To the Editor:
Recombination Activating Genes (RAG) 1 and 2 encode proteins necessary for T and B cell antigen receptor rearrangement. Complete deficiency of either RAG1 or RAG2 results in classical severe combined immunodeficiency (SCID) lacking T and B cells, since RAG1 mediates DNA binding and cleavage, while RAG2 is an essential cofactor for RAG1 function.1 Hypomorphic missense mutations that preserve residual RAG activity and allow the development of oligoclonal T cells, but virtually no B cells, result in recurrent infections, erythroderma, hepatomegaly, colitis, and αβ T cell expansion (Omenn syndrome).2 RAG1/2 mutations can also cause γδ T cell expansion and immunodeficiency with granulomas.3
Hyper-IgM syndrome is characterized by normal or increased IgM with decreased IgG and IgA levels. It results from defects in class switch recombination caused by mutations in CD40 ligand, CD40, Activation-Induced Deaminase, Uracil-DNA glycosylase, and Nuclear Factor Kappa B Essential Modifier (NEMO).4 In addition to defective humoral immunity, T cell function is affected in CD40L, CD40, and NEMO deficiency and is associated with opportunistic infections.4
We report a novel homozygous mutation in RAG2 resulting in two different phenotypes: Omenn syndrome and hyper-IgM syndrome.
Patient 1 was born to first-degree consanguineous parents and presented at 4 months of age with lymphopenia (absolute lymphocyte count of 869 cells/μl), erythroderma, Pseudomonas aeruginosa pneumonia, and Coombs’ positive hemolytic anemia. She had recurrent pulmonary infections, and developed onchomycosis by 1 year of age. Lymphocyte subset analysis at 16 and 19 months of age revealed lymphopenia with severely decreased T and B cells and normal NK cell numbers (Table I). The majority of her T cells were CR45RO+ activated cells (not shown). HLA typing revealed no evidence of maternal cell engraftment. Lymphocyte proliferation to phytohemagglutinin (PHA) and anti-CD3 mAb was severely decreased (Table I). She had low serum IgG and undetectable serum IgA, while serum IgM was significantly decreased by 19 months of age (Table I). Because her phenotype was consistent with Omenn syndrome, RAG1 and RAG2 were sequenced. A previously unreported homozygous missense mutation in RAG2 (c.1375A>C) was identified, causing a methionine to leucine change at position 459 (M459L) in the protein’s plant homeodomain (PHD). Patient 1 subsequently underwent a successful matched sibling bone marrow transplant.
TABLE I.
Immune profiles
| Pt. 1 | Pt. 2 | |||
|---|---|---|---|---|
|
| ||||
| 16 months | 19 months | 16 months | 5 years 5 months | |
| Lymphocyte counts (cells/μl)1 | ||||
| CD3+ | 149 (1900-6200) | 205(1900-6200) | 691(1900-6200) | 777 (1400-6200) |
| CD3+CD4+ | 85 (1300-3400) | 149 (1300-3400) | 311 (1300-3400) | 443 (700-2200) |
| CD3+CD8+ | 61 (620-2000) | 42 (620-2000) | 173 (620-2000) | 293 (490-1300) |
| CD4+/CD8+ ratio | 1.4 (1.3-3.9) | 3.5 (1.3-3.9) | 1.7 (1.3-3.9) | 1.5 (0.9-3.7) |
| CD19+ | 4 (610-2600) | 1 (610-2600) | 173 (610-2600) | 15 (390-1400) |
| CD16+/CD56+ | 279 (160-1100) | 208 (160-1100) | 657 (160-1100) | 1111 (130-720) |
| Immunoglobulins (mg/dl)2 | ||||
| IgG | 193 (400-1300) | 147 (400-1300) | <152 (400-1300) | 213 (600-1500) |
| IgA | Undetectable (20-230) |
Undetectable (20-230) |
Undetectable (20-230) |
1 (50-150) |
| IgM | 54.9 (30-120) | <17.3 (30-120) | 171 (30-120) | 1048 (22-100) |
| Proliferation (c.p.m.) | ||||
| PHA | ND | 16700 (116187) | ND | 1980 (57182) |
| Anti-CD3 mAb | ND | 14310 (107265) | ND | 12558 (82957) |
The values in parentheses represent the normal range for age of lymphocyte counts, immunoglobulin concentrations, and 3H-thymidine incorporation into DNA measured as radioactive counts per minute (c.p.m.). For lymphocyte proliferation studies, a normal healthy control was studied the same day as the patient. Pt, patient; ND, not determined.
Comans-Bitter WM, de Groot R, van der Beemd R, Neijens HJ, Hop WC, Groeneveld K, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr 1997; 130:388-393.
Jolliff CR, Cost KM, Stivrins PC, Grossman PP, Nolte CR, Franco SM, et al. Reference intervals for serum IgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. Clin Chem 1982; 28:126-128.
Patient 2 was born to first-degree consanguineous parents. He presented at 4 months of age with recurrent skin abscesses. He subsequently developed recurrent pneumonias, Pseudomonas aeruginosa sepsis with disseminated intravascular coagulopathy, colitis, CMV viremia, oral candidiasis, hepatosplenomegaly, autoimmune hemolytic anemia, and antiphospholipid antibodies. Lymphocyte subset analysis at 16 months of age revealed lymphopenia with low T and B cells and normal NK cell numbers (Table I). Serum IgG was very low, serum IgA undetectable, and serum IgM normal. By 5 years 5 months of age, his IgM increased to 1048 mg/dl (Table I), prompting a diagnosis of hyper-IgM syndrome. The patient was referred to us for further investigation. His T cells upregulated CD40L normally following activation with phorbol 12-myristate 13-acetate and ionomycin (not shown). However, he had low numbers of T cells and virtually no B cells (Table I). The majority of his T cells were CR45RO+ activated cells (not shown). T cell proliferation to PHA and anti-CD3 mAb was severely decreased (Table I). These results suggested that Patient 2 had a combined immunodeficiency rather than hyper-IgM syndrome.
A detailed family history revealed that the great-grandparents of Patients 1 and 2 were cousins. This raised the possibility that they might share the same RAG2 mutation. Patient 2 was indeed homozygous for the RAG2 mutation found in Patient 1. The healthy sister of Patient 1 has a normal RAG2 sequence, while the parents of both patients and two healthy brothers of Patient 2 are heterozygous for the RAG2 mutation.
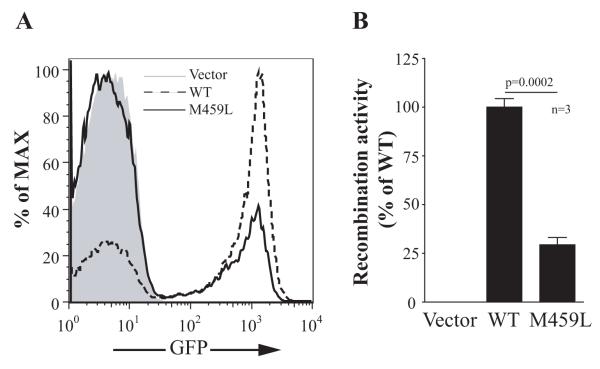
The M459 residue is highly conserved and located in the zinc-binding loop region of the RAG2 PHD domain. Mutations in this domain impair RAG2 protein stability, translocation to the nucleus, interaction with histones, and recombination capacity, resulting in SCID or Omenn syndrome.5 The recombination activity of the M459L RAG2 mutant was analyzed using Abelson-transformed Rag2−/− tg.bcl2 pro-B cells containing an intrachromosomal inverted GFP cassette flanked by recombination signal sequences. Cells were transduced with retroviral vectors encoding either wild-type or mutant RAG2 and human CD2 as a reporter, then treated with the Abl inhibitor STI-571 to promote cell differentiation and induce RAG activity. The expressed RAG2, in combination with endogenous RAG1, causes inversion of the GFP cassette and the resultant GFP expression correlates with the level of recombination activity.6,7 The recombination activity of the RAG2(M459L) mutant was 29.7±3.3% of WT RAG2 (Fig 1).
FIG 1.
Recombination activity of RAG2(M459L) mutant. Abelson-transformed Rag2−/− tg.bcl2 pro-B cells containing an inverted GFP cassette were transduced with retroviral vectors encoding wild-type or M459L mutant human RAG2, and human CD2 as a reporter. An empty vector encoding CD2 was used as a negative control and resulted in no GFP expression. A, GFP expression on CD2+ cells indicate the recombinase activity level. Shown are representative histograms from three independent experiments. B, Bar graph represents the average recombination activity from three independent experiments (n=3). The recombination activity is calculated as percent activity of wild-type RAG2.
We describe two related patients with a novel mutation in RAG2 causing different clinical phenotypes. Patient 1 had Omenn syndrome with erythroderma, low T and B cells, and opportunistic infections. Patient 2 lacked erythroderma and had persistently high IgM levels, leading to a presumptive diagnosis of hyper-IgM syndrome. Of note, the phenotype of Patient 2 also evolved over time; the progressive loss of B cells and lack of T cell proliferation to PHA and anti-CD3 seen at five years of age are consistent with a mutation in RAG2. Phenotypic heterogeneity due to the same RAG2 mutation has been attributed to differences in genetic background, epigenetic factors, and environmental exposures.8,9 The low level recombinase activity in vitro, and the presence of residual autologous T and B lymphocytes in the patients, indicate that the M459L RAG2 variant may retain some V(D)J recombination activity in vivo. Combined with different antigen exposure, this may contribute to our patients’ different phenotypes. Thirty percent recombination activity was also found in a patient with RAG2 mutations resulting in hypogammaglobulinemia, lymphopenia, and recurrent infections, indicating that this level of reduced recombination activity results in clinically significant disease.3
To our knowledge, hyper-IgM has not been previously reported with mutations in RAG2. It is therefore important to consider RAG mutations in patients with elevated levels of IgM and progressive lymphopenia.
ACKNOWLEDGMENTS
We thank Dr. Barry P. Sleckman for providing the Abelson-transformed Rag2−/− tg.bcl2 pro-B cells.
Supported by USPHS grants 1P01AI076210-01A1 (RSG, LDN), T32AI007512 (RSG, JC), and a grant from the Dubai Harvard Foundation for Medical Research (RSG), and by a grant provided by the Jeffrey Modell Foundation (to LDN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: R.S. Geha and L.D. Notarangelo have received research support from the National Institutes of Health. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Schatz DG, Ji Y. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol. 2011;11:251–63. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- 2.Niehues T, Perez-Becker R, Schuetz C. More than just SCID--the phenotypic range of combined immunodeficiencies associated with mutations in the recombinase activating genes (RAG) 1 and 2. Clin Immunol. 2010;135:183–92. doi: 10.1016/j.clim.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Schuetz C, Huck K, Gudowius S, Megahed M, Feyen O, Hubner B, et al. An immunodeficiency disease with RAG mutations and granulomas. N Engl J Med. 2008;358:2030–8. doi: 10.1056/NEJMoa073966. [DOI] [PubMed] [Google Scholar]
- 4.Vale AM, Schroeder HW., Jr Clinical consequences of defects in B-cell development. J Allergy Clin Immunol. 2010;125:778–87. doi: 10.1016/j.jaci.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couedel C, Roman C, Jones A, Vezzoni P, Villa A, Cortes P. Analysis of mutations from SCID and Omenn syndrome patients reveals the central role of the Rag2 PHD domain in regulating V(D)J recombination. J Clin Invest. 2010;120:1337–44. doi: 10.1172/JCI41305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Ravin SS, Cowen EW, Zarember KA, Whiting-Theobald NL, Kuhns DB, Sandler NG, et al. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood. 2010;116:1263–71. doi: 10.1182/blood-2010-02-267583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gapud EJ, Lee BS, Mahowald GK, Bassing CH, Sleckman BP. Repair of chromosomal RAG-mediated DNA breaks by mutant RAG proteins lacking phosphatidylinositol 3-like kinase consensus phosphorylation sites. J Immunol. 2011;187:1826–34. doi: 10.4049/jimmunol.1101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrella V, Poliani PL, Casati A, Rucci F, Frascoli L, Gougeon ML, et al. A hypomorphic R229Q Rag2 mouse mutant recapitulates human Omenn syndrome. J Clin Invest. 2007;117:1260–9. doi: 10.1172/JCI30928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalal I, Tabori U, Bielorai B, Golan H, Rosenthal E, Amariglio N, et al. Evolution of a T-B-SCID into an Omenn syndrome phenotype following parainfluenza 3 virus infection. Clin Immunol. 2005;115:70–3. doi: 10.1016/j.clim.2004.08.016. [DOI] [PubMed] [Google Scholar]