Abstract
Background
Tissue factor (TF), a primary initiator of blood coagulation, also plays a pivotal role in angiogenesis. TF expression in the airways is associated with asthma, a disease characterized in part by subepithelial angiogenesis.
Objectives
To determine potential sources of TF and the mechanisms of its availability in the lung microenvironment.
Methods
Normal Human Bronchial Epithelial (NHBE) cells grown in air-liquid interface (ALI) culture were subjected to a compressive stress of 30 cmH2O; this is comparable to that generated in the airway epithelium during bronchoconstriction in asthma. Conditioned media and cells were harvested to measure TF mRNA and TF protein. We also tested bronchoalveolar lavage fluid (BALF) and airway biopsies from asthmatics and healthy controls for TF.
Results
TF mRNA was upregulated 2.2-fold after 3 hours of stress compared to unstressed cells. Intracellular and secreted TF proteins were enhanced 1.6-fold and over 50-fold, respectively, compared to that of control cells after onset of compression. The amount TF in BALF from patients with asthma was found at mean concentrations that were 5 times greater than that of healthy controls. Immunohistochemical staining of endobronchial biopsies identified epithelial localization of TF with increased expression in asthma.
Exosomes isolated from the conditioned media of NHBECs and BALF of asthmatic subjects by ultracentrifugation contained TF.
Conclusions
Our in vitro and in vivo studies show that mechanically-stressed bronchial epithelial cells are a source of secreted TF and that exosomes are potentially a key carrier of the TF signal.
Keywords: Asthma, tissue factor, exosomes, bronchoconstriction, mechanotransduction, bronchial epithelium
INTRODUCTION
During asthmatic bronchoconstriction, airways experience altered physical forces that result in the airway epithelial cells experiencing compressive mechanical stress (1). We have previously shown that compressive stress applied to normal human bronchial epithelial cells (NHBECs) grown in air-liquid interface (ALI) culture, recapitulates many key features of airway remodeling (2). This includes collagen production (3), goblet cell hyperplasia (4), and release of inflammatory markers and mediators (5, 6). Herein we expand these studies to show that compressive mechanical stress on NHBECs in ALI culture results in the release of tissue factor (TF) containing exosomes; we also show that TF-bearing exosomes can be isolated from human asthmatic bronchoalveolar lavage fluid (BALF) and that epithelial cells express TF in vivo with enhanced immunohistochemical staining in asthma.
Although TF is best known as an initiator of the blood coagulation cascade upon its binding to factor VIIa followed by PAR-2 activation, the TF–factor VIIa complex promotes angiogenesis (7-9). Therefore, given its role in promotion of angiogenesis (10) and the observation that there are increased levels of TF in asthmatic compared to normal sputum (11), it is important to understand whether airway epithelial cells are capable of producing TF, and if so the mechanism and form of its secretion.
METHODS
Culture of Bronchial Epithelial Cells
Primary normal human bronchial epithelial (NHBE) cells were expanded and maintained in a humidified environment containing 95% air, 5% CO2 as described previously (6). Passage-2 NHBE cells were plated at a density of 2×104 cells/cm2 on 12-well Transwell plates with polyester membranes, 0.4μm pores (Corning, Inc., Corning, NY) coated with 50ng/ml of type 1 rat tail collagen (BD Biosciences, San Jose, CA). Primary cells from three different non-smoker donors were used.
Exposure of NHBE cells to Compressive Mechanical Stress
To expose cells to compressive stress, non-toxic tapered silicon plugs were pressed into the top of each Transwell with an access port for pressure application. This creates a sealed pressure chamber over the apical surface of the NHBE cells (1, 5). Each plugged well was connected to a 5% CO2 (balance room air) pressure cylinder via a humidified chamber maintained at 37°C with gas pressure regulated to the pressure of interest. For most experiments, the pressure in the apical chamber was increased to 30 cm H2O above atmospheric for the indicated duration, while the basal surface and medium remained at atmospheric pressure. The compressive stress (30 cm H2O) is comparable to that generated in the airway epithelium during bronchoconstriction and orders of magnitude higher than the stress experienced by the airway epithelium during normal breathing (1, 12). Application of transcellular compressive stress on the epithelial cells has been routinely used in our laboratory (1, 2) and others (13) and the physiologic relevance of this system in intact humans has been documented recently (14).
NHBE cells were exposed to compressive stress (30 cm H2O) for 3hrs and cells and conditioned media were harvested immediately or at the indicated time point post-pressure application. Control cells were treated identically to compressive stress samples, including placement of the wells in the experimental apparatus, but were not exposed to the pressure gradient.
Pharmacological Manipulation
To determine whether compressive stress induced TF transcription through the synthesis of new RNA, cells were pretreated with 5μg/ml of actinomycin D for 30 minutes prior to compressive stress. To determine whether compressive stress induced intracellular TF protein in a manner dependent on new protein synthesis, cells were pre-treated with cycloheximide (10μg/ml). The effect of actinomycin D or cycloheximide on compressive stress provoked TF secretion was determined as well. To investigate the role of PKC in TF expression or secretion, bisindoylmaleimide 1(Bis 1)(IC50 = 8-20 nM), was pre-incubated in the basal media for 30 minutes prior to application of compressive stress. We have previously (6) shown that 1μM Bis 1 completely inhibited PKC activity as indicated by phosphorylation of the PKC substrate, myristoylated alanine-rich C kinase substrate (MARCKS).
All inhibitors were initially dissolved in DMSO as stock solutions and dissolved to final concentrations in minimal media at the concentration described in each Figure. DMSO was used for a vehicle control for all inhibitors.
Western Blot Analysis
Western Blot analysis of proteins was performed using 20~50μg of protein lysates per lane of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (6).
Intracellular proteins were collected from the cells in Transwells at the indicated period of time following compressive stress. E-Cadherin was used for a loading control for Western blot analysis of intracellular TF protein. To detect secreted TF into the conditioned media (CM) in response to compressive stress, CM was collected from the basal compartment of the ALI cultures and concentrated by trichloroacetic acid (TCA) precipitation and examined by Western blot analysis (6).
Enzyme-linked Immunosorbent Assay (ELISA)
The amount of TF protein in the conditioned media was quantified by ELISA using a commercially available Quantikine® Kit (R&D Systems) following the manufacturer’s instructions.
Real time PCR Analysis
RNA was isolated from cell lysates with RNeasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. The forward and reverse primers for TF (Gene bank ID: NM_001993.4) and GAPDH (6) endogenous control were generated by Primer Express 3.0 software (Applied Biosystems) (Table I). TF primers were generated in the region spanning Exon 3. The primer sets used were validated against GAPDH using various amount of cDNA to ensure similar levels of PCR efficiency. Fold changes compared to the control were calculated by the comparative delta-delta Ct method (15).
Table I.
Primer sets for Real-time PCR analysis
| Gene | Primer sequence | |
|---|---|---|
| TF(F3) | Forward | 5’- ACAGAGTGTGACCTCACCGACGA -3’ |
| Reverse | 5’- CCTGCAGGGTAGGAGAAGACCC -3’ | |
| GAPDH | Forward | 5’-TGGGCTACACTGAGCACCAG-3’ |
| Reverse | 5’-GGGTGTCGCTGTTGAAGTCA-3’ | |
Isolation and Identification of Exosomes
Exosomes were isolated by differential ultracentrifugation and confirmed by transmission electron microscopy (TEM). We performed ultracentrifugation as described (16). CM from the basal compartment of the ALI culture was centrifuged sequentially at 2000g and 10,000g for 10 minutes and 30 minutes, respectively to remove cell debris. The saved supernatant was further ultracentrifuged at 100,000g for 70 minutes and the supernatant removed. PBS was added to the pellet followed by a second round of ultracentrifugation at 100,000g for 70 minutes; the pellet was used as the exosome fraction.
Negative Staining Procedure for TEM
The pellet from ultracentrifugation was suspended in 50μl of PBS and vortexed briefly and then 5μl was adsorbed for 1 minute to a carbon coated grid that had been made hydrophilic by a 30 second exposure to a glow discharge. Excess liquid was removed with filterpaper (Whatman #1) and the samples were stained with 0.75% uranyl formate for 30 seconds. After removing the excess uranyl formate the grids were examined in a JEOL 1200EX Transmission electron microscope or a TecnaiG2 Spirit BioTWIN and images were recorded with an AMT 2k CCD camera.
Bronchoscopic Airway Sampling and Processing
Fiberoptic bronchoscopy was undertaken under local anesthesia to obtain bronchoalveolar lavage fluid (BALF) and endobronchial biopsies (EBB) as summarized in Table II. The bronchoscopic procedure, BALF harvest and processing, and EBB airway sampling were as previously described (14). All subjects gave written informed consent and the study had full ethics and research participation at the University of Southampton.
Table II.
FEV1 of participants from whom bronchoalveolar lavage fluid (BALF) or airway biopsies were obtained.
| Type of Sample | Healthy volunteers | Asthmatics |
|---|---|---|
|
| ||
| BAL fluids | n=5 | n=5 |
| Mean age : 31.6 yrs [range 20-55 yrs] | Mean age : 32.0 yrs [range 20-37 yrs] | |
| FEV1% predicted : 102.0 ± 5.5 [±sem] | FEV1% predicted : 97.1 ± 7.3 [±sem] | |
|
| ||
| No medications | Albuterol prn only | |
|
| ||
| Endobronchial biopsy | n=5 | n=10 |
| Mean age : 24.4yrs [range 18-35 yrs] | Mean age : 37.9 yrs [range 18-56 yrs] | |
| FEV1% predicted : 104.8 ± 3.5 [±sem] | FEV1% predicted : 83.7 ± 3.9 [±sem] | |
|
| ||
| No medications | Inhaled steroids (at least 2000 ug beclometasone or equivalent), inhaled long acting beta agonists and at least one other asthma therapy (oral LTRA, theophylline, or oral steroids) | |
All human research was conducted under permission granted by Southampton and South West Hampshire Research Ethics Committees.
BALF Processing
On removal from the subject, BALF was filtered using a 100μm nylon filter (BD Falcon cell strainer, Marathon Lab. Supplies. London, UK) and then centrifuged at 1300G for 10 minutes at 4°C. The supernatant was removed and aliquoted prior to storage at -80°C for later analysis.
EBB Processing and Immunohistochemical Staining
On removal from the subject EBBs were processed into glycolmethacrylate (GMA) resin as previously described (17) and stored at -20 °C until 2μm tissue sections were cut for immunohistochemical staining for TF expression. Immunostaining was undertaken as described (14, 17) using a murine monoclonal antibody against human tissue factor (American Diagnostica inc, Stamford, USA) with parallel staining undertaken in the absence of primary antibody and in the presence of an isotype control to verify the specificity of the TF staining. Immunohistochemical staining of TF within the epithelium was quantified in well orientated areas of intact epithelium by computer assisted image analysis (Zeiss KS400 image analysis system, Zeiss, Welyn Garden City, UK) and expressed as percentage epithelial staining.
Statistical Analysis
Data are presented as mean ± SEM. Results were evaluated by Student’s t-tests with Bonferroni posttest correction for multiple comparisons. A P value of < 0.05 was considered significant.
RESULTS
Compressive mechanical stress induces TF mRNA expression and intracellular TF protein in well differentiated NHBE cells
Well differentiated NHBE cells grown in ALI conditions were exposed to 30 cm H2O transcellular compressive stress for, 3 hours. As shown in Figure 1A, this duration of compressive stress resulted in an increase in TF mRNA expression by 2.2-fold compared to time matched controls. By 8hrs the magnitude of the induction had fallen to 1.8-fold; both differences were significant compared to their time matched controls. Intracellular TF protein (Fig 1B) was detected constitutively and its expression was increased by 1.6- and 1.3-fold, at 3 or 24hrs, respectively, following onset of compressive stress when compared to time matched controls.
Figure 1. Compressive stress induces tissue factor (TF) production from human bronchial epithelial cells.
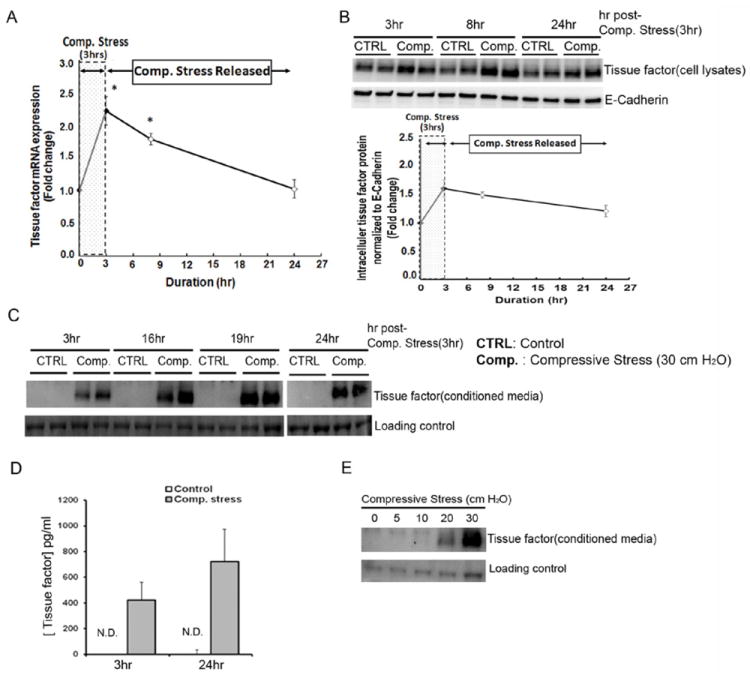
A-C, E. TF mRNA expression (A), intracellular TF (B), and secreted TF (C, E) in response to compressive stress (mean ± SD, n=3). Loading controls: E-Cadherin (B), Coomassie staining of SDS-PAGE (C and E). D. TF measured by ELISA in the CM (mean ± SD, n=3).
Compressive stress induces secretion of TF protein from NHBE cells
We next examined TF protein secretion into the basal CM from ALI cultures of NHBE cells in the presence or absence of compressive stress. Western blot analysis of CM collected 3, 16, 19, or 24hrs after the onset of 3 hours of compressive stress for TF is shown in Figure 1C. Secretion of TF protein was undetectable under baseline culture conditions; it was detected as early as 3hrs after onset of compressive stress and sustained for 24hrs. As basal amounts of TF were undetectable by Western Blot, we measured the concentration of TF in CM by ELISA. CM were collected from control and compressed cells at 3hr or 24hrs after onset of compressive stress. As illustrated in Figure 1D, the concentration of TF was 420pg/ml and 722pg/ml in the CM at 3hrs and 24hrs after onset of compressive stress, respectively, while it was below the detection limit (7.8pg/ml) in the conditioned media from control wells at any time point. We found the amount of TF secretion at 3hrs was about 58% (420pg/ml) of that observed at 24hrs in the compressed CM; this difference was consistent with that we observed by western blot analysis. The recovery of significant amounts of TF in CM at the completion of 3hrs compressive stress suggested that there could be early TF secretion from an intracellular store followed by the release of newly synthesized protein.
There was a graded amount of TF released into the CM following application of graded application of compressive stress from 5 to 30 cm H2O. Secretion of TF protein was not detected by Western Blot analysis after application of 5 or 10 cm H2O pressure as shown in Figure 1E. There was slight induction of TF by 20 cm H2O pressure, with extensive induction generated by 30 cm H2O pressure.
Initial TF secretion is not dependent on new mRNA or protein synthesis
To test whether new transcription was involved in the TF expression response to compressive stress, we incubated cells in the presence or absence of the transcription inhibitor, actinomycin D (5μg/ml), for 30 minutes prior to exposure to the compressive stress. This pretreatment completely attenuated the compressive stress induced TF mRNA expression (Fig 2A) indicating that compressive stress leads to new RNA synthesis. Next, to test whether induction of TF is the result of newly synthesized protein production, cells were pre-incubated with cycloheximide (10μg/ml) for 30 minutes prior to exposure to the compressive stress. As illustrated in Figure 2B, intracellular TF protein induction in response to compressive stress was largely attenuated in the presence of cycloheximide (10μg/ml). Taken together these data suggest that compressive stress is followed by both new TF protein synthesis and new RNA synthesis.
Figure 2. Secretion of TF elicited by compressive stress is independent of TF transcription or protein synthesis.
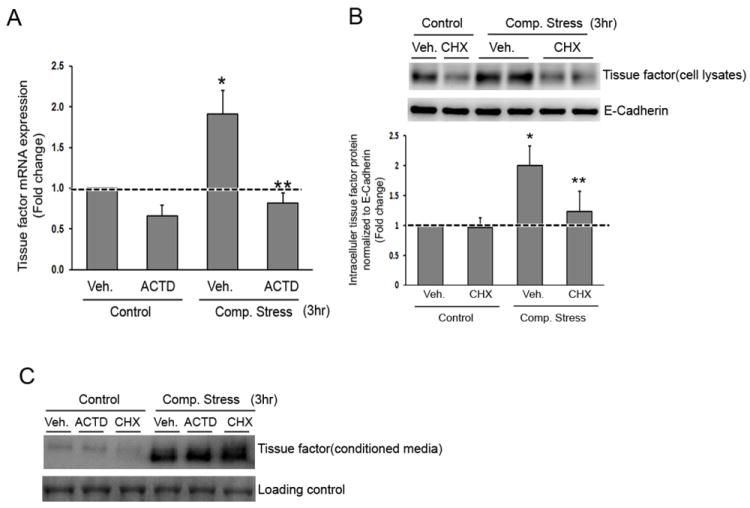
A-C. TF mRNA expression (A), intracellular TF protein (B), or TF secretion (C) was determined in the absence or presence of inhibitors (ACTD: actinomycin D, CHX: cycloheximide D). Loading controls: E-Cadherin in B, Coomassie staining of SDS-PAGE in C.
Surprisingly, as illustrated in Figure 2C, pretreatment with actinomycin D or cycloheximide did not affect TF secretion in response to compressive stress at 3hrs, while transcription or intracellular TF protein was attenuated as we described above (Fig 1A, B), further supporting the concept of TF secretion from a preformed intracellular store.
Compressive stress induces TF bearing exosomes in differentiated NHBE cells
TF is a transmembrane protein and is known to be secreted in various membrane vesicles (18-20). Therefore, we isolated exosomes by differential ultracentrifugation from CM collected from NHBE cells following compressive stress. As illustrated in Figure 3A, we identified membrane vesicles from ~50nm to 200nm in diameter, with most of them smaller than 100nm in diameter; the vesicles had the characteristic cup-shape of exosomes (21). As shown in Figure 3B, TF was detected in the whole cell lysates (WCL) as well as in the exosomal fraction (pellet) from ultracentrifugation. We further detected the exosomal marker proteins, GAPDH (22), actin (22, 23), annexin V (22, 24), and EGFR (22, 25, 26). Lamin A/C, which is expressed in the nuclear envelope and E-cadherin and beta 5 integrin, which are expressed in the plasma membrane, were not found in the exosome fraction. We detected trace amount of PCNA in the exosomes as found in colon cancer cells (27). Annexin V was also detected in the supernatant of ultracentrifugation suggesting that it is contained in the non-exosomal fraction as well.
Figure 3. Human bronchial epithelial cells release exosomes in response to compressive stress.
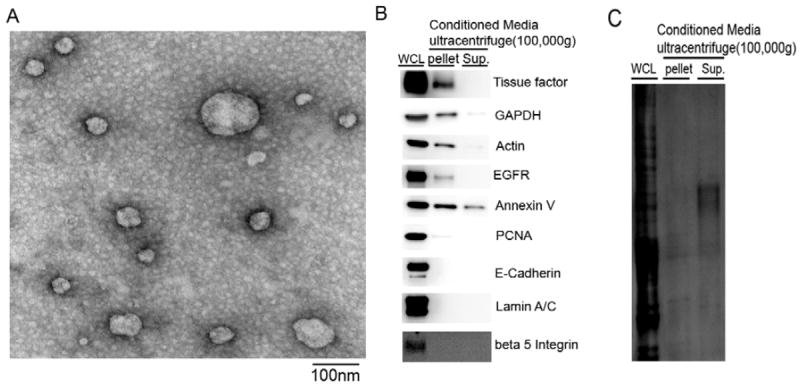
A. Detection of exosomes from compressed NHBECs by transmission electron microscopy. B. Western blot analysis of TF, exosome markers (GAPDH, actin, annexin V, and EGFR), and non-exosomal proteins (Lamin A/C, E-cadherin, and beta 5 integrin). C. Coomassie staining of SDS-PAGE gel corresponding to the blots shown in B.
TF secretion in response to compressive stress is mediated partially via PKC pathway
One of the known mechanisms triggering membrane vesicle secretion is an increase in intracellular calcium concentration potentially followed by PKC activation (26). To determine if compressive stress activates PKC and thus controls secretion of TF contained in the exosomes, we inhibited PKC activity using Bis 1. First to determine the efficacy of Bis 1, NHBE cells were pretreated with increasing concentrations of Bis 1 (0, 0.1, 1, and 10μM) for 30 minutes prior to application of compressive stress for 3hrs. Conditioned media were collected at the end of application of compressive stress. As illustrated in Figure 4A, pretreatment with Bis1 attenuated compressive stress induced TF secretion in a concentration-dependent manner; the effect plateaued at a concentration of 1μM. Inhibition of PKC did not attenuate compressive stress induced TF mRNA expression as shown in Figure 4B but substantially attenuated secretion of TF in response to compressive stress (72% reduced from maximum) shown in Figure 4C.
Figure 4. Inhibition of PKC activity attenuates compressive stress induced TF secretion.
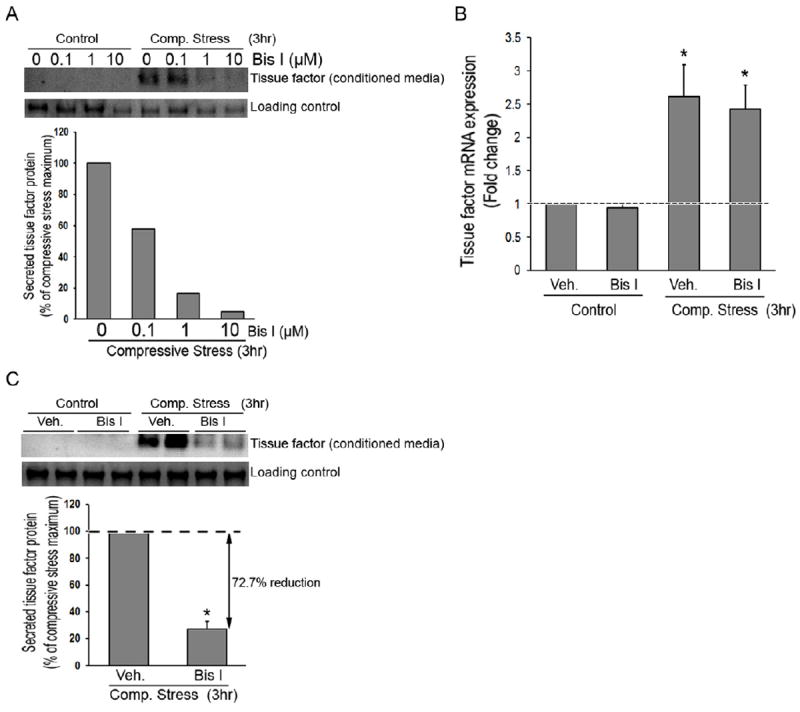
A. TF secretion in the presence of increasing concentration of Bis1. B-C. TF mRNA expression (* P < 0.05 vs. control) (means ± SEM, n=4) and TF secretion (* P < 0.01 vs. control) (means ± SEM, n=3) in the presence of absence of Bis1 (1μM).
TF in human BAL fluid is correlated with asthma and detected in exosomes
To test if there is a differential availability of TF in patients with asthma as compared with healthy volunteers, we tested BALF for TF. As shown in Figure 5A, we detected small amounts of TF in the BALF from healthy volunteers but in BALF from patients with asthma the mean concentration was 5 times greater. We confirmed that human BALF contained exosomes by ultracentrifugation and TEM as described above. As shown in Figure 5B, the size of the exosomes in human BALF is more homogenous than that of exosomes from NHBE cells ; in exosomes from human BALF the most commonly occurring vesicle has an approximate diameter of 50nm. We confirmed the presence of the exosome markers, GAPDH, actin, EGFR, and annexin V (Fig. 5C) in vesicles isolated from asthmatic BALF.
Figure 5. TF is elevated in human bronchoalveolar lavage fluid (BALF) from patients with asthma, and is detected in exosomes.
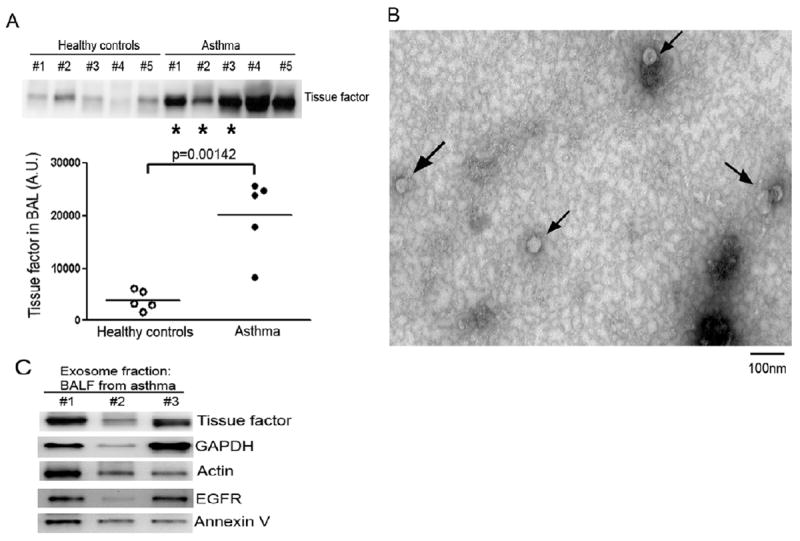
A. TF in human BAL (mean ± SD, n=5/condition). B. Human BALF-derived exosomes detected by transmission electron microscopy. C. TF and exosome markers in exosomes isolated from BALF of asthmatics (using the three asterisked samples in panel A).
TF in EBB is expressed in epithelial cells
Immunohistochemical staining for TF was present in all samples tested and localized to the airway epithelium (Fig 6). The percentage of the epithelium that had positive immunostaining for TF (Fig 6) was significantly greater (p=0.037) in asthma (median 13.6% [range 2.0-24.0%]) than in the healthy controls (6.0% [range 3.8-12.4%]).
Figure 6. TF localized in the airway epithelium is associated with the diagnosis of asthma.
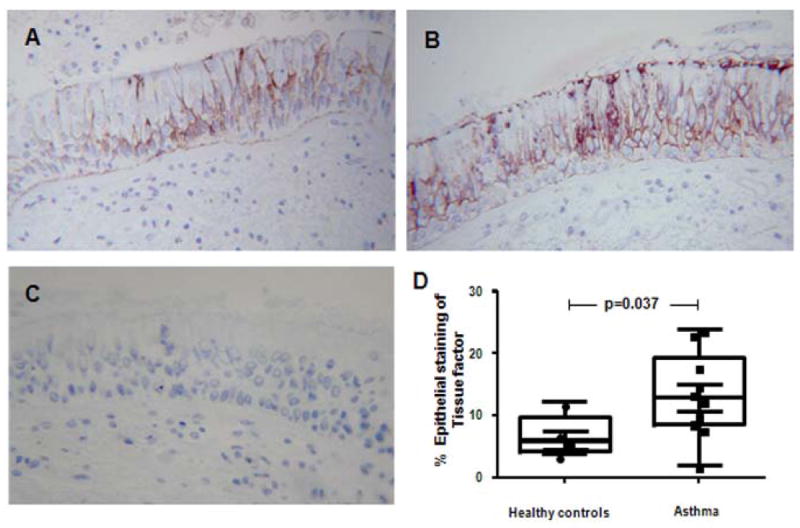
A-B. Immunohistochemical staining of TF in sections of endobronchial biopsies from a healthy control (A) and an asthmatic volunteer (B). C. Immunohistocehmical staining using an isotype control antibody. D. The percentage of epithelial cells stained for TF in healthy controls (n=5) and asthmatics subjects (n=10).
DISCUSSION
Our experiments show that NHBE cells constitutively express TF protein expression in cell lysates, but there is no detectable secretion into its culture medium under basal ALI culture conditions. Compressive mechanical stress (30 cm H2O), comparable in magnitude to that associated with asthmatic bronchoconstriction, led to modestly enhanced TF transcription and intracellular protein production, and dramatically increased TF secretion. New RNA synthesis was required for the upregulated TF transcription in response to compressive stress. While intracellular TF production induced by compressive stress is dependent on new protein synthesis, secretion of TF during the first three hours of stress application is independent of either new RNA synthesis or intracellular protein synthesis. We isolated exosomes in the conditioned media collected from compressed bronchial epithelial cells and detected TF in the membrane vesicle fraction along with a panel of exosomal marker proteins (18-20, 23-26). We found that release of TF in response to compressive stress was attenuated by inhibition of PKC activity suggesting that TF-bearing exosomes are released in a PKC-dependent manner. Our data were extended to samples from humans by our demonstration that the TF concentration in human BALF from asthmatics is significantly higher than that from controls. The TF was largely contained in exosomes; these findings are consistent with the hypothesis that bronchoconstriction could serve as a source of TF-bearing exosomes. In concordance with these findings, we have also demonstrated that TF is immunolocalised in airway biopsies to the epithelium and that its immunoreactivity at this site is significantly increased in asthma in comparison to that in healthy controls. Our combined in vivo and in vitro data establish unequivocally that mechanically perturbed airway epithelial cells are a potential source of TF in humans.
There are few data to provide information about the role of TF in pulmonary diseases. Immunohistochemical analysis has shown abundant TF protein expression in basal cells of normal bronchi (28). It is also known that exposure to urokinase plasminogen activator (uPA) induces TF mRNA and protein expression in Beas2B cells and in mouse lungs (29). The expression of TF in the lung has been implicated in the mechanism of fibrin deposition in an experimental model of asthma (30). Moreover, others have shown that the expression of TF in type 2 alveolar epithelial cells may be related to the pathogenesis of idiopathic pulmonary fibrosis (31). There is a higher TF concentration in the sputum from patients with asthma when compared to healthy controls (11, 32), but its origin is not known. In our in vitro experiments TF was found in the fluid bathing the baso-lateral surface of airway epithelial cells, but we recovered TF in BALF from asthmatics, which presumably reflects the composition of fluid on the airway epithelial surface. It is likely that TF translocation took place from the base of airway epithelial cells to the apical surface through the known “leaky” asthmatic airway epithelium(33). However, other mechanisms, such as production by type 2 alveolar epithelial cells and macrophages cannot be ruled out.
While TF is best known for its role as an initiator of blood coagulation, it is also a well-established proangiogenic factor (8, 9, 34, 35). It is known that increased subepithelial angiogenesis is associated with reduced lung function in patients with asthma (36-38). Our data provide strong evidence that mechanical stress enhances the availability of this angiogenic factor. We speculate that such angiogenesis could be a link between mechanical stress and the persistent airway obstruction observed in patients with chronic persistent asthma.
We have shown that primary bronchial epithelial cells secrete TF-bearing exosomes upon compressive stress. Since TF is a transmembrane protein and in our assays we used a polyclonal TF antibody, which detects epitopes distributed along the entire protein, the amount of TF measured was insensitive to the orientation of TF within exosomes. TF has been found in membrane vesicles fraction isolated from platelets, monocytes, or macrophages (39-41). TF-bearing exosomes secreted from the human epithelial cell line (A431) have been shown to translocate TF to the plasmalemma of mouse brain endothelial cells (42). Thus exosomes may serve as a mechanism whereby TF can be translocated from one cell type to another cell type by vesicle trafficking. Indeed, in patients with the acute respiratory distress syndrome (ARDS), alveolar epithelial-derived microparticles enriched with TF likely play a role in transferring pro-coagulant activity (43-45).
Our finding that TF is contained in exosomes, potentially confounds previously published immunohistochemical analysis indicating that TF is located within and, thus by inference, produced by epithelial cells. It was possible that TF could have been produced by cells other than airway epithelial cells and simply identified in these cells as a result of exosomal transport. However our combined in vitro and in vivo data establish that airway epithelial cells themselves are a potential source of TF in humans.
In summary, our findings demonstrate for the first time that NHBE cells are a source of TF-bearing exosomes. These exosomes are released when cells are stimulated by compressive mechanical stress comparable to that experienced during bronchoconstriction. Activation of this PKC-dependent pathway represents the first link between this pivotal signaling pathway and secretion of mediator-bearing exosomes from human bronchial epithelial cells.
Acknowledgments
Authors thank Maria Ericsson (Harvard Medical School electron microscopy facility) for her expert help in electron microscopy.
Declaration of all sources of funding
NIH HL88028(JMD), T32-HL007118(JP), and MRCgrant-G0900453(PH)
Abbreviations used
- NHBECs
Normal human bronchial epithelial cells
- BALF
Bronchoalveolar lavage fluid
- TF
tissue factor
- PKC
Protein Kinase C
- EGFR
Epidermal growth factor receptor
Footnotes
Clinical Implications
Subepithelial angiogenesis is a feature of airway remodeling. Expression of the proangiogenic mediator, TF, is associated with asthma, but its source and mechanism of secretion in asthma are not understood.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ressler B, Lee RT, Randell SH, Drazen JM, Kamm RD. Molecular responses of rat tracheal epithelial cells to transmembrane pressure. Am J Physiol Lung Cell Mol Physiol. 2000;278(6):L1264–1272. doi: 10.1152/ajplung.2000.278.6.L1264. [DOI] [PubMed] [Google Scholar]
- 2.Tschumperlin DJ, Dai G, Maly IV, Kikuchi T, Laiho LH, McVittie AK, et al. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429(6987):83–86. doi: 10.1038/nature02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swartz MA, Tschumperlin DJ, Kamm RD, Drazen JM. Mechanical stress is communicated between different cell types to elicit matrix remodeling. Proc Natl Acad Sci U S A. 2001;98(11):6180–6185. doi: 10.1073/pnas.111133298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JA, Tschumperlin DJ. Chronic intermittent mechanical stress increases MUC5AC protein expression. Am J Respir Cell Mol Biol. 2009;41(4):459–466. doi: 10.1165/rcmb.2008-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tschumperlin DJ, Shively JD, Kikuchi T, Drazen JM. Mechanical Stress Triggers Selective Release of Fibrotic Mediators from Bronchial Epithelium. Am J Respir Cell Mol Biol. 2003;28(2):142–149. doi: 10.1165/rcmb.2002-0121OC. [DOI] [PubMed] [Google Scholar]
- 6.Park JA, Drazen JM, Tschumperlin DJ. The chitinase-like protein YKL-40 is secreted by airway epithelial cells at base line and in response to compressive mechanical stress. J Biol Chem. 2010;285(39):29817–29825. doi: 10.1074/jbc.M110.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Bierhaus A, Schiekofer S, Andrassy M, Chen B, Stern DM, et al. Tissue factor--a receptor involved in the control of cellular properties, including angiogenesis. Thromb Haemost. 2001;86(1):334–345. [PubMed] [Google Scholar]
- 8.Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, et al. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383(6595):73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 9.Belting M, Dorrell MI, Sandgren S, Aguilar E, Ahamed J, Dorfleutner A, et al. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nat Med. 2004;10(5):502–509. doi: 10.1038/nm1037. [DOI] [PubMed] [Google Scholar]
- 10.van den Berg YW, Osanto S, Reitsma PH, Versteeg HH. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood. 2011 doi: 10.1182/blood-2011-06-317685. [DOI] [PubMed] [Google Scholar]
- 11.Gabazza EC, Taguchi O, Tamaki S, Takeya H, Kobayashi H, Yasui H, et al. Thrombin in the airways of asthmatic patients. Lung. 1999;177(4):253–262. doi: 10.1007/pl00007645. [DOI] [PubMed] [Google Scholar]
- 12.Wiggs BR, Hrousis CA, Drazen JM, Kamm RD. On the mechanism of mucosal folding in normal and asthmatic airways. J Appl Physiol. 1997;83(6):1814–1821. doi: 10.1152/jappl.1997.83.6.1814. [DOI] [PubMed] [Google Scholar]
- 13.Button B, Boucher RC. Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Respir Physiol Neurobiol. 2008;163(1-3):189–201. doi: 10.1016/j.resp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, et al. The influence of bronchoconstriction on airway remodeling in asthma. New Egland Journal of Medicine. 2011;364(21):2006–2015. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3(Unit 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 17.Britten KM, Howarth PH, Roche WR. Immunohistochemistry on resin sections: a comparison of resin embedding techniques for small mucosal biopsies. Biotech Histochem. 1993;68(5):271–280. doi: 10.3109/10520299309105629. [DOI] [PubMed] [Google Scholar]
- 18.Lee TH, D’Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer—the emerging science of cellular ‘debris’. Semin Immunopathol. 2011;33(5):455–467. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 19.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 21.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101(36):13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ExoCarta:Exosome markers. http://www.exocarta.org/exosome_markers.
- 23.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011 doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Cheruvanky A, Zhou H, Pisitkun T, Kopp JB, Knepper MA, Yuen PS, et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol Renal Physiol. 2007;292(5):F1657–1661. doi: 10.1152/ajprenal.00434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106(10):3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanderson MP, Keller S, Alonso A, Riedle S, Dempsey PJ, Altevogt P. Generation of novel, secreted epidermal growth factor receptor (EGFR/ErbB1) isoforms via metalloprotease-dependent ectodomain shedding and exosome secretion. J Cell Biochem. 2008;103(6):1783–1797. doi: 10.1002/jcb.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathivanan S, Lim JW, Tauro BJ, Ji H, Moritz RL, Simpson RJ. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics. 2010;9(2):197–208. doi: 10.1074/mcp.M900152-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134(5):1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 29.Shetty S, Bhandary YP, Shetty SK, Velusamy T, Shetty P, Bdeir K, et al. Induction of Tissue Factor by Urokinase in Lung Epithelial Cells and in the Lungs. Am J Respir Crit Care Med. 2010;181(12):1355–1366. doi: 10.1164/rccm.200901-0015OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagers SS, Norton RJ, Rinaldi LM, Bates JH, Sobel BE, Irvin CG. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest. 2004;114(1):104–111. doi: 10.1172/JCI19569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imokawa S, Sato A, Hayakawa H, Kotani M, Urano T, Takada A. Tissue factor expression and fibrin deposition in the lungs of patients with idiopathic pulmonary fibrosis and systemic sclerosis. Am J Respir Crit Care Med. 1997;156(2 Pt 1):631–636. doi: 10.1164/ajrccm.156.2.9608094. [DOI] [PubMed] [Google Scholar]
- 32.Brims FJH, Chauhan AJ, Higgins B, Shute JK. Coagulation factors in the airways in moderate and severe asthma and the effect of inhaled steroids. Thorax. 2009;64(12):1037–1043. doi: 10.1136/thx.2009.114439. [DOI] [PubMed] [Google Scholar]
- 33.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120(6):1233–1244. doi: 10.1016/j.jaci.2007.10.025. quiz 1245-1236. [DOI] [PubMed] [Google Scholar]
- 34.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003;9(4):458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Deng Y, Luther T, Muller M, Ziegler R, Waldherr R, et al. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest. 1994;94(3):1320–1327. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156(1):229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 37.Paredi P, Barnes PJ. The airway vasculature: recent advances and clinical implications. Thorax. 2009;64(5):444–450. doi: 10.1136/thx.2008.100032. [DOI] [PubMed] [Google Scholar]
- 38.Orsida BE, Li X, Hickey B, Thien F, Wilson JW, Walters EH. Vascularity in asthmatic airways: relation to inhaled steroid dose. Thorax. 1999;54(4):289–295. doi: 10.1136/thx.54.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu X, Harris SL, Levine AJ. The Regulation of Exosome Secretion: a Novel Function of the p53 Protein. Cancer Res. 2006;66(9):4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 40.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 41.Aharon A, Brenner B. Microparticles, thrombosis and cancer. Best Practice & Research Clinical Haematology. 2009;22(1):61–69. doi: 10.1016/j.beha.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Yu J, May L, Milsom C, Anderson GM, Weitz JI, Luyendyk JP, et al. Contribution of host-derived tissue factor to tumor neovascularization. Arterioscler Thromb Vasc Biol. 2008;28(11):1975–1981. doi: 10.1161/ATVBAHA.108.175083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bastarache JA, Fremont RD, Kropski JA, Bossert FR, Ware LB. Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2009;297(6):L1035–1041. doi: 10.1152/ajplung.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mineo M, Garfield SH, Taverna S, Flugy A, De Leo G, Alessandro R, et al. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis. 2012;15(1):33–45. doi: 10.1007/s10456-011-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


