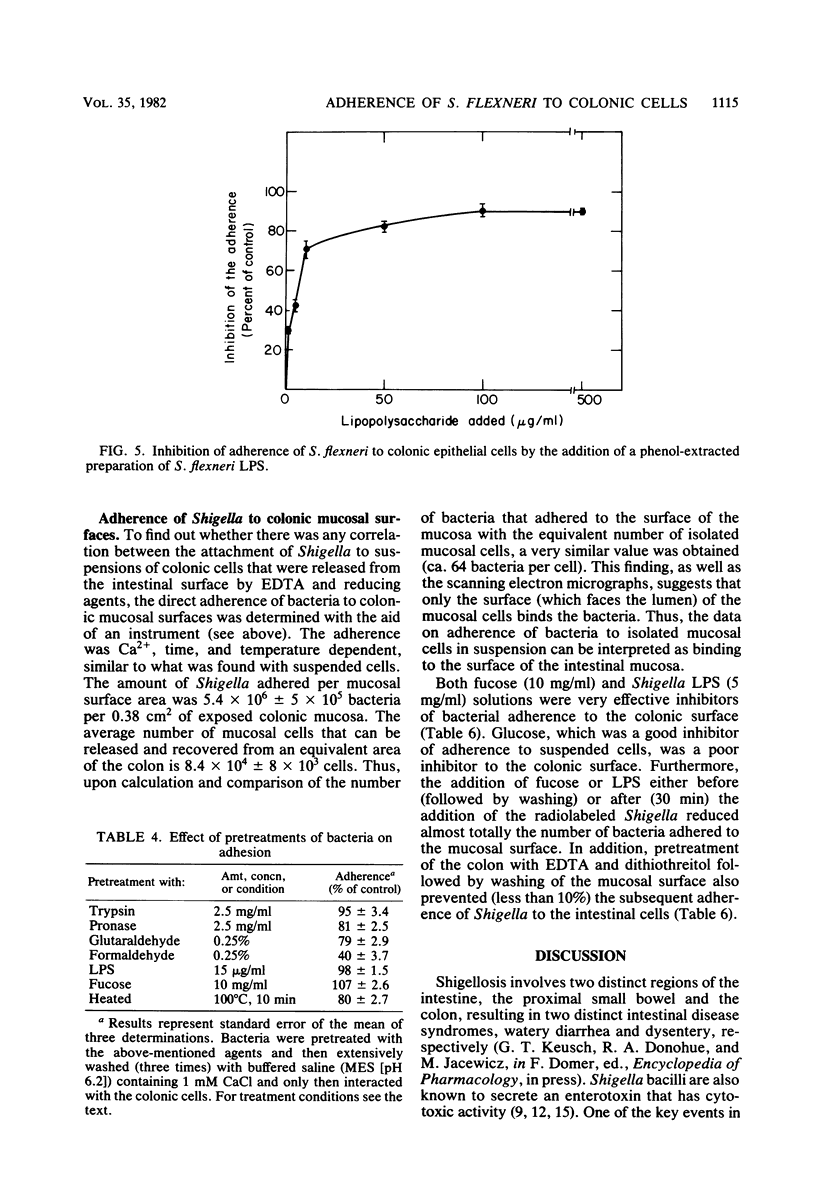
Guinea pig colonic epithelial cells released by treating sections of the colon with solutions containing EDTA, dithiothreitol, and citrate avidly adhered Shigella flexneri bacteria. Separation of the intestinal cells from nonbound bacteria was achieved by differential sedimentation on a Percoll gradient. Adherence of S. flexneri to the colonic cells was Ca2+ (1 mM) and time dependent. The pH optimum was pH 6.2, and almost no attachment (less than 5%) was observed at low temperature (4 degrees C). The average number of bacteria which bound to colonic cells was 70 bacteria per cell, whereas attachment to cells isolated from the ileum region was 6 bacteria per cell. Colonic cells obtained from the intestine of rabbits or rats did not adhere Shigella. Adherence to guinea pig colonic cells was inhibited (50%) by several carbohydrates, such as 0.1% fucose or 0.5% glucose, as well as by a lipopolysaccharide preparation (10 micrograms /ml) isolated from S. flexneri. Fixation of the bacteria with glutaraldehyde or preincubation of the bacteria with lectins or proteolytic enzymes did not affect their adherence. Proteolytic digestions or fixation of the epithelial cells, as well as pretreatments with lipopolysaccharide or fucose solutions, abolished their ability to adhere bacteria. These results indicate that a carbohydrate-binding substance on the surface of guinea pig colonic epithelial cells is responsible for the attachment of the Shigella bacilli.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. C., Zweig S. E., Barondes S. H. Two lactose binding lectins from chicken tissues. Purified lectin from intestine is different from those in liver and muscle. J Biol Chem. 1980 May 10;255(9):4236–4239. [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Bhatti T., Chambers R. E. The determination of carbohydrate in biological materials by gas-liquid chromatography. Methods Biochem Anal. 1971;19:229–344. doi: 10.1002/9780470110386.ch3. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Ofek I., Yashouv-Gan Y., Sharon N., Mirelman D. Isolation of a mannose-specific lectin from Escherichia coli and its role in the adherence of the bacteria to epithelial cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1551–1559. doi: 10.1016/0006-291x(78)91179-8. [DOI] [PubMed] [Google Scholar]
- FORMAL S. B., DAMMIN G. J., LABREC E. H., SCHNEIDER H. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J Bacteriol. 1958 May;75(5):604–610. doi: 10.1128/jb.75.5.604-610.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Jr, Takeuchi A., Washington O., Formal S. B. Shigellosis due to Shigella dysenteriae. 1. Relative importance of mucosal invasion versus toxin production in pathogenesis. J Infect Dis. 1972 Nov;126(5):523–530. doi: 10.1093/infdis/126.5.523. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M. Pathogenesis of Shigella diarrhea. VII. Evidence for a cell membrane toxin receptor involving beta1 leads to 4-linked N-acetyl-D-glucosamine oligomers. J Exp Med. 1977 Aug 1;146(2):535–546. doi: 10.1084/jem.146.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., DuPont H. L., Formal S. B., Hornick R. B., Takeuchi A., Gangarosa E. J., Snyder M. J., Libonati J. P. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis. 1973 Mar;127(3):261–270. doi: 10.1093/infdis/127.3.261. [DOI] [PubMed] [Google Scholar]
- Mazurier J., Spik G., Montreuil J. Isolation and characterization of the cyanogen bromide fragments from human lactotransferrin. FEBS Lett. 1974 Nov 15;48(2):262–265. doi: 10.1016/0014-5793(74)80482-5. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Altmann G., Eshdat Y. Screening of bacterial isolates for mannose-specific lectin activity by agglutination of yeasts. J Clin Microbiol. 1980 Apr;11(4):328–331. doi: 10.1128/jcm.11.4.328-331.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J. B. Shigellosis in nonhuman primates: a review. Lab Anim Sci. 1971 Oct;21(5):734–738. [PubMed] [Google Scholar]
- SERENY B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4(4):367–376. [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Eshdat Y., Silverblatt F. J., Ofek I. Bacterial adherence to cell surface sugars. Ciba Found Symp. 1981;80:119–141. doi: 10.1002/9780470720639.ch9. [DOI] [PubMed] [Google Scholar]
- Shepherd V. L., Lee Y. C., Schlesinger P. H., Stahl P. D. L-Fucose-terminated glycoconjugates are recognized by pinocytosis receptors on macrophages. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1019–1022. doi: 10.1073/pnas.78.2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. A. The immunochemistry of Shigella flexneri O-antigens. The structure and biosynthesis of the O-specific side chains of some representative serotypes. Eur J Biochem. 1969 Dec;11(3):554–575. doi: 10.1111/j.1432-1033.1969.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]



