Abstract
Background
Higher mortality for patients with aneurysmal subarachnoid hemorrhage has been reported.
Aims
In families with intracranial aneurysms, we sought to determine whether mortality among subjects with intracranial aneurysm (affecteds) was higher and related to rupture, compared with unaffected family members.
Methods
Subjects enrolled in the Familial Intracranial Aneurysm protocol, were contacted yearly and their status was obtained. If reported to be deceased, the cause of death was verified by available records. A Cox proportional hazards model was utilized to compare mortality rates.
Results
Of the 2,794 subjects, 1,073 were affected and 1,721 were unaffected. There were 8,525 person-years of follow-up (mean 3.05±1.73 years) and 85 deaths. Age at study entry for affecteds (58.4±11.9 years) was significantly older (p<.0001) than for unaffecteds (52.2±16.1). After adjusting for age, the overall mortality rate for affected subjects was not significantly different from that for unaffecteds (RR 1.26, 95% CI 0.82–1.93, p=0.292). There was a strong effect modification due to age. The mortality rate ratio of affecteds to unaffecteds who were ≤60 years of age was RR=3.48 (95% CI: 1.59–7.63, p=0.002), the rate for affected subjects who were ≥ 60 was less than the rate for unaffecteds (RR=0.69, 95% CI: 0.404–1.19, p=0.178). Affecteds who had ruptures had 2.62 times the mortality rate as those without ruptures (95% CI 1.43–4.80, p=0.002).
Conclusion
The overall mortality was similar for affected and unaffected subjects in this cohort. Among affecteds only, those with ruptured IA had a higher mortality rate than those without ruptured.
Keywords: Intracranial Aneurysm, Subarachnoid Hemorrhage, Mortality
Introduction
Studies have described higher mortality for patients with intracranial aneurysm (IA).1,2 Much of this higher mortality rate is related to aneurysm rupture. The 30-day mortality rate of patients with SAH is 35%–44% 2–4 Family history is a significant risk factor for aneurysm formation and rupture, with first degree relatives of subjects with a ruptured IA having a three- to seven-fold higher risk of SAH than the general population.5–10
Aim
In a cohort of families with a history of IA, we sought to determine whether mortality among IA cases (“affected”) was higher and related to aneurysmal rupture, compared with family members without an IA at study entry (“unaffected”).
Methods
The Familial Intracranial Aneurysm (FIA) study is an international, multicenter study that includes 41 recruitment sites in 26 clinical centers in the United States, Canada, New Zealand, and Australia. The study was approved by the institutional review board/ethics committee at each of the study centers and recruitment sites. The overall aim of this National Institute of Neurological Disorders and Stroke-funded study is to identify specific gene variants that are related to the formation and rupture of intracranial aneurysms. The study protocol and initial genetic findings have been previously reported.11–14
Consenting members of families with multiple members who were diagnosed saccular IAs were enrolled into the FIA protocol. Individuals with IAs, whether ruptured or unruptured, constituted the “affected” group, while subjects with no known diagnosis of IA made up the “unaffected” group. Exclusion criteria were employed to eliminate subjects who had IAs due to known genetic causes such as polycystic kidney disease, Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, or moyamoya disease. Subjects were also excluded if they had a fusiform IA, or an IA associated with an intracranial arteriovenous malformation.
Verification of Intracranial Aneurysms
All medical records and information acquired by screening of affected family members with a reported history of IA, subarachnoid hemorrhage, or intracranial hemorrhage were reviewed by the study’s Verification Committee. Two neurologists independently reviewed the records and determined whether the participant met all the inclusion and exclusion criteria. In cases of disagreement, a third neurologist was consulted. Characteristics of the aneurysms were recorded including: location, number, size, rupture status and treatment. Aneurysm size, when available, was classified into four categories: 3–7mm, 7–12 mm, 13–25 mm, and >25 mm. Unaffected family members ≥ 30 years of age with a history of smoking and/or hypertension were deemed high-risk for the presence of an IA at the time of study enrollment and were offered a study magnetic resonance angiogram (MRA). As previously reported, study MRAs were performed on 541 high-risk family members, and an additional 7 subjects had non-study imaging performed. Of these 548 subjects, 114 (20.8%) had an IA on imaging.15,16 Two neuroradiologists at the Study Imaging Center at the Mayo Clinic, Rochester MN, independently reviewed all screening MRAs and non-study imaging, and cases were adjudicated to define the level of certainty of aneurysm presence. If a consensus could not be reached, a third blinded interpretation was performed to verify the presence of an IA.
Risk Factors
Potential risk factors for aneurysm formation and rupture including age, gender, cigarette smoking history, and history of hypertension prior to diagnosis (for affected subjects) or study entry (for unaffected subjects) were ascertained at the time of study entry. Smoking history was recorded as “ever” (current or former smoker) and “never,” and if the subject ever smoked, the number of pack years was calculated.
Follow-Up
Participants (or their proxies) were administered a yearly follow-up questionnaire on the anniversary of their study entry (±30 days). This questionnaire provided a means of maintaining contact with participants as well as collecting information concerning deaths, rupture of known IAs, or IAs newly diagnosed after study enrollment. All documented newly diagnosed IAs and ruptured IAs underwent a detailed review by the Verification Committee. If a subject was reported to be deceased, the date and cause of death was recorded, and available death certificates and medical records were used to verify the cause of death.
Statistical Methods
A Cox proportional hazards model was utilized to compare mortality rates between subjects with and without IA. We examined possible confounding by age, race, gender, affected status, rupture status, smoking history and hypertension. Two subjects who expired within 24 hours of study entry were excluded from the analysis.
Results
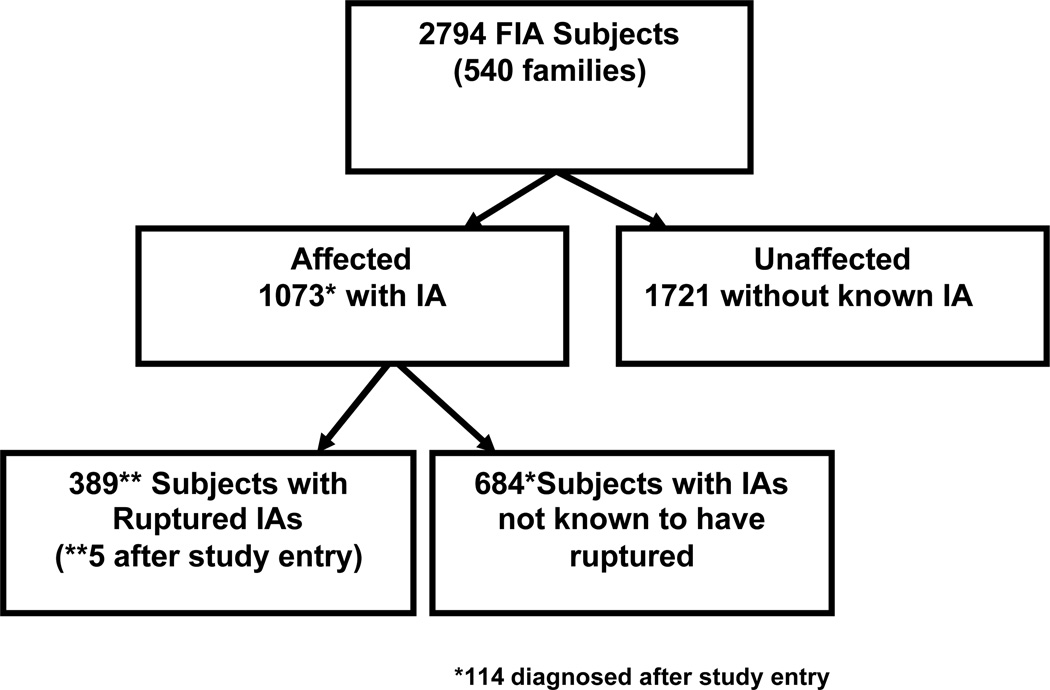
The FIA study enrolled 2,794 subjects between August 2002 and December 2007; 959 had a known aneurysm at the time of study entry, and an additional 114 subjects were diagnosed after study entry (Figure 1). Of those with a known IA, 384 subjects had rupture prior to study entry, and an additional 5 subjects ruptured after study entry. Of the 1073 subjects with IA, 361 (33.6%) had more than one aneurysm, 134 of whom had ruptures. No subject had more than one rupture. Size of aneurysm was unknown for 336 (21.3%) of the 1073 subjects. Of the remaining 737 subjects, the largest aneurysm exceeded 7mm in 259 subjects (35.1%). The complete distribution of aneurysm multiplicity and largest aneurysm size is shown in Table 1. The 684 subjects who did not have known rupture had a total of 1052 aneurysms. Of these unruptured IAs 506 were clipped, 128 had coiling, 4 had wrapping or other procedure, 397 were managed medically, and management for 17 subjects is unknown. Only 229 subjects without a rupture had medical management only for all identified aneurysms.
Figure 1.
Flow diagram for FIA study and affected status
Table 1.
Distribution of aneurysm multiplicity and size of largest aneurysm for 1073 affected subjects
| Subjects with Ruptured IA (n=389) |
No known rupture (n=684) |
Total (n=1073) |
|
|---|---|---|---|
| Number of aneurysms |
|||
| 1 | 255 | 457 | 712 |
| 2 | 90 | 147 | 237 |
| 3 | 26 | 45 | 71 |
| 4 | 13 | 19 | 32 |
| 5 | 3 | 8 | 11 |
| 6 | 6 | 6 | |
| 7 | 2 | 2 | |
| 8 | 2 | 2 | |
| Size of largest aneurysm |
|||
| < 7mm | 108 | 370 | 478 |
| 7–12 mm | 70 | 117 | 187 |
| 13–25 mm | 22 | 34 | 56 |
| > 25 mm | 8 | 8 | 16 |
| unknown | 181 | 155 | 336 |
Baseline potential risk factors for the formation and rupture of IAs age, female gender, hypertension and cigarette smoking were statistical significantly higher among affected subjects (Table 2).
Table 2.
Baseline subject characteristics of 1073 subjects with IA compared with 1721 without a known IA
| Risk Factors | Known IA (n=1073) |
Unaffected | P Value |
|---|---|---|---|
| Age at study entry | 58.4 years | 52.2 years | <.0001 |
| Female gender | 75% | 58% | <.0001 |
| Hypertension | 49% | 33% | <.0001 |
| Never Smoked | 21% | 42% | <.0001 |
| Current | 46% | 25% | |
| Former | 33% | 33% | |
| Pack-years (mean, SD) |
22.4 (23.3) | 12.9 (20.7) | <.0001 |
Through August 2009, there were 8,525 person-years of follow-up and 85 deaths from all causes in this prospectively followed cohort; mean follow-up time was 3.05 years (± 1.73 years). There was no significant difference (p=.11) in length of follow-up between affected subjects (3.12 ±1.68 years) and unaffected subjects (3.01 ±1.75 years). For subjects with a diagnosis of IA prior to study entry, the mean (± SD) time from diagnosis to study entry was 5.5 (± 7.5) years. For subjects who had a rupture of an IA prior to study entry, the mean (± SD) time from rupture to study entry was 8.0 (± 9.2) years.
The crude annual mortality rate was 13.2 per 1000 person-years for affected subjects and 8.3 per 1000 person-years for unaffected subjects. After adjusting for age, pack-years of smoking, and history of hypertension at study entry, these rates were not significantly different (RR1.26, 95% CI 0.82-1.93, p=0.292). However, there was a strong effect modification due to age at entry. The overall mortality rate ratio of affecteds to unaffecteds who were less than 60 years of age at study entry was RR=3.48 (95% CI: 1.59–7.63, p=0.002), while the mortality rate for affected subjects who were 60 and over at entry was actually less than the mortality rate for unaffected subjects (RR=0.69, 95% CI:0.404–1.19, p=0.178). Affecteds who had ruptures had 2.62 times the mortality rate as those without known ruptures (95% CI 1.43–4.80, p=0.002). When the analysis was restricted to subjects who had an unruptured IA upon entry into the study the results were similar, RR=3.25 for subjects who were less than 60 years of age at study entry and RR=0.69 for subjects 60 or older at study entry.
There were 4 deaths attributed to a ruptured IA among the 1073 affected subjects, 3 of these occurred within 10 days of study entry. Of the remaining 81 subjects who died during the follow-up period, 23 died of cancer, 13 of other neurological diseases, 10 of respiratory disease, 8 of cardiac conditions, 17 died of other causes, and 10 deaths were of unknown cause. The causes of death are listed in Table 3.
Table 3.
Causes of Death within the FIA Cohort (n=85)
| Ruptured IA (n=389) % of Events |
Unruptured IA/ Unknown rupture (n=684) % of Events |
No History of IA (n=1721) % of Events |
All subjects | ||
|---|---|---|---|---|---|
| Cancer | 6(1.54) | 4 (0.58) | 13 (0.75) | 23 | |
| Lung | 2 | 4 | |||
| Breast | 1 | 2 | |||
| Pancreatic | 2 | ||||
| Ovarian | 1 | ||||
| Brain | 1 | ||||
| Blood | 1 | 1 | |||
| Unspecified | 4 | 2 | 2 | ||
| Neurological | 7(1.79) | 2(0.29) | 8(0.46) | 17 | |
| AIS | 1 | 1 | 3 | ||
| Alzheimer’s | 1 | 3 | |||
| Ruptured IA | 4 | ||||
| ICH | 1 | 2 | |||
| Epileptic Sz | 1 | ||||
| Respiratory | 3(0.77) | 2(0.29) | 5(0.29) | 10 | |
| COPD | 1 | 1 | |||
| Failure | 1 | 3 | |||
| Pneumonia | 3 | ||||
| P E | 1 | ||||
| Cardiac | 3(0.77) | 0 | 5(0.29) | 8 | |
| CHF | 3 | ||||
| MI | 3 | 2 | |||
| Other Causes | 3(0.77) | 6(0.87) | 8(0.46) | 17 | |
| Drug OD | 2 | 1 | |||
| Natural | 1 | 2 | |||
| MVA | 2 | ||||
| Other single events* |
2 | 2 | 5 | ||
| Unknown | 3(0.77) | 3(0.43) | 4(0.23) | 10 | |
| Totals | 25 | 17 | 43 | 85 |
Abbreviations: AIS, acute ischemic stroke; IA, intracranial aneurysm; ICH, intracerebral hemorrhage; Sz, seizure; COPD, chronic obstructive pulmonary disease; PE, pulmonary embolus; CHF, congestive heart failure; MI, myocardial infarction; OD, over dose; MVA, motor vehicle accident
Other single events include: complications from diabetes, pelvic fracture, liver failure, rare disease unrelated to IA, sepsis, renal failure, lupus erythematosus, sudden collapse, ruptured abdominal aortic aneurysm
Discussion
We have previously reported a possible higher risk of rupture of small IAs among subjects in the FIA study as compared with sporadic unruptured IAs of similar size.16 Despite this increased risk of aneurysm rupture and the associated high mortality, the overall cause of death in this high-risk prospectively followed cohort was not significantly elevated due to intracranial aneurysm across all ages. The ability to detect IA before rupture could decrease mortality by surgical or endovascular treatment of IAs. While the rupture rate for familial aneurysms may be higher than sporadic IA, the overall number of aneurysm ruptures in our cohort was small, and other causes of death were more frequent. We did note that younger affected subjects who were enrolled before age 60 had a significantly high overall mortality rate compared to unaffected subjects of the same age range.
Our study had several limitations. We were unable to confirm the cause of reported death in 10 subjects, which were divided relatively evenly between the groups (3 whose IA ruptured and 3 whose IAs were not known to have ruptured in the affected group, and 4 in the unaffected group). Only 25% of the unaffected family members (n=435) had study imaging completed to confirm their status with respect to IA or no IA. However, screening criteria included the presence of significant risk factors such as age ≥ 30 years of age; and history of smoking and/or hypertension to enrich the yield. For subjects without imaging, information about their IAs was abstracted from medical records, which did not always provide details regarding size, hence the missing size data for 336 subjects. Additionally, the mean follow-up time (3.05 years) was relatively short, which limits the generalizability of our findings over longer time periods. The predominant known cause of death in this cohort was cancer, followed by non-aneurysmal neurological conditions, respiratory disease, and cardiac disease. There were no significantly elevated mortality rate ratios associated with IA and these conditions. The percentage of current smokers at the time of study entry was 33%, which is higher than current tobacco use reported by the individual countries included in the study, according to the World Health Organization for the same time period, which ranged from 18%–25.1%.17 The significantly higher mortality rate in younger affected subjects unrelated to IA rupture suggests that addressing other health related issues such as cigarette smoking and hypertension is needed to impact mortality for all causes such as cancer, cerebrovascular, respiratory and cardiovascular conditions.
Acknowledgments
Funding: This study was funded by a grant from the National Institute of Neurological Disease and Stroke (NINDS R01 NS39512) National Institutes of Health, Bethesda, MD.
Footnotes
Other Information
Registration www.clinicaltrials.gov NCT00071565
Protocol
Broderick JP, Sauerbeck LR, Foroud T, et al. Familial intracranial aneurysm (FIA) study protocol. BMC Med Genet. 2005;6:17. www.biomedcentral. com/1471-2350/6/17
References
- 1.Olafsson E, Hauser WA, Gudmundsson G. A population-based study of prognosis of ruptured cerebral aneurysm: Mortality and recurrence of subarachnoid hemorrhage. Neurology. 1997;48:1191–1195. doi: 10.1212/wnl.48.5.1191. [DOI] [PubMed] [Google Scholar]
- 2.Sandvei MS, Mathiesen EB, Vatten LJ, et al. Incidence and mortality of aneurysmal subarachnoid hemorrhage in two norwegian cohorts 1984–2007. Neurology. 2011;77:1833–1839. doi: 10.1212/WNL.0b013e3182377de3. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 1993;78:188–191. doi: 10.3171/jns.1993.78.2.0188. [DOI] [PubMed] [Google Scholar]
- 4.Ingall T, Asplund K, Mahonen M, Bonita R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke. 2000;31:1054–1061. doi: 10.1161/01.str.31.5.1054. [DOI] [PubMed] [Google Scholar]
- 5.Sundquist J, Li X, Sundquist K, Hemminki K. Risks of subarachnoid hemorrhage in siblings: A nationwide epidemiological study from sweden. Neuroepidemiology. 2007;29:178–184. doi: 10.1159/000111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaist D, Vaeth M, Tsiropoulos I, et al. Risk of subarachnoid haemorrhage in first degree relatives of patients with subarachnoid haemorrhage: Follow up study based on national registries in denmark. BMJ. 2000;320:141–145. doi: 10.1136/bmj.320.7228.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kissela BM, Sauerbeck L, Woo D, et al. Subarachnoid hemorrhage: A preventable disease with a heritable component. Stroke. 2002;33:1321–1326. doi: 10.1161/01.str.0000014773.57733.3e. [DOI] [PubMed] [Google Scholar]
- 8.Schievink WI, Schaid DJ, Michels VV, Piepgras DG. Familial aneurysmal subarachnoid hemorrhage: A community-based study. J Neurosurg. 1995;83:426–429. doi: 10.3171/jns.1995.83.3.0426. [DOI] [PubMed] [Google Scholar]
- 9.Teasdale GM, Wardlaw JM, White PM, et al. The familial risk of subarachnoid haemorrhage. Brain. 2005;128:1677–1685. doi: 10.1093/brain/awh497. [DOI] [PubMed] [Google Scholar]
- 10.Bromberg JE, Rinkel GJ, Algra A, et al. Familial subarachnoid hemorrhage: Distinctive features and patterns of inheritance. Ann Neurol. 1995;38:929–934. doi: 10.1002/ana.410380614. [DOI] [PubMed] [Google Scholar]
- 11.Broderick JP, Sauerbeck LR, Foroud T, et al. Familial intracranial aneurysm (FIA) study protocol. BMC Med Genet. 2005;6:17. doi: 10.1186/1471-2350-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foroud T, Sauerbeck L, Brown R, et al. Genome screen to detect linkage to intracranial aneurysm susceptibility genes: The familial intracranial aneurysm (FIA) study. Stroke. 2008;39:1434–1440. doi: 10.1161/STROKEAHA.107.502930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foroud T, Sauerbeck L, Brown R, et al. Genome screen in familial intracranial aneurysm. BMC Med Genet. 2009;10:3. doi: 10.1186/1471-2350-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deka R, Koller DL, Lai D, et al. The relationship between smoking and replicated sequence variants on chromosomes 8 and 9 with familial intracranial aneurysm. Stroke. 2010;41(6):1132–1137. doi: 10.1161/STROKEAHA.109.574640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown RD, Jr, Huston J, Hornung R, et al. Screening for brain aneurysm in the familial intracranial aneurysm study: Frequency and predictors of lesion detection. J Neurosurg. 2008;108:1132–1138. doi: 10.3171/JNS/2008/108/6/1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broderick JP, Brown RD, Jr, Sauerbeck L, et al. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke. 2009;40:1952–1957. doi: 10.1161/STROKEAHA.108.542571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Statistics 2007. www.WHO.org downloaded March 1, 2011.