Abstract
The preschool years represent a time of expansive psychological growth, with the initial expression of many psychological abilities that will continue to be refined into young adulthood. Likewise, brain development during this age is characterized by its “blossoming” nature, showing some of its most dynamic and elaborative anatomical and physiological changes. In this article, we review human brain development during the preschool years, sampling scientific evidence from a variety of sources. First, we cover neurobiological foundations of early postnatal development, explaining some of the primary mechanisms seen at a larger scale within neuroimaging studies. Next, we review evidence from both structural and functional imaging studies, which now accounts for a large portion of our current understanding of typical brain development. Within anatomical imaging, we focus on studies of developing brain morphology and tissue properties, including diffusivity of white matter fiber tracts. We also present new data on changes during the preschool years in cortical area, thickness, and volume. Physiological brain development is then reviewed, touching on influential results from several different functional imaging and recording modalities in the preschool and early school-age years, including positron emission tomography (PET), electroencephalography (EEG) and event-related potentials (ERP), functional magnetic resonance imaging (fMRI), magnetoencephalography (MEG), and near-infrared spectroscopy (NIRS). Here, more space is devoted to explaining some of the key methodological factors that are required for interpretation. We end with a section on multimodal and multidimensional imaging approaches, which we believe will be critical for increasing our understanding of brain development and its relationship to cognitive and behavioral growth in the preschool years and beyond.
Introduction
Just as the preschool years represent a time of great cognitive and behavioral growth, with the emergence in early form of many quintessentially human psychological abilities, they likewise constitute a period of “blossoming” within the brain, during which anatomical and physiological substrates show some of their most dynamic and elaborative developmental changes. Here, we present a review of human brain development during the preschool years that we hope will be particularly informative for readers interested in a pediatric neuropsychological perspective. Although a comprehensive review of all relevant research is beyond the scope of our current purpose, we have nevertheless sought to present a wide range of evidence gathered from a variety of research methods that are relevant to an understanding of preschool brain development, including both seminal older work and important new findings from cellular to systems levels. While wider than deep, hopefully this approach will be useful in conveying some of the major progress, prospects, and remaining questions within this still growing scientific area. Our goal is to inform the reader about the growing, working preschool brain within a broad developmental context.
The organization of this article will be first to cover the early postnatal neurobiological foundations of human brain growth, explaining some of the primary cellular mechanisms that underlie what will subsequently be presented at a larger scale within imaging studies. Next, we review noninvasive structural and functional neuroimaging research, which has significantly augmented scientific knowledge derived from postmortem studies and forms the bulk of our current understanding of human brain development. Within the anatomical imaging section, we focus on studies of brain morphology and tissue properties, including diffusion imaging of the developing cerebral white matter fiber tracts. We also present new data on changes during the preschool years in cortical area, thickness, and volume provided by the multisite Pediatric Imaging, Neurocognition, and Genetics (PING) study. Physiological brain development in young children is then reviewed, touching on influential results that have come from several different functional imaging and recording modalities, including positron emission tomography (PET), resting and event-related electroencephalography (EEG/ERP), resting and event-related functional magnetic resonance imaging (fMRI), magnetoencephalography (MEG), and near-infrared spectroscopy (NIRS). Since measures of brain activity are more dynamic and more dependent on immediate contextual factors than anatomical imaging measures (such as cortical thickness or hippocampal volume), we devote more space in this section to explaining some of the principal experimental and methodological factors that are required for interpretation. Before summary and conclusions, we end with a section on multimodal and multidimensional imaging approaches, which we believe will be particularly important for increasing our understanding of brain development and its relationship to cognitive and behavioral growth in the preschool years and beyond.
Early Postnatal Neurobiological Development
Though many brain patterning processes are complete at birth, the human brain exhibits further dramatic biological development during the preschool years, and roughly quadruples in weight before the age of six (Dobbing and Sands, 1973), when it has acquired approximately 90% of its adult volume (Courchesne et al., 2000; Iwasaki et al., 1997; Kennedy et al., 2002; Kennedy and Dehay, 2001; Lenroot and Giedd, 2006; Paus et al., 2001; Reiss et al., 1996). In vivo brain imaging of infants and young children has provided much new information about the nature and timing of alterations that attend this exuberant period of brain growth, but the specific biological processes that give rise to the imaging effects remain obscure. Basic neurodevelopmental research has added much to our knowledge of postnatal brain development, but importantly, estimates of the extent and time course of human developmental processes generally rely upon extrapolation from data acquired in other species, often rodents, and from very limited human postmortem material. Unfortunately, the result is much remaining uncertainty about the scale and temporal extent of cell proliferation, migration, differentiation, and regression, and about the relationship of these processes to each other, during the human postnatal period.
Progressive and regressive processes in postnatal brain development
The production and migration of neurons are largely prenatal events, however neurogenesis continues to a very limited degree in the subventricular zone, where new neurons continue to emerge and migrate to the olfactory bulb, and in the dentate gyrus of the hippocampus, where they migrate a short distance from the subgranular to the granular layer. These exceptional forms of neurogenesis appear to continue throughout adult life but produce only a small percentage of the neuronal population. In contrast, proliferation and migration of glial progenitors is particularly exuberant during the immediate postnatal and preschool years, and continues for a protracted period as oligodendrocytes and astrocytes differentiate; in fact, glial progenitors (particularly oligodendrocyte progenitor cells, or OPCs) persist indefinitely in the adult brain in a wide anatomical distribution, and can differentiate in response to injury. Glial progenitors mostly proliferate in the forebrain subventricular zone and migrate radially and laterally into the overlying white matter and cortex, striatum, and hippocampus, where they differentiate into oligodendrocytes and astrocytes. Unlike neural progenitors, glial progenitors continue to proliferate as they migrate (Cayre et al., 2009).
Upon reaching their destinations, many OPCs begin to differentiate by extending processes and up-regulating myelin protein expression. The new processes then begin to form membrane wraps around nearby axons. Eventually the oligodendrocytes form tightly wrapped multi-layered sheaths from which most of the cytoplasm has been extruded. The best understood functional consequence of myelination is the effect on axonal conduction velocity; however, functional interactions between oligodendrocytes and neurons appear to extend far beyond the effects of the electrically insulating sheath. Oligodendrocytes synthesize a number of trophic factors that appear to contribute to the maintenance of axonal integrity and neuronal survival, and neuron-oligodendrocyte interactions have been shown to influence neuronal size and axon diameter (McTigue and Tripathi, 2008). An intriguing new line of evidence also suggests that a subset of the OPCs dispersed throughout the brain form excitatory and inhibitory connections with neurons, and thus may contribute actively and directly to neural signaling (Lin and Bergles, 2004).
In summary, proliferation and migration of glial precursors and differentiation of astrocytes and oligodendrocytes are largely postnatal processes. While there is little doubt that these processes play a critical role in the functional maturation of developing neural circuits, the full scope of their impact on neural dynamics may be much greater than was previously appreciated. Ongoing research continues to uncover additional molecular interactions between neurons, oligodendrocytes, and astrocytes. The existence of these interactions implies that the late maturation of glial populations during the preschool years probably has widespread functional implications.
Brain development is characterized by early overproduction of neurons and glial cells, neural processes, and synapses. Although programmed loss of neurons has its peak during prenatal life, apoptosis in glial cell populations has a time course corresponding to the protracted postnatal time course of differentiation from glial precursors. During the period of initial myelination, many excess oligodendrocytes undergo apoptosis within a few days after differentiating, and there is evidence that this process depends on signals from nearby axons, such that the number of surviving oligodendrocytes matches the local axonal surface area (see (McTigue and Tripathi, 2008), for review).
Much of the regressive remodeling that occurs in postnatal brain involves elimination or pruning of neuronal processes, i.e., axonal and dendritic processes, spines, and synapses. Studies of monkeys and humans reveal early excess connections throughout distributed brain regions in the early postnatal period (Bourgeois et al., 1994; Bourgeois and Rakic, 1993; Huttenlocher and Dabholkar, 1997; Huttenlocher and de Courten, 1987; Zecevic et al., 1989). Exuberant connectivity has been documented in several pathways, and is especially well described in the corpus callosum, but there is also work showing early excess connectivity in thalamocortical pathways, corticospinal tract, and pathways linking the temporal lobe and the limbic system (Innocenti and Price, 2005; Stanfield and O’Leary, 1985; Stanfield et al., 1982). Pruning of excess connectivity is still not fully understood, but factors such as competition for neurotrophic factors and the presence or absence of afferent input have been implicated in this process. Recent studies using video microscopy have begun to record the processes of growth and pruning at a microscopic level. These studies suggest that as axons seek their targets they continuously sample the surrounding space, forming and retracting synaptic connections dynamically (Hua and Smith, 2004).
Structural Brain Development
Imaging studies of brain morphology
MRI reveals dramatic changes in the tissues of the developing brain during the postnatal brain growth spurt. These MRI signal changes reflect alterations in tissue chemistry that are presumed to mark the proliferation of oligodendrocytes and deposition of myelin, and they reveal much about the timing and anatomical distribution of these processes (Barkovich, 2000, 2005). The visual appearance of the brain on MR images changes appreciably over the first 2 to 3 years of life, mirroring an orderly pattern of myelination in white matter regions. In general, MR signal changes associated with myelination appear earliest in sensorimotor pathways and commissural tracts, later in other ascending pathways of the corona radiata, and latest in cortico-cortical association tracts. However, across different neural systems there is temporal overlap of these patterns, and early conventional MR studies lacked the anatomical specificity to map the pattern of myelination precisely. The first MR morphometry studies comparing gray matter morphology in children and adults revealed that gray matter volumes estimated using these methods, both in the cerebral cortex and in subcortical nuclei, are considerably larger in school-aged children than in young adults (Jernigan and Tallal, 1990; Jernigan et al., 1991; Pfefferbaum et al., 1994). These early studies suggested that tissue alterations related to brain maturation might be much more protracted during childhood than was generally supposed, and that some of these alterations might be regressive; that is, they might involve tissue loss. These findings were confirmed and extended by later studies (see Toga et al., 2006 for a review), but the underlying tissue alterations remain a matter of speculation. MRI measurements of volume of the cranial vault increase dramatically with age after birth but very little after the first decade. However, the MRI results suggest that throughout childhood and adolescence effects of waning progressive changes, associated with continuing maturation of glial populations and neurotrophic effects, are opposed by concurrent regressive changes, perhaps in part associated with “pruning” of neuronal processes. These observations are consistent with ample histological evidence for ongoing myelination across this period (Yakovlev and Lecours, 1967), and more limited, but persuasive, evidence for reduction of synaptic density in cortex during childhood, but it remains unclear to what extent these factors, and perhaps others, contribute to the changing morphology observed with MRI.
Recent MR morphometry studies have provided more anatomical detail by employing mapping methods for visualizing the pattern of age-related change across the late childhood range (Giedd et al., 1996b; Sowell et al., 1999a; Sowell et al., 1999b; Sowell et al., 2002). Studies of developing children describe the protracted course of postnatal white matter growth and establish that the volume of tissue with the MR signal characteristics of “gray matter” declines concurrently in locations throughout the brain, e.g., in cerebral cortex and deep nuclei. The most detailed studies, employing both high-resolution mapping techniques and longitudinal assessments (Gogtay et al., 2004; Sowell et al., 2004) have revealed a modal pattern of childhood and adolescent change in the cerebral cortex and some studies have suggested not only widespread, regionally specific, apparent cortical thinning, but more limited areas of cortical thickening as well. One challenge in interpreting these early studies is the differentiation of change in surface area versus cortical thickness, especially across the first decade of life, as this distinction is difficult from a computational point of view, and the relationship between surface and thickness change may be nonmonotonic during the preschool years. On average, however, it appeared from early studies that cortical thinning occurred first in primary sensory-motor cortex and then progressed into secondary, then multimodal, and then supramodal cortical areas throughout childhood and adolescence. A recent study (Ostby et al., 2009) confirmed these observations in a large cross-sectional sample and provided concurrent estimates of cortical surface area and cortical thickness. This is an important contribution since studies of cortical volume conflate these factors, and no previous studies had addressed whether the changes in cortical thickness are accompanied by alterations of surface area as well. Østby et al. (2009) report that between the ages of 8 and 30 years more modest decreases in cortical surface area accompany the dramatic decreases in cortical thickness; but unfortunately this study provided no information about the preschool period.
An important issue germane to the interpretation of these effects is their relationship to myelination. At the most basic level, cortical “thinning” could simply reflect increased myelination in the white matter tracts coursing within and near the deepest layer of cortex. In other words the “gray” signal of the unmyelinated fibers could simply be becoming more “white” as myelin is deposited. This is clearly a part of what is measured as cortical thinning with morphometry, especially in preschool aged children. However, there is evidence that true regressive changes also occur in some structures – probably due to loss or simplification of neuronal processes (dendrites and/or axons). This can be inferred from the fact that the progressive changes that would be expected to result from continuing myelination do not seem to increase cranial volume in late childhood (as though they were opposed by some regressive factor); and from the fact that there are modest but significant CSF volume increases adjacent to the cortical surface and in the ventricular system over this age-range, as might be expected, ex vacuo, in the wake of the loss of neural elements in the adjacent tissues (Jernigan et al., 1991; Sowell et al., 2002).
Using volume-mapping methods, Sowell et al. (2004) reported similarities in the patterns of brain growth and cortical density reductions and interpreted this as evidence that local cortical thinning might bear a direct relationship to myelination of nearby fiber tracts; but the nature of this relationship remains unclear. It is possible that functional changes resulting from maturation of fiber tracts stimulate cortical thinning (or thickening), or, conversely, that increasing activity due to intrinsic cortical maturation stimulates myelination of the axons in the maturing network. Neuron-glia signaling mechanisms that mediate effects of action potentials on oligodendrocyte differentiation and myelination have been reported (see Fields and Burnstock, 2006 for review). However, again, the interactions among these factors in developing brain tissues of young children are still poorly understood. In summary, early MR morphometry studies reveal a complex pattern of development in brain structure during childhood and hint that ongoing maturation of fiber tracts probably plays a key role. Only recently, however, has it been possible to examine the maturation of fiber tracts directly, using diffusion tensor imaging (DTI) (Basser et al., 1994; Mori and van Zijl, 1995).
Diffusion imaging of fiber tract development
Diffusion imaging measures the diffusion of water molecules through the tissue. A common use of diffusion imaging involves fitting, for each voxel, a tensor that estimates proton diffusion (movement) along each of the principal axes. Tensors from free water voxels exhibit high, isotropic, diffusion values; i.e., protons diffuse freely in all directions. Diffusion in gray matter is lower but also relatively isotropic. However, in voxels that contain fiber bundles, the diffusion is higher along the long axis of the fibers. This phenomenon is measured as an index of anisotropy, usually as fractional anisotropy (FA). It has been shown that proton diffusion in the cerebral white matter of human newborns is high, and exhibits low anisotropy (Hermoye et al., 2006). As the fiber tracts mature, and myelination proceeds, diffusion declines, and anisotropy increases. By examining the change in detail, i.e., by measuring the three tensor eigenvalues, it has been shown that developmental increase in FA often reflects a decrease in all three diffusivities, which is, however, less acute for the principal eigenvalue (i.e., in diffusion along the long axis of the tracts). The interpretation (Suzuki et al., 2003) is that unrestricted water in extra-axonal space declines, resulting in decreased tissue diffusivity overall, while diffusion within and/or along the membranes of the axons remains relatively constant or increases. The denser packing of axons that results from myelination and growth of axonal diameter is likely to reduce diffusion by decreasing extra-axonal water. Whether and how alterations of fiber morphology or intra-axonal diffusion contribute to changing tensor values is unknown. Nevertheless, there is growing evidence that alterations reflected in and measurable with diffusion imaging continue throughout childhood and adolescence (Barnea-Goraly et al., 2005; Schneider et al., 2004; Snook et al., 2005). The pattern of FA increases, for example, suggests that FA reaches asymptote earliest in long projection, then commissural, and finally association fibers, the latter continuing to exhibit age-related FA increases well into adulthood (see Cascio et al., 2007; Huppi and Dubois, 2006; Mukherjee and McKinstry, 2006 for reviews). Lebel et al. (2009; Lebel et al., 2008) reported diffusion imaging results in a large group of typically developing children and young adults. Robust increases in FA across the age-range from 5–12 years were observed within fiber tracts defined with manual and semi-automated tractography. This study revealed the rapid change in FA in young school-aged children and also demonstrated that different tracts varied in the pace with which adult values of FA are approached. This group recently reported individual trajectories of tract FA obtained with repeated imaging of school-aged children (Lebel and Beaulieu, 2011). These data clearly demonstrated that over 2 to 4 years substantial increases in FA occurred within individual children, and they also highlighted wide individual differences in the pace of these changes. Again, however, these studies provide little information about the pattern of change during the earlier preschool age-range.
In summary, in vivo brain imaging is opening a window on continuing brain development during infancy and early childhood. However, few studies have focused on the important preschool years, due to the difficulty of imaging young, unsedated children. Ironically, the ability to image sleeping infants under one year of age has led to some information from imaging about this perinatal period, but leaves a substantial gap in the evidence for the preschool period. Studies of older children combined with those of infants point strongly to the presence of highly dynamic biological change in brain tissues, with a complex regional pattern, in the preschool period. Fortunately, imaging techniques are maturing, and are becoming more robust to motion in the scanner. These improvements, combined with better validation studies establishing the biological significance of MR signals, promise to reveal more about the relationship between alterations observed in developing children on MRI and the molecular and microstructural events described above.
Annualized cortical changes from the Pediatric Imaging, Neurocognition, and Genetics (PING) Study
Although the preschool years are still under-characterized in brain imaging research, recent advances are making it increasingly feasible for scientists to look at brain development within this important age range and make meaningful comparison to other periods across development. One recent effort is the Pediatric Imaging, Neurocognition and Genetics (PING) Study, funded by the National Institute on Drug Abuse and the National Institute of Child Health and Human Development to establish a large-scale, multisite, multimodal brain imaging, genetics, and behavioral database on approximately 1400 typically developing individuals between 3 and 20 years old. Several aspects of the PING study promise to set it apart in addressing questions about the developmental trajectories of human brain maturation from preschool into young adulthood. Imaging data can vary significantly between scanners, even from the same model by the same manufacturer, and quality can drift over time within scanners. This leads to large differences across scanners in image contrast, field homogeneity, artifacts, and spatial distortions depending on where and when the data were collected (Holland et al., 2010). Through PING, recently developed technology is applied specifically to address these problems. First, a standardized multimodal image acquisition protocol is used, and correction and normalization is applied to remove remaining distortion and scanner effects. This allows MRI-derived biomarkers to be directly compared across individuals, even when their data are obtained in different cities on different scanners. Secondly, the PING Study approach allows for data from across different imaging modalities (e.g., T1-weighted, T2-weighted, diffusion-weighted, functional imaging) to be fully integrated into a shared analytic space by the use of sophisticated new nonlinear image registration and alignment methods (Holland and Dale, 2011). These advances provide an unprecedented degree of precision in making comparisons across different types of brain measures, such as relating levels of brain activity to the growth and tissue property changes of white matter fiber tracts within adjacent regions. Thirdly, the PING post-processing protocol takes advantage of an automated, probabilistic atlas-based method for characterizing white matter development, which avoids the subjectivity of commonly used manual tractography techniques (Hagler et al., 2009). Finally, PING employs prospective head motion correction, which is particularly useful when collecting data with young and clinical subject populations, significantly reducing lost data and artifacts from subject motion, improving the reliability of automated morphological measures, and increasing the clinical diagnostic utility of scans (Brown et al., 2010; Kuperman et al., 2011; White et al., 2010a).
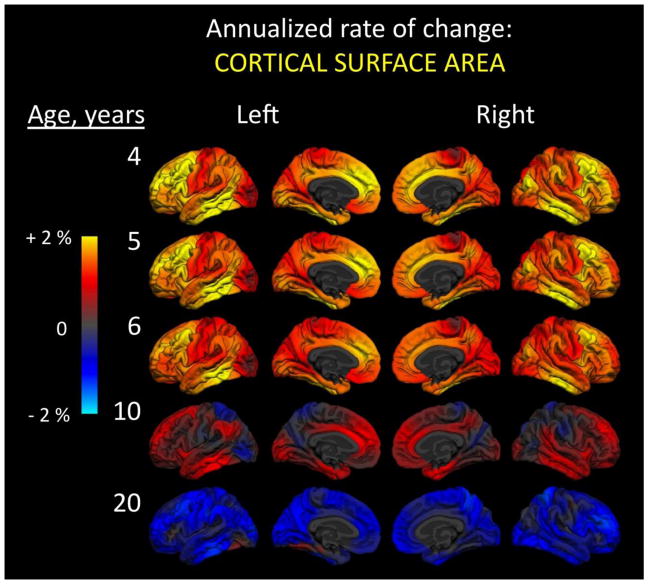
This collection of developmentally targeted imaging advances is already beginning to provide a more complete view of the brain within the preschool years and beyond, augmenting previous work with both new information and greater details. From data collected for the PING project so far, some of the most dramatic developmental changes are evident in the morphological characteristics of the cerebral cortex as children develop from preschool ages into early school age (Brown et al., 2012). Annualized rate of change, a measure of the age-varying developmental slope across a one-year age band, can be calculated at every point across the brain surface and displayed as a map of changing characteristics for different features, such as cortical surface area, thickness, and volume. As Figure 1 shows, there is significant expansion of cortical surface area during preschool ages, extending into the early school years. By age 4, the greatest changes in area are occurring within higher order cortical regions such as prefrontal cortex and temporal association areas, still increasing but to a lesser extent are areas within primary sensory (visual, auditory) and sensorimotor cortex bilaterally. In a change of direction, by the age of 10 some cortical regions begin to show decreases in area, especially within occipital and superior parietal lobes. By about the age of 20, essentially the entire cortex shows decreases in area, which continue through adulthood.
Figure 1.
Annualized rate of change in cortical surface area. At every vertex across the reconstructed cortical surface, age-dependent annualized rate of change is shown at five different ages. Left and right hemisphere lateral (outside) and medial (inside) surfaces are shown. Color scale ranges from two percent increases (yellow) to two percent decreases (cyan) in area. Change was calculated using 202 subjects (102 male, 100 female): 26 four-year-olds (12 males, 14 females); 20 five-year-olds (11 males, 9 females); 37 six-year-olds (20 males, 17 females); 48 10-year-olds (26 males, 22 females); and 71 20-year-olds (33 males, 38 females). Data come from the Pediatric Imaging, Neurocognition, and Genetics (PING) Study, adapted from Brown et al., 2012.
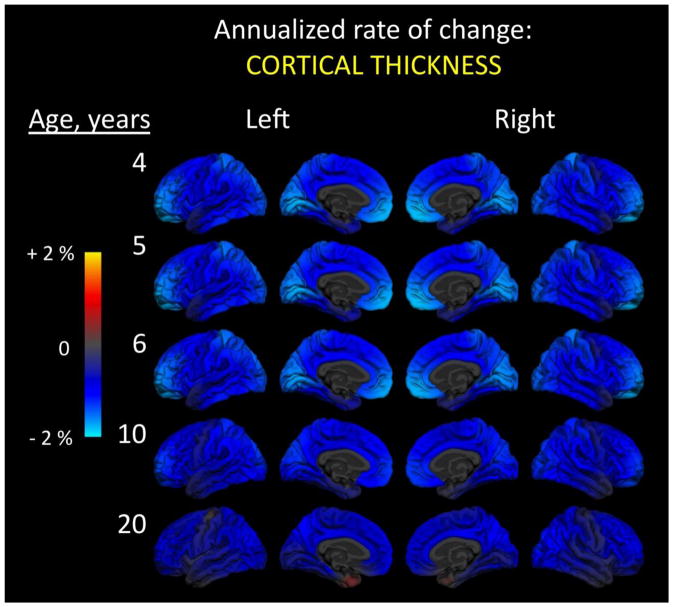
In stark contrast to cortical area, cortical thickness shows no developmental increases during the preschool period and, in fact, decreases throughout the cortex all the way into young adulthood (Figure 2). Between the ages of 4 and 6, cortical thickness decreases the most within medial and polar occipital and prefrontal regions, as well as within parietal cortex, declining by about two percent each year. At these ages, the remainder of the cortical surface shows an annual decrease in thickness of about one percent, which continues all the way to age 20 (and likely beyond). Although many previous imaging studies have characterized developmental changes in the volume of cortical gray matter (Caviness et al., 1996; Courchesne et al., 2000; Giedd et al., 1996a; Jernigan and Tallal, 1990; Jernigan et al., 1991; Sullivan et al., 2011), the results shown here demonstrate how it is informative to deconstruct volume into thickness and area, since they have quite different developmental trajectories overall and within different cortical regions (see also (Sowell et al., 2004). These findings are consistent with what is known about the separate neurobiological origins of cortical area and thickness, as well as recent evidence for their distinct genetic influences (Panizzon et al., 2009). For all of these reasons, cortical area and thickness may also relate to cognitive and behavioral development in different ways.
Figure 2.
Annualized rate of change in cortical thickness. At every vertex across the reconstructed cortical surface, age-dependent annualized rate of change is shown at five different ages. Left and right hemisphere lateral (outside) and medial (inside) surfaces are shown. Color scale ranges from two percent increases (yellow) to two percent decreases (cyan) in thickness. Change was calculated using 202 subjects (102 male, 100 female): 26 four-year-olds (12 males, 14 females); 20 five-year-olds (11 males, 9 females); 37 six-year-olds (20 males, 17 females); 48 10-year-olds (26 males, 22 females); and 71 20-year-olds (33 males, 38 females). Data come from the Pediatric Imaging, Neurocognition, and Genetics (PING) Study, adapted from Brown et al., 2012.
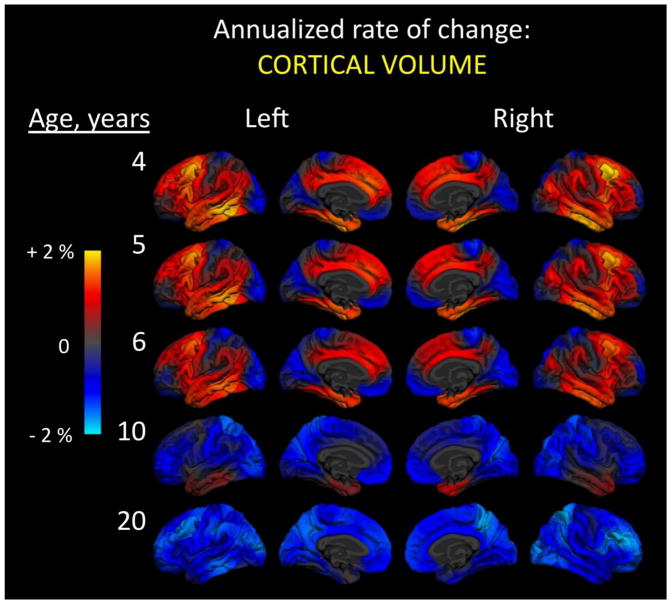
Annualized changes in cortical volume during the preschool years represent development of the product of surface area and thickness, and these reveal striking regional variation even within the same age (Figure 3). At age four, for example, some regions of the cortex are notably decreasing in volume while others are strongly increasing. Here, the greatest differences are evident within most of the occipital lobe, primary somatosensory areas of the parietal lobe, and medial and lateral aspects of the frontal pole, all of which show declining volume in contrast to increasing volume within temporal and frontal lobes, particularly inferior temporal regions and dorsal motor and premotor portions of frontal cortex. Interestingly, the anterior temporal lobes bilaterally are some of the last cortical regions to shift from increasing to decreasing volume with maturation. Consistent with the theme of dramatic architectural “blossoming” in the brain within the preschool years, changes in cortical volume show an early period of striking, widespread expansion that eventually gives way to selective reductions across the cortex by around the ages of puberty.
Figure 3.
Annualized rate of change in cortical volume. At every vertex across the reconstructed cortical surface, age-dependent annualized rate of change is shown at five different ages. Left and right hemisphere lateral (outside) and medial (inside) surfaces are shown. Color scale ranges from two percent increases (yellow) to two percent decreases (cyan) in volume. Change was calculated using 202 subjects (102 male, 100 female): 26 four-year-olds (12 males, 14 females); 20 five-year-olds (11 males, 9 females); 37 six-year-olds (20 males, 17 females); 48 10-year-olds (26 males, 22 females); and 71 20-year-olds (33 males, 38 females). Data come from the Pediatric Imaging, Neurocognition, and Genetics (PING) Study, adapted from Brown et al., 2012.
Functional Brain Development
The complex cascade of neuroanatomical changes in the preschool years is paralleled by concomitant changes in the dynamic physiological activity of the brain. Along with advances in structural imaging have come new tools and strategies for measuring brain activity in young children noninvasively. Using a variety of functional brain imaging and recording technologies, some in use for decades and some very new, we are still learning much about the growing, working brain during the preschool years. In this section, we present a very selective review of studies of functional brain development, introducing the reader to several of the primary methods that have been used most successfully for studying the working brain during the early childhood years.
Positron emission tomography
Positron emission tomography (PET) is an imaging modality that is used to measure chemical and physiological activity in a variety of body organs and has been applied to the study of child brain function down through neonatal ages (Phelps and Mazziotta, 1985). This technique uses radiotracers injected into the bloodstream that contain positron-emitting isotopes, which can be detected with a ring of sensors and used to quantify blood flow or metabolic rates for specific elements or compounds associated with localized changes in brain activity. PET can also be used to track the synthesis of specific proteins or the uptake and binding of neurotransmitters. Since glucose and oxygen are fundamental to meeting the energy demands of the brain, many PET studies of early development have measured age changes and differences in these substrates. Local cerebral metabolic rates for glucose (lCMRGlc) undergo dramatic maturational changes in most parts of the brain, particularly in the cerebral cortex, and these changes continue over a protracted period (Chugani and Phelps, 1991; Chugani et al., 1987). At birth, whole brain glucose metabolism rates are about 30% lower than adult rates but rapidly increase to adult levels by about the second year of life. These increases in lCMRGlc continue through the preschool ages, exceed adult levels by about the age of three, and plateau from about four to nine years old. At their peak, glucose metabolic rates are highest within the cerebral cortex, where they are twice the value of adult rates. In phylogenetically older structures, such as the brainstem and cerebellum, lCMRGlc does not exceed adult values and appears to be relatively metabolically mature at birth. Other subcortical structures, such as the thalamus and basal ganglia, show intermediate increases in glucose metabolic rates over adult values, suggesting a hierarchical ordering of energy demands. This hierarchy, shifting from phylogenetically older to newer structures, corresponds to early aspects of behavioral development, which is dominated at birth by primitive reflexes and gradually shows the exertion of greater cortical control. At around the ages of eight to 10, lCMRGlc begins to decline and comes to resemble adult levels by about 16 to 18 years of age.
The maturational curve shown by changing rates of glucose (and oxygen) metabolism in the brain is postulated to be directly linked to a similar trajectory for specific neuroanatomical events. As detailed above, structural brain development is characterized by periods of both progressive overproduction and subsequent regressive elimination. These processes, which are integral to the functional specialization of neocortical areas, involve components of individual neurons, such as their dendritic arborization and synaptic contact patterns, and affect multi-cell circuits and systems of neuronal networks. Based on the developmental trajectories of synaptic proliferation and elimination, as well as on clinical observations of behavioral plasticity in children with brain damage, Chugani and colleagues have proposed that early increasing lCMRGlc rates are directly related to the period of rapid overproduction of synapses and nerve terminals thought to occur within a similar timeframe. The plateau period during which glucose metabolic rates far exceed adult levels is thought to be caused by the transient increased cerebral energy demand from this overly elaborated connectivity. Likewise, the developmental decline in metabolic rates is hypothesized to correspond to the later period of selective elimination (i.e., activity-dependent “withering”) of many of these connections, marking a time when plasticity seems to be notably diminished. Chugani and colleagues have tested and found support for these hypotheses in developmental studies with nonhuman animals (Chugani et al., 1991).
Electroencephalography
Electroencephalography (EEG) methods have been used with humans for nearly 100 years and have been applied to the study of normal childhood brain activity for several decades (Nelson and McCleery, 2008). EEG, requiring the placement of scalp electrodes, offers particularly sensitive measures of the timing aspects of brain activity since it records with millisecond temporal resolution the electric potentials generated by neurons. Scalp-recorded EEG activity is thought to reflect the intermittent synchronization of extracellular current flows within small populations of neurons predominantly on the gyral surfaces of the cortex (Nunez, 1981). Despite its excellent temporal sensitivity, EEG cannot precisely localize activity to its cerebral sources because of inherent biophysical limitations; electric potentials are smeared, distorted, and deflected in difficult-to-model ways as they conduct through different tissue types (e.g., brain, dura, skull, scalp; (Cuffin and Cohen, 1979). So, by the time these signals reach electrodes at the scalp, it is difficult to determine exactly where they originated (Pascual-Marqui and Biscay-Lirio, 1993).
Research on developmental differences in resting EEG coherence, a measure of the degree of correlation and synchronization across different electrodes, provides fundamental information about the neurophysiological dynamics of the maturing brain. In a series of studies involving up to more than 500 children and adolescents, Thatcher and colleagues identified several major cycles and nested “microcycles” of resting network coherence across the ages of about 6 months and 16 years, separated by phase transitions (Thatcher, 1992; Thatcher et al., 1987). Over this age span, quantitative age differences were identified across three major axes of developmental change representing anterior-posterior, lateral-medial, and left-right gradients. Up until around 1.5 years old, growth spurts in coherence were mostly localized to immediately neighboring electrodes and occurred most prominently within the left hemisphere. At about 2.5 years of age, coherence was evident across greater distances, especially along the anterior-posterior dimension involving sensory areas and frontal regions. At age three, there was further lengthening of coherence distances anterior-to-posterior, with significant coupling of dorsal mediofrontal cortex to posterior regions and lengthening coherence distances in the lateral-medial direction for temporal and parietal lobes. Interestingly, in two cycles occurring at about 9 years old and again at around age 14, the right hemisphere showed anterior-posterior coherence “contractions”, changing this time from longer to shorter distances. Thatcher has suggested that the early left hemisphere expanding sequence reflects a process of functional integration of differentiated subsystems, whereas the later coherence contraction in the right hemisphere relates to processes of functional differentiation of previously integrated subsystems. Despite these observed hemispheric differences in the developing coherence patterns, complex systems analysis shows that in general there is significantly increasing dimensional complexity and nonlinearity in the resting EEG signal between infancy and school-age (Meyer-Lindenberg, 1996).
One advantage of EEG is that the data can be analyzed in continuous fashion, extracting effects within the frequency domain as discussed for coherence, or it can be averaged in relation to the repeated presentation of some time-locked stimulus of interest, referred to as evoked or event-related potentials (ERP). ERP approaches allow for stronger inferences to be made about specific mental operations by relying on more heavily constrained experimental and behavioral circumstances. Different sensory, perceptual, and cognitive processes produce unique ERP components, which are traditionally labeled according to the polarity (positive or negative) and timing of their peaks in relation to the stimulus (Polich, 1993). For example, the N170 is a negative-going peak typically observed at around 170 milliseconds (in adults) during the processing of faces. The P600 is a positive polarity, language-related deflection that occurs at about 600 milliseconds. Typically, ERP components are examined for changes in amplitude, latency, and scalp topography in relation to the manipulation of sensory, cognitive, or subject factors of interest such as clinical group or age.
ERP techniques have been used to examine many aspects of information processing during the preschool and school-age years, including attention, memory, language, visuospatial cognition, and learning, primarily using visual and auditory stimuli (Nelson and McCleery, 2008). In general, preschool children show differences in components characterized by longer latencies (i.e., slower timing of peaks in relation to stimuli) as compared to older children and young adults, and they may show larger or smaller activity amplitudes depending on the specific component. Developmental differences are not evident for all components, especially early sensory (sometimes called exogenous) components, but are common and particularly pronounced for middle and late latency waves tied to perceptual and cognitive (or endogenous) processing. For example, the mismatch negativity (MMN) is a negative-going, sensory wave evident at about 175 ms after the presentation of a rare or novel auditory stimulus, most prominent in centrofrontal electrodes. This component is present from birth through adulthood, is robust to attentional effects (i.e., shows little differences among being attended to, ignored, or doing another task), and shows few measurable changes from the preschool years into adulthood (Lyytinen et al., 1992; Naatanen and Alho, 1995a, 1995b). As an early established and fundamental sensory response, the MMN is thought to reflect an automatic, obligatory mechanism that compares current auditory inputs to recent traces of previous signals received through the same modality (Winkler et al., 1996).
In contrast to this developmentally stable auditory component, the visual N170 shows significant changes over maturation. This component, elicited by faces and face-like stimuli, is most prominent at occipito-parietal electrodes, begins to emerge during the preschool years by about age four (Nelson and McCleery, 2008), and reaches an adult-like amplitude and latency in late adolescence (Taylor et al., 2004). The large amplitude of the N170 in response to faces compared to other objects is believed to reflect the recruitment of a collection of specialized mechanisms for identifying and distinguishing faces from one another, tuned from extensive long-term visual experience (Rossion et al., 2004).
The P300 (or P3) is the quintessential “cognitive” ERP component, being the first such wave discovered to relate not directly to the physical attributes of stimuli, but instead to a person’s evaluation of them within the context of a task. More specifically, the P300 is thought to reflect active processes involved in stimulus categorization, novelty detection, and the updating of working memory (Chapman and Bragdon, 1964; Dien et al., 2003; Polich, 2007; Sutton et al., 1965; Sutton et al., 1967). The P300 can be elicited by stimuli delivered through the visual, auditory, somatosensory (Yamaguchi and Knight, 1991), or even olfactory (Geisler and Murphy, 2000) modalities, usually presented as a sequence of discrete, frequently occurring events (e.g., tones, pictures, scents) with rarer and sporadically interspersed “oddball” stimuli that are manipulated by the experimenter to differ from these to varying degrees. Early P3 studies suggested a topography with generators within the parietal lobes, but subsequent research has identified two separable subcomponents: the earlier P3a, which peaks at around 250–280 ms near frontal and central electrodes and the more posterior classic P3b, which peaks just after, around 250–500 ms. The amplitude and latency of the P300 in adults has been shown to relate to a number of factors. Particularly, the latency of the peak increases (i.e., the response is slower) and the amplitude of the peak decreases as the difficulty of discriminating the frequent and infrequent stimuli increases (McCarthy and Donchin, 1981).
During the preschool and kindergarten years, the timing of the P300 is significantly slower, peaking on average around 700 ms and ranging between about 600 and 900 ms (Courchesne, 1977). Both children and young adults show greater P3 amplitudes to target, attended stimuli relative to non-target, unattended stimuli, and their topographical organizations are qualitatively similar in distribution across posterior electrodes. For unattended novel stimuli, however, the scalp distribution changes with age. In young children, sources are parietal, similar to target P3 waves, but in adults the P3 to novel stimuli appears more anteriorly, believed to reflect the developmental emergence of a new frontal generator related to the processing of novel events (Courchesne, 1978). The transition to adult-like forms of the P3a and P3b components appears to occur between the ages of about 12 to the mid-teens (Fuchigami et al., 1995; Pearce et al., 1989).
Functional magnetic resonance imaging
Since the earliest experiments with children in the mid 1990’s, functional magnetic resonance imaging (fMRI) has become the mainstay of studies of localized brain activity in typical development (Casey et al., 1995; Casey et al., 1997b; Casey et al., 1997c; Hertz-Pannier et al., 1997); for review, see (Casey et al., 2005). This has been due in large part to the wide availability of MRI scanners and to the fact that, unlike its predecessor and fellow tomographic (slice-based) imaging method PET, fMRI requires no intravenous injection and no exposure to ionizing radiation. In contrast to EEG, fMRI techniques allow for the precise spatial localization of brain activity. However, because it uses a sluggish vascular response as a proxy for neural activity, fMRI cannot measure real-time temporal dynamics (Bandettini et al., 1993; Belliveau, 1990; Belliveau et al., 1991; DeYoe et al., 1994). While neuronal action potentials and current flows vary on the order of milliseconds, fMRI relies on a regionally correlated blood oxygenation level-dependent (BOLD) response that fluctuates over the course of seconds and adds a level of interpretation that is required for making inferences about brain activity (Devor et al., 2005; Logothetis and Wandell, 2004).
Despite an increasing amount of published work on infants, particularly on language development (Dehaene-Lambertz et al., 2002; Friederici, 2006), there are still relatively few fMRI studies that have focused specifically on typical development within the preschool years, similar to the paucity of studies in the structural MRI literature. Research on school-age children, in contrast, is rather extensive, likely because of age differences in the practical feasibility of scanning (i.e., beginning at about the age of seven, children are noticeably better able to lie still within the scanner and comply with task instructions over a prolonged period). School-age studies have looked at developmental changes in attention (Vaidya et al., 2011; Velanova et al., 2008; Wendelken et al., 2011a), memory (Ghetti et al., 2010; Nelson et al., 2000; Thomas et al., 2004), reading, language, and semantics (Booth et al., 1999; Brown et al., 2005; Chou et al., 2006; Gaillard et al., 2000; Holland et al., 2007; Schlaggar et al., 2002; Turkeltaub et al., 2003), executive functions (Bunge et al., 2002; Dumontheil and Klingberg, 2011; Somerville et al., 2010; Wendelken et al., 2011b), and even social, moral, and emotional cognition (Decety et al., 2011; McRae et al., 2012; Pfeifer et al., 2009).
It is somewhat difficult and scientifically risky to try to extract broad developmental characterizations across experiments that use such a wide array of behavioral tasks, imaging methods, conceptual approaches, and age ranges. Nevertheless, it is fair to say that many fMRI studies have found, in comparison with adolescents and young adults performing the same tasks, that young children tend to recruit a larger number and greater spatial extent of active brain regions, that these cross-sectional differences and longitudinal changes include areas of both relatively higher and lower activity amplitudes in children, and that young children often show significantly greater activity within primary and secondary modality-specific sensoriperceptual cortical areas than older participants (Booth et al., 2004; Booth et al., 2003; Brown et al., 2005; Casey et al., 1995; Casey et al., 1997a; Durston et al., 2006; Gaillard et al., 2000; Gaillard et al., 2003; Schlaggar et al., 2002). However, in using fMRI to make inferences about developmental changes in cerebral functional organization, it is as critical as it is difficult to control certain factors that are known to be confounded with age and, therefore, to affect functional image properties, statistical parametric maps, and their interpretations. These factors relate to subject head motion, statistical issues, task compliance, performance accuracies and response times, experimental designs and task assumptions, head size and image registration methods, and imaging data analysis strategies (Brown et al., 2003, 2006; Burgund et al., 2002; Church et al., 2010; Cohen and DuBois, 1999; Crone et al., 2010; Fair et al., 2006; Kang et al., 2003; Kotsoni et al., 2006; Murphy and Garavan, 2004a, 2004b; Palmer et al., 2004; Poldrack, 2010). So, studies where the strongest inferences can be made about developmental brain changes usually include experimentally constraining features such as overt task performance measures, modeling of hemodynamic timecourses, accounting for performance-related effects on brain activity, correction for multiple statistical tests, a priori selection of regions of interest, validation of a common stereotactic space across the ages under study, and direct voxel-wise statistical comparisons between groups.
Recent event-related fMRI studies of preschool-age children have added to our understanding of the neural underpinnings of several important emerging behaviors at these ages. Using an imaging-compatible version of Piaget’s classic number conservation task, for example, Houde et al. have shown a correlation between the preschool-age behavioral shift from using a visuospatial intuitive task approach to the successful conservation of number, implicating a bilateral network of parietal and frontal brain regions that likely support emerging number concepts and executive functions (Houde et al., 2011). Another important set of milestones occurring from toddlerhood to preschool involves the rapid acceleration of spoken language production and comprehension abilities. Using fMRI, Redcay and colleagues compared toddlers (mean age: 21 months) with preschoolers (mean age: 39 months) during the presentation of forward and backward spoken auditory passages during natural sleep. Interestingly, the younger group showed relatively greater activity for forward speech than the 3-year-olds in frontal, occipital, and cerebellar brain regions. The preschoolers, on the other hand, showed comparatively greater activity than the younger subjects in superior temporal regions typically involved in receptive speech in adults. This finding of precocial frontal lobe involvement in the earlier organization for language is consistent with certain models of early functional brain development, particularly ones that predict the involvement of higher level (and attention-related) regions at younger ages required as “scaffolding” while forming the mature organization (see (Johnson, 2000, 2011; McClelland et al., 2010; Plunkett et al., 1997; Quartz and Sejnowski, 1997).
One intriguing new methodological aspect of this study was its use of the passive presentation of stimuli during sleep. From a practical perspective, this novel approach seems promising for dealing with some of the behavioral difficulties of scanning toddlers and preschoolers, potentially (though not necessarily) reducing head motion and altogether avoiding the typically cumbersome task compliance and performance issues since no responses are required. At the same time, in completely passive and unconstrained behavioral circumstances with no objective response measures of information processing, it is not possible to determine how these resting brain differences might relate to developing cognitive operations. Also, known differences in thalamic and cortical tone, inhibition, and synchronization during sleep raise the question as to whether these differences in brain activity are solely attributable to the stimuli that were presented and whether they would generalize to the waking state (Davis et al., 2004; Hobson and McCarley, 1971; Steriade et al., 2001).
Relatedly, researchers using fMRI have recently developed techniques to look at network properties of the brain that express themselves as slowly modulating inter-regional hemodynamic activity correlations measured at waking rest, in the absence of the presentation of any time-locked stimuli. Over about the past five years, resting state functional connectivity MRI (rs-fcMRI), as it is called, has come to play a prominent role in fMRI research on both adults and children (Biswal et al., 2010; Snyder and Raichle, 2012). Resting functional connectivity studies of young children, although again not commonly measured with preschool-age participants, have nevertheless produced interesting developmental findings comparing school-age children to adolescents and young adults. These results have been broadly consistent with at least some of the changing activity correlation patterns found in resting EEG coherence studies, but they also include aspects that seem both consistent and inconsistent with common developmental findings reported in PET and event-related fMRI studies. For example, from about seven to 30 years old, distributed, linked networks of brain activity known to be important in adult attentional control show both “integrated” (added) and “segregated” (lost) network nodes with increasing age (Fair et al., 2007). Across the same age range, this study and several others have suggested that, in general, linked resting functional networks shift from a predominantly “local” organization in young children to a more “distributed” architecture in young adults (Fair et al., 2009), with greater overall connectivity at older ages (Fair et al., 2010) and significant weakening of short-range and strengthening of long-range functional connections with development (Dosenbach et al., 2010; Power et al., 2010). Based on several published studies, other rs-fcMRI experiments have replicated these connectional differences going from short- to long-range with age and also have concluded that resting inter-regional activity correlations undergo a developmental shift from “diffuse to focal activation patterns” (Supekar et al., 2010; Uddin et al., 2010b; Uddin et al., 2011).
Some of the primary developmental characterizations across resting state fMRI studies appear to be contradictory. For example, how exactly does the developing cerebral functional organization change from being both “diffuse” and “local” in childhood to both “focal” and “distributed” at older ages? This apparent discrepancy may simply reflect an imprecision in the descriptive terminology that has been adopted, or it may relate more directly to the results themselves, but some reconciliation seems warranted. This inconsistency is strongly reminiscent of a similar issue in event-related fMRI studies of development, where qualitative, side-by-side characterizations of group statistical maps (instead of direct, voxel-wise comparisons) have led to similar contradictions in the results and interpretations (for discussion of the use of the term “diffuse”, for example, see (Brown et al., 2003, 2006).
As a separate but equally important issue, recently it has been discovered that head motion may cause particularly insidious artifacts in resting state connectivity studies (Van Dijk et al., 2011) and that systematic differences in head motion from early childhood into young adulthood may, in fact, underlie some of the major developmental effects that have been reported and replicated (Carp, 2012; Power et al., 2011, 2012). These methodological studies have shown that in both adults and children, the degree of head motion that is present in resting activity correlation maps can drive effects that mimic some of the main developmental findings: When subjects move more during scanning, they show stronger short-range connections (the “immature” pattern), and those that move less show stronger long-range connections (the “mature” pattern). Although rs-fcMRI measures have demonstrated reasonably good test-retest reliability (Shehzad et al., 2009), this fact does not resolve the developmental issue; age-related artifacts themselves may, in fact, replicate robustly across different studies, scanners, and methods. Head motion in particular is well known to be problematic in developmental structural and functional neuroimaging, and even children aged nine and older commonly show more than a centimeter of translation and 15 degrees of rotation, significantly affecting quantitative and qualitative structural imaging measurements (Brown et al., 2010; Kuperman et al., 2011). This magnitude of head motion is much larger than what is suspected to cause artifacts in resting state results, which can be seen in individual subjects with less than 1 mm root mean square of motion (Power et al., 2011). Speculatively, rs-fcMRI might be especially sensitive to motion artifacts because the low frequency range within which resting inter-regional BOLD correlations are measured may strongly overlap with the range within which subjects tend to move continuously inside the scanner (i.e., < 0.1 Hz). Regardless, if head motion is sufficiently tied to subject age, spurious developmental effects could be independently replicated time and again.
A rigorous characterization of the nature and scope of this problem is especially important since resting state functional connectivity methods are already being applied to a wide range of child and adult clinical groups. These include studies of autism (Di Martino et al., 2011; Lee et al., 2009), attention disorders (Mennes et al., 2011), schizophrenia (Alonso-Solis et al., 2012; White et al., 2010b), early deprivation (Behen et al., 2009), childhood epilepsy (Mankinen et al., 2012), fetal alcohol spectrum disorders (Wozniak et al., 2011), pre-term and low birth weight children (Gozzo et al., 2009), pediatric Tourette syndrome (Church et al., 2009), and childhood depression and bipolar disorder (Cullen et al., 2009; Dickstein et al., 2010). If children and adults with clinical problems tend to have more trouble holding still in the scanner, as one might easily suspect for many of these diagnostic categories, they may spuriously appear to have more “immature” patterns of functional connectivity. As is always the case with promising new scientific techniques, researchers within the field will need to resolve these issues, and the devil will be in the technical details. Hopefully, enthusiasm for reconciling inconsistencies and for continuing methodological validation will match the vigor with which initial rs-fcMRI techniques have been applied.
Magnetoencephalography
Magnetoencephalography (MEG) is a neurophysiological technique similar to EEG in that it measures neural activity directly with millisecond temporal resolution. As opposed to electric potentials, however, the Dewar of MEG sensors detects fluctuations in the magnetic fields that are induced by neuronal current flows (Cohen and Cuffin, 1983). Because magnetic fields pass through biological tissues with essentially no perturbation as they emanate from the brain (Hämäläinen et al., 1993), this biophysical characteristic produces a relatively straightforward spatial relationship between activity sources and the sensors, allowing for much more precise localization of brain activity than EEG (Cohen and Cuffin, 1991; Cohen et al., 1990). However, unlike PET and fMRI, which are tomographic or slice-based approaches, MEG is still like EEG in that it relies on making inferences about what’s going on inside the head using measurements made at the scalp. So, MEG activity maps still depend on the modeling assumptions that are made about the number and complexity of the activity sources. Nevertheless, noise-normalized, anatomically constrained statistical parametric maps of MEG-derived brain activity show strong spatial correspondence with recordings from intracranial EEG for a variety of stimulus types and sensory and cognitive components (Halgren, 2004; Halgren et al., 1994).
Although the first magnetometer was developed in the late 1960’s (Cohen, 1968, 1972), high-density, whole-head MEG recording devices have only been available relatively recently as technologies related to electronic circuits and superconducting quantum interference devices (SQuIDs) have advanced. While MEG is still emerging as a tool for studying child development, it shows great promise for expanding our understanding of maturational changes in the real-time spatiotemporal dynamics of brain activity. In an impressive recent MEG study of 78 right-handed children, Kikuchi and colleagues demonstrated a clear link between left-lateralized functional activity coherence and behavioral performance on language tasks among (primarily) 3- to 4-year-old preschoolers (Kikuchi et al., 2011). During the presentation of audio stories with moving images, children showed prominent theta band activity (6–8 Hz) within parieto-temporal regions of the left hemisphere that was strongly correlated with higher behavioral performance on a number of language tasks administered outside the scanning environment, including subtests of the Kaufman Assessment Battery for Children. This synchronized activity was not related to nonverbal cognitive performance, head circumference, or chronological age. Other recent studies using MEG with preschool-age children have demonstrated changes in auditory cortical-evoked fields after musical training (Fujioka et al., 2006), age-related decreases in the latency of the MMN response of about one millisecond per month (Morr et al., 2002), localization of the temporal lobe systems involved in speech sound discrimination (Pihko et al., 2005), and developmental increases between the ages of 7 and 16 years in the lateralization of activity for verb generation and vowel identification tasks (Ressel et al., 2008). As is apparent from this work and from a growing body of infant studies (Cheour et al., 2004; Imada et al., 2006; Travis et al., 2011), MEG is being applied with particular enthusiasm to address questions about early language development. With increasing availability and with conceptual and methodological advances for use with younger populations, we expect MEG to become an important new tool for improving our understanding of the active preschool brain.
Near-infrared spectroscopy
Near-infrared spectroscopy (NIRS) is an optical imaging method that was originally developed as a clinical tool for monitoring tissue oxygenation (Jobsis, 1977). In recent years, its use has expanded into the realm of cognitive physiological brain imaging, often called functional NIRS or optical tomography (Hoshi, 2003). NIRS is noninvasive and requires the placement of electrode-like sensors onto the scalp, called optodes. Coupled with a light source that projects into the scalp, optodes measure changes in the transmitted intensity of light within the near-infrared spectral band. Typically measured on the order of seconds or tenths of seconds depending on the camera system used, these signals reflect the state of hemoglobin oxygenation within a banana-shaped section of the brain arcing beneath the illuminator and detector, tied to regional levels of cerebral blood flow and cortical activity. In comparison with other functional brain imaging tools for studying child development, NIRS has several potentially useful advantages. First, the recording systems are quite portable and allow for subjects to move relatively freely during data collection. Also, they operate quietly and require very little setup and calibration time. These factors alone set NIRS apart from most other brain activity recording techniques and give it particular appeal for research with infants and young children. Nevertheless, several limitations exist. One of the most persistent and consequential problems in the scientific application of NIRS has been difficulty in developing standard source models that allow for the quantification (and therefore direct comparison and interpretation) of signals across different subjects and even across different brain regions within the same subject. Also, it is impossible to know exactly which brain regions are being recorded from with a given optode array, and idiosyncratic factors such as skull and skin thickness and even skin color may affect recordings (Hoshi, 2003).
These drawbacks may be currently limiting the widespread application of NIRS to developmental cognitive neuroscience but enthusiasm remains about its potential, and several studies of young children demonstrate its capabilities. One recent NIRS experiment compared 48 typically developing children from preschool-age into adolescence with 22 young adults during the performance of a letter fluency task (Kawakubo et al., 2011). Focusing on developmental differences in frontal regions, Kawakubo and colleagues found significant increases in oxygenated hemoglobin within polar prefrontal regions, correlating strongly with age between 4 and 18 years old but not among the older, young adult subjects. Remjin et al. compared 19 preschoolers and 19 young adults during the presentation of static pictures and video stimuli, measuring from striate, left and right middle temporal, and left and right temporo-parietal areas (Remijn et al., 2011). Preschool participants showed significantly greater increases in oxy-hemoglobin over all regions of interest in response to visual motion stimuli. Static visual stimuli also caused a significant oxy-Hb increase over striate and left middle temporal areas that was larger in children than in young adults.
Multimodal and multidimensional developmental imaging
At this point, it should be abundantly clear that there is a diverse and growing collection of scientific tools available for noninvasively studying human brain maturation and relating it to cognitive and behavioral growth. Using these technologies, substantial progress has been made in characterizing structural and functional brain development during the preschool years and comparing it with other phases of maturity. Despite significant advances, an obvious characteristic of imaging studies to date is that the vast majority have investigated developmental differences or changes within a single imaging or recording modality, comparing brain features only within structural MRI, EEG, fMRI, DTI, or MEG. In order to understand the complex interplay of anatomical and physiological growth, and to better comprehend the biological significance of our imaging measures, it will be necessary to study brain changes using integrated multimodal approaches that relate different kinds of measures to one another. Done rigorously, these kinds of studies typically require more than just the addition of more variables to statistical models; the accurate spatial and temporal interrelation of multiple structural and functional brain measures often demands collaborative expertise in signal processing, biophysics, computational neuroscience, multivariate statistics, and mathematical modeling, as well as behavioral science. Multimodal imaging approaches over the past two decades have successfully integrated EEG and MEG data with structural MRI data (Dale and Sereno, 1993), MRI and MEG with fMRI data (Dale and Halgren, 2001; Dale et al., 2000), PET and fMRI (Gerstl et al., 2008), EEG and fMRI (De Martino et al., 2010; Oun et al., 2009), fMRI, MEG, and intracranial EEG (McDonald et al., 2010), resting state fMRI with DTI tractography (Uddin et al., 2010a), and both resting fMRI and DTI with voxel-based morphometry (Supekar et al., 2010). In addition to relating different kinds of measures, a major goal in integrating approaches is to capitalize on the relative strengths and neutralize the relative weaknesses of each modality, for example combining the superior spatial resolution of fMRI with the millisecond-wise temporal resolution of MEG, creating fMEG (Dale and Halgren, 2001). Although usually applied first to studies of adults, integrated forms of multimodal structural and functional neuroimaging constitute an exciting prospect for future studies of child development.
A separate but related collection of emerging advances involves the ability to model simultaneously the developmental change of a large number of biological or behavioral variables and relate them to each other in interpretable ways. Just as often as published papers on brain development use only measures from one type of imaging, they also commonly only characterize brain features and maturational trajectories in isolation, as a list of separate, univariate dimensions along which development proceeds. In developmental research, it remains a critical challenge to characterize the multidimensional nature of brain development in a way that accurately conveys complex relations among many variables. Capitalizing on key advances in multisite, multimodal MRI in our own work with the PING study, and using a regularized nonlinear modeling and cross-validation method, we recently developed an approach that quantifies the age-varying contributions of different biological change measures to the prediction of age, within a multidimensional space. Using this technique, different components of the developing anatomy and physiology can be quantified and directly compared, showing their relative roles in the dynamic cascade of changing brain characteristics. We found that the composite developmental phase of the brain for an individual can be captured with much greater precision than has been possible using other biological measures or approaches. Using a multidimensional set of 231 brain biomarkers assessed in 885 subjects between the ages of 3 to 20 years old, we were able to predict the age of every individual within about one year on average (Brown et al., 2012). More than just an age-predicting “carnival trick” of sorts, this composite developmental phase metric based on brain morphology, diffusivity, and signal intensity addresses a longstanding question about the degree of biological variability that exists among children of the same age. It reveals that there exists within the brain a latent phenotype for which the timing of maturation is more tightly controlled and more closely linked to chronological age than was previously known. This multidimensional biological signal cuts through the many other differences among young individuals and explains more than 92% of the variance in chronological age.
Our results also show how the neuroanatomical features that contribute most strongly to predicting age change over the course of development. Interestingly, from the preschool years until about the age of 11, changes in normalized MR signal intensities within subcortical regions, including gray matter, explained the most variance in age. From the ages of about 11 to 15, changes in the diffusivity (such as FA) of white matter tracts was the strongest age predictor, while volumetric measures of subcortical structures explained the most variance in age from about the age of 15 to 17. Surprisingly, since many researchers aren’t measuring diffusion within these structures, diffusivity within subcortical regions, including gray matter, was the strongest contributor to the prediction of age between 17 and 20 years old. Future applications of this flexible, new approach will examine whether cognitive, behavioral, and clinical variables can also be reliably predicted using multidimensional metrics of developmental brain phase.
Summary and Conclusions
In surveying current scientific knowledge about brain development during the preschool years, several recurring themes become apparent. First, during these ages, the brain shows some of its largest annualized changes in both its anatomical and physiological characteristics. Structural growth is accompanied by significant morphological changes with increases in cortical area, decreases in cortical thickness, and changing cortical volume that varies widely by region, as well as nonmonotonic increases in the volumes of cerebral subcortical structures, deep nuclei, and the cerebellum. Gray and white matter tissue properties also exhibit pronounced changes, for example in the form of decreasing diffusivity and increasing diffusion anisotropy in major cerebral fiber tracts. Functional changes include equally dramatic increasing metabolic demands and region- and hemisphere-specific changes in inter-regional activity correlations that both add and subtract network nodes with development. Although varying heavily according to the specific task at hand, there commonly appears to be a greater number of brain regions required at younger ages than at older ages for successful completion of the same cognitive task, suggesting the existence of “scaffolding” mechanisms in the early cerebral functional organization that are eliminated with increasing age. Anatomical and physiological development from infancy into young adulthood reflects a complex cascade of cyclical progressive (additive) and regressive (subtractive) types of changes. However, the preschool years can be thought of as a developmental period generally dominated by dynamic and robust progressive processes, with an emphasis on growth, expansion, “construction”, and “blossoming” that will later be pruned and tuned with continued maturation and experience.
Noninvasive structural and functional neuroimaging methods have revolutionized the way we study the human brain and have provided a wealth of new information about its typical development. Nevertheless, the immediacy of brain images can cause us to forget that there exist levels of interpretation between imaging results and their neuroanatomical and neurophysiological bases. Despite great advances in knowledge made possible by these techniques, many of the specific biological processes that produce the effects we measure with imaging remain poorly understood. Within developmental functional imaging, and fMRI in particular, results can be driven by technical and methodological factors that affect what are inherently dynamic and heavily state-dependent activity measures. In order to make sense of a literature that sometimes reports widely varying results and interpretations, or even potentially replicated spurious findings, it is increasingly necessary to have a technical understanding of the methods and to take into account factors such as head motion, experimental design, behavioral performance, task parameters, and statistical approaches. Because of its importance to our collective scientific understanding of the developing brain, we devoted some effort to explaining some of these considerations so that the reader may be an informed consumer moving forward. With so many potentially powerful tools now available for studying the active brain during the preschool years, it is as important as ever to continue our validation and refinement of these exciting techniques.
Finally, across all types of brain imaging and recording methods, the preschool and early childhood years remain relatively undercharacterized as compared to other periods of development. Although certainly improving, the lag in published studies for this age range is especially noticeable as it recently appears to be outpaced by some areas of imaging research on infancy. This is likely driven by the fact that preschoolers represent a particularly challenging group for the practical behavioral demands of successful imaging data collection. Infants can be more readily controlled in most imaging environments, lacking certain abilities for willful defiance and refusal. They can also be counted on to more readily sleep through the procedures. Within the young school-age range, on the other hand, children become imageable for other reasons. Despite limited ability for sustained attentive vigilance, they have at least the rudimentary psychological skills required for understanding and following task instructions for a short period of time. In contrast, the much greater difficulty collecting imaging data with preschool-age children may actually tell us something important about the nature of brain-behavior relationships during these years. It appears that the burgeoning preschool brain must express itself in outward behaviors that are similarly bursting forth, unrestrained, and difficult to contain. All the more reason we need to keep studying this fascinating and important time in child development.
Contributor Information
Timothy T. Brown, Email: ttbrown@ucsd.edu.
Terry L. Jernigan, Email: tjernigan@ucsd.edu.
References
- Allen P, Stephan KE, Mechelli A, Day F, Ward N, Dalton J, Williams SC, McGuire P. Cingulate activity and fronto-temporal connectivity in people with prodromal signs of psychosis. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Solis A, Corripio I, de Castro-Manglano P, Duran-Sindreu S, Garcia-Garcia M, Proal E, Nunez-Marin F, Soutullo C, Alvarez E, Gomez-Anson B, Kelly C, Castellanos FX. Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophrenia Research. 2012;139:13–18. doi: 10.1016/j.schres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magnetic Resonance in Medicine. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ. Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol. 2000;21:1099–1109. [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ. Magnetic resonance techniques in the assessment of myelin and myelination. J Inherit Metab Dis. 2005;28:311–343. doi: 10.1007/s10545-005-5952-z. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behen ME, Muzik O, Saporta AS, Wilson BJ, Pai D, Hua J, Chugani HT. Abnormal fronto-striatal connectivity in children with histories of early deprivation: A diffusion tensor imaging study. Brain Imaging and Behavior. 2009;3:292–297. doi: 10.1007/s11682-009-9071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau JW. PhD Thesis. Harvard University; Cambridge, MA: 1990. Functional NMR Imaging of the Brain. [Google Scholar]
- Belliveau JW, Cohen MS, Weisskoff RM, Buchbinder BR, Rosen BR. Functional studies of the human brain using high-speed magnetic resonance imaging. Journal of Neuroimaging. 1991;1:36–41. doi: 10.1111/jon19911136. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Development of brain mechanisms for processing orthographic and phonologic representations. Journal of Cognitive Neuroscience. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Choy J, Gitelman DR, Parrish TB, Mesulam MM. Modality-specific and -independent developmental differences in the neural substrate for lexical processing. Journal of Neurolinguistics. 2003;16:383–405. [Google Scholar]
- Booth JR, Macwhinney B, Thulborn KR, Sacco K, Voyvodic J, Feldman HM. Functional organization of activation patterns in children: whole brain fMRI imaging during three different cognitive tasks. Progress in Neuropsychopharmacology and Biological Psychiatry. 1999;23:669–682. doi: 10.1016/s0278-5846(99)00025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Kuperman JM, Chung Y, Erhart M, McCabe C, Hagler DJ, Jr, Venkatraman VK, Akshoomoff N, Amaral DG, Bloss CS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kaufmann WE, Kenet T, Kennedy DN, Murray SS, Sowell ER, Jernigan TL, Dale AM. Neuroanatomical assessment of biological maturity. Current Biology. 2012;22:1–6. doi: 10.1016/j.cub.2012.07.002. http://dx.doi.org/10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Kuperman JM, Erhart M, White NS, Roddey JC, Shankaranarayanan A, Han ET, Rettmann D, Dale AM. Prospective motion correction of high-resolution magnetic resonance imaging data in children. Neuroimage. 2010;53:139–145. doi: 10.1016/j.neuroimage.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Brown TT, Petersen SE, Schlaggar BL. Functional neuroimaging approaches to the study of human brain development. Neurophysiology and Neurogenic Speech and Language Disorders. 2003;13:3–10. [Google Scholar]
- Brown TT, Petersen SE, Schlaggar BL. Does human functional brain organization shift from diffuse to focal with development? Developmental Science. 2006;9:9–11. doi: 10.1111/j.1467-7687.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Carp J. Optimizing the order of operations for movement scrubbing: Comment on Power et al. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2011.12.061. [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Gerig G, Piven J. Diffusion tensor imaging: Application to the study of the developing brain. J Am Acad Child Adolesc Psychiatry. 2007;46:213–223. doi: 10.1097/01.chi.0000246064.93200.e8. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Giedd J, Kaysen D, Hertz-Pannier L, Rapoport JL. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, King SW, Franzen PL, Nystrom LE, Badgaiyan RD, Schubert AB, Noll DC. A developmental functional MRI study of cortical activation during a spatial working memory task. Neuroimage. 1997a;5:S69. [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Current Opinion in Neurobiology. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, Kozuch P, Rapoport JL. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Developmental Psychobiology. 1997b;30:61–69. [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Orendi J, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997c;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Meyer J, Makris N, Kennedy DN. MRI-based topographic parcellation of the human neocortex: An anatomically specified method with estimate of reliability. Journal of Cognitive Neuroscience. 1996:8. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Cayre M, Canoll P, Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog Neurobiol. 2009;88:41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RM, Bragdon HR. Evoked Responses to Numerical and NonNumerical Visual Stimuli While Problem Solving. Nature. 1964;203:1155–1157. doi: 10.1038/2031155a0. [DOI] [PubMed] [Google Scholar]
- Cheour M, Imada T, Taulu S, Ahonen A, Salonen J, Kuhl P. Magnetoencephalography is feasible for infant assessment of auditory discrimination. Experimental Neurology. 2004;190(Suppl 1):S44–51. doi: 10.1016/j.expneurol.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Burman DD, Bitan T, Bigio JD, Lu D, Cone NE. Developmental changes in the neural correlates of semantic processing. Neuroimage. 2006;29:1141–1149. doi: 10.1016/j.neuroimage.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Hovda DA, Villablanca JR, Phelps ME, Xu WF. Metabolic maturation of the brain: a study of local cerebral glucose utilization in the developing cat. Journal of Cerebral Blood Flow and Metabolism. 1991;11:35–47. doi: 10.1038/jcbfm.1991.4. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME. Imaging human brain development with positron emission tomography. Journal of Nuclear Medicine. 1991;32:23–26. [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Annals of Neurology. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Church JA, Fair DA, Dosenbach NU, Cohen AL, Miezin FM, Petersen SE, Schlaggar BL. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain. 2009;132:225–238. doi: 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Petersen SE, Schlaggar BL. The “Task B problem” and other considerations in developmental functional neuroimaging. Human Brain Mapping. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. Magnetic field measurements of human alpha rhythm. Science. 1968;161:784–786. doi: 10.1126/science.161.3843.784. [DOI] [PubMed] [Google Scholar]
- Cohen D. Magnetoencephalography: detection of the brain’s electrical activity with a superconducting magnetometer. Science. 1972;175:664–666. doi: 10.1126/science.175.4022.664. [DOI] [PubMed] [Google Scholar]
- Cohen D, Cuffin BN. Demonstration of useful differences between magnetoencephalogram and electroencephalogram. Electroencephalography and Clinical Neurophysiology. 1983;56:38–51. doi: 10.1016/0013-4694(83)90005-6. [DOI] [PubMed] [Google Scholar]
- Cohen D, Cuffin BN. EEG versus MEG localization accuracy: theory and experiment. Brain Topography. 1991;4:95–103. doi: 10.1007/BF01132766. [DOI] [PubMed] [Google Scholar]
- Cohen D, Cuffin BN, Yunokuchi K, Maniewski R, Purcell C, Cosgrove GR, Ives J, Kennedy JG, Schomer DL. MEG versus EEG localization test using implanted sources in the human brain. Annals of Neurology. 1990;28:811–817. doi: 10.1002/ana.410280613. [DOI] [PubMed] [Google Scholar]
- Cohen MS, DuBois RM. Stability, repeatability, and the expression of signal magnitude in functional magnetic resonance imaging. Journal of Magnetic Resonance Imaging. 1999;10:33–40. doi: 10.1002/(sici)1522-2586(199907)10:1<33::aid-jmri5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Event-related brain potentials: comparison between children and adults. Science. 1977;197:589–592. doi: 10.1126/science.877575. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Neurophysiological correlates of cognitive development: changes in long-latency event-related potentials from childhood to adulthood. Electroencephalography and Clinical Neurophysiology. 1978;45:468–482. doi: 10.1016/0013-4694(78)90291-2. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Crone EA, Poldrack RA, Durston S. Challenges and methods in developmental neuroimaging. Human Brain Mapping. 2010;31:835–837. doi: 10.1002/hbm.21053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuffin BN, Cohen D. Comparison of the magnetoencephalogram and electroencephalogram. Electroencephalography and Clinical Neurophysiology. 1979;47:132–146. doi: 10.1016/0013-4694(79)90215-3. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, Camchong J, Bell CJ, Houri A, Kumra S, Lim KO, Castellanos FX, Milham MP. A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Halgren E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. Current Opinion in Neurobiology. 2001;11:202–208. doi: 10.1016/s0959-4388(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Davis KF, Parker KP, Montgomery GL. Sleep in infants and young children: Part one: normal sleep. Journal of Pediatric Health Care. 2004;18:65–71. doi: 10.1016/s0891-5245(03)00149-4. [DOI] [PubMed] [Google Scholar]
- De Martino F, Valente G, de Borst AW, Esposito F, Roebroeck A, Goebel R, Formisano E. Multimodal imaging: an evaluation of univariate and multivariate methods for simultaneous EEG/fMRI. Magnetic Resonance Imaging. 2010;28:1104–1112. doi: 10.1016/j.mri.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Kinzler KD. The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study. Cerebral Cortex. 2011;22:209–220. doi: 10.1093/cercor/bhr111. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Devor A, Ulbert I, Dunn AK, Narayanan SN, Jones SR, Andermann ML, Boas DA, Dale AM. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proceedings of the National Academy of Sciences. 2005;102:3822–3827. doi: 10.1073/pnas.0407789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoe EA, Bandettini P, Neitz J, Miller D, Winans P. Functional magnetic resonance imaging (FMRI) of the human brain. Journal of Neuroscience Methods. 1994;54:171–187. doi: 10.1016/0165-0270(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, Lord C, Castellanos FX, Milham MP. Aberrant striatal functional connectivity in children with autism. Biological Psychiatry. 2011;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Gorrostieta C, Ombao H, Goldberg LD, Brazel AC, Gable CJ, Kelly C, Gee DG, Zuo XN, Castellanos FX, Milham MP. Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biological Psychiatry. 2010;68:839–846. doi: 10.1016/j.biopsych.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Localization of the event-related potential novelty response as defined by principal components analysis. Brain Research: Cognitive Brain Research. 2003;17:637–650. doi: 10.1016/s0926-6410(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Archives of Disease in Childhood. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR, Jr, Barch DM, Petersen SE, Schlaggar BL. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I, Klingberg T. Brain activity during a visuospatial working memory task predicts arithmetical performance 2 years later. Cerebral Cortex. 2011;22:1078–1085. doi: 10.1093/cercor/bhr175. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, Zhang D, Snyder AZ, Raichle ME, Stevens AA, Nigg JT, Nagel BJ. Maturing thalamocortical functional connectivity across development. Frontiers in Systems Neuroscience. 2010;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Brown TT, Petersen SE, Schlaggar BL. A comparison of analysis of variance and correlation methods for investigating cognitive development with functional magnetic resonance imaging. Developmental Neuropsychology. 2006;30:531–546. doi: 10.1207/s15326942dn3001_2. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. The neural basis of language development and its impairment. Neuron. 2006;52:941–952. doi: 10.1016/j.neuron.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Fuchigami T, Okubo O, Ejiri K, Fujita Y, Kohira R, Noguchi Y, Fuchigami S, Hiyoshi K, Nishimura A, Harada K. Developmental changes in P300 wave elicited during two different experimental conditions. Pediatric Neurology. 1995;13:25–28. doi: 10.1016/0887-8994(95)00086-u. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Ross B, Kakigi R, Pantev C, Trainor LJ. One year of musical training affects development of auditory cortical-evoked fields in young children. Brain. 2006;129:2593–2608. doi: 10.1093/brain/awl247. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, Grandin CB. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Human Brain Mapping. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler MW, Murphy C. Event-related brain potentials to attended and ignored olfactory and trigeminal stimuli. International Journal of Psychophysiology. 2000;37:309–315. doi: 10.1016/s0167-8760(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Gerstl F, Windischberger C, Mitterhauser M, Wadsak W, Holik A, Kletter K, Moser E, Kasper S, Lanzenberger R. Multimodal imaging of human early visual cortex by combining functional and molecular measurements with fMRI and PET. Neuroimage. 2008;41:204–211. doi: 10.1016/j.neuroimage.2008.02.044. [DOI] [PubMed] [Google Scholar]
- Ghetti S, DeMaster DM, Yonelinas AP, Bunge SA. Developmental differences in medial temporal lobe function during memory encoding. Journal of Neuroscience. 2010;30:9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996a;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996b;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J, Schneider KC, Peterson BS, Rajeevan N, Makuch RW, Constable RT, Ment LR. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009;48:458–463. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Ahmadi ME, Kuperman J, Holland D, McDonald CR, Halgren E, Dale AM. Automated white-matter tractography using a probabilistic diffusion tensor atlas: Application to temporal lobe epilepsy. Human Brain Mapping. 2009;30:1535–1547. doi: 10.1002/hbm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E. How can intracranial recordings assist MEG source localization? Neurology and Clinical Neurophysiology. 2004;2004:86. [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Chauvel P, Clarke M. Spatio-temporal stages in face and word processing. 2. Depth-recorded potentials in the human frontal and Rolandic cortices. Journal of Physiology, Paris. 1994;88:51–80. doi: 10.1016/0928-4257(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography - theory, instrumentation, and applications to noninvasive studies of the working human brain. Reviews of Modern Physics. 1993;65:413–497. [Google Scholar]
- Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, Menten R, Clapuyt P, Donohue PK, Hua K, Wakana S, Jiang H, van Zijl PC, Mori S. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Gaillard WD, Mott SH, Cuenod CA, Bookheimer SY, Weinstein S, Conry J, Papero PH, Schiff SJ, Le Bihan D, Theodore WH. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology. 1997;48:1003–1012. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW. Cortical unit activity in sleep and waking. Electroencephalography and Clinical Neurophysiology. 1971;30:97–112. doi: 10.1016/0013-4694(71)90271-9. [DOI] [PubMed] [Google Scholar]
- Holland D, Dale AM. Nonlinear registration of longitudinal images and measurement of change in regions of interest. Medical Image Analysis. 2011;15:489–497. doi: 10.1016/j.media.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, Kuperman JM, Dale AM. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. Neuroimage. 2010;50:175–183. doi: 10.1016/j.neuroimage.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema JM, Karunanayaka PR, Schmithorst VJ, Yuan W, Plante E, Byars AW. Functional MRI of language lateralization during development in children. International Journal of Audiology. 2007;46:533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi Y. Functional near-infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology. 2003;40:511–520. doi: 10.1111/1469-8986.00053. [DOI] [PubMed] [Google Scholar]
- Houde O, Pineau A, Leroux G, Poirel N, Perchey G, Lanoe C, Lubin A, Turbelin MR, Rossi S, Simon G, Delcroix N, Lamberton F, Vigneau M, Wisniewski G, Vicet JR, Mazoyer B. Functional magnetic resonance imaging study of Piaget’s conservation-of-number task in preschool and school-age children: a neo-Piagetian approach. Journal of Experimental Child Psychology. 2011;110:332–346. doi: 10.1016/j.jecp.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nature Neuroscience. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11:489–497. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Human Neurobiology. 1987;6:1–9. [PubMed] [Google Scholar]
- Imada T, Zhang Y, Cheour M, Taulu S, Ahonen A, Kuhl PK. Infant speech perception activates Broca’s area: a developmental magnetoencephalography study. Neuroreport. 2006;17:957–962. doi: 10.1097/01.wnr.0000223387.51704.89. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6:955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- Iwasaki N, Hamano K, Okada Y, Horigome Y, Nakayama J, Takeya T, Takita H, Nose T. Volumetric quantification of brain development using MRI. Neuroradiology. 1997;39:841–846. doi: 10.1007/s002340050517. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Tallal P. Late childhood changes in brain morphology observable with MRI. Developmental Medicine and Child Neurology. 1990;32:379–385. doi: 10.1111/j.1469-8749.1990.tb16956.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114(Pt 5):2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in infants: elements of an interactive specialization framework. Child Development. 2000;71:75–81. doi: 10.1111/1467-8624.00120. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Interactive specialization: a domain-general framework for human functional brain development? Developmental Cognitive Neuroscience. 2011;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kawakubo Y, Kono T, Takizawa R, Kuwabara H, Ishii-Takahashi A, Kasai K. Developmental changes of prefrontal activation in humans: a near-infrared spectroscopy study of preschool children and adults. PLoS One. 2011;6:e25944. doi: 10.1371/journal.pone.0025944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DN, Makris N, Herbert MR, Takahashi T, Caviness VS., Jr Basic principles of MRI and morphometry studies of human brain development. Developmental Science. 2002;5:268–278. [Google Scholar]
- Kennedy H, Dehay C. Gradients and boundaries: limits of modularity and its influence on the isocortex. [Commentary] Developmental Science. 2001;4:147–148. [Google Scholar]
- Kikuchi M, Shitamichi K, Yoshimura Y, Ueno S, Remijn GB, Hirosawa T, Munesue T, Tsubokawa T, Haruta Y, Oi M, Higashida H, Minabe Y. Lateralized theta wave connectivity and language performance in 2- to 5-year-old children. Journal of Neuroscience. 2011;31:14984–14988. doi: 10.1523/JNEUROSCI.2785-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsoni E, Byrd D, Casey BJ. Special considerations for functional magnetic resonance imaging of pediatric populations. Journal of Magnetic Resonance Imaging. 2006;23:877–886. doi: 10.1002/jmri.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman JM, Brown TT, Ahmadi ME, Erhart MJ, White NS, Roddey JC, Shankaranarayanan A, Han ET, Rettmann D, Dale AM. Prospective motion correction improves diagnostic utility of pediatric MRI scans. Pediatric Radiology. 2011 doi: 10.1007/s00247-011-2205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lee PS, Yerys BE, Della Rosa A, Foss-Feig J, Barnes KA, James JD, VanMeter J, Vaidya CJ, Gaillard WD, Kenworthy LE. Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: a fcMRI study of response inhibition. Cerebral Cortex. 2009;19:1787–1794. doi: 10.1093/cercor/bhn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annual Review of Physiology. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Lyytinen H, Blomberg AP, Naatanen R. Event-related potentials and autonomic responses to a change in unattended auditory stimuli. Psychophysiology. 1992;29:523–534. doi: 10.1111/j.1469-8986.1992.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Mankinen K, Jalovaara P, Paakki JJ, Harila M, Rytky S, Tervonen O, Nikkinen J, Starck T, Remes J, Rantala H, Kiviniemi V. Connectivity disruptions in resting-state functional brain networks in children with temporal lobe epilepsy. Epilepsy Research. 2012;100:168–178. doi: 10.1016/j.eplepsyres.2012.02.010. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: a comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Botvinick MM, Noelle DC, Plaut DC, Rogers TT, Seidenberg MS, Smith LB. Letting structure emerge: connectionist and dynamical systems approaches to cognition. Trends in Cognitive Science. 2010;14:348–356. doi: 10.1016/j.tics.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Thesen T, Carlson C, Blumberg M, Girard HM, Trongnetrpunya A, Sherfey JS, Devinsky O, Kuzniecky R, Dolye WK, Cash SS, Leonard MK, Hagler DJ, Jr, Dale AM, Halgren E. Multimodal imaging of repetition priming: Using fMRI, MEG, and intracranial EEG to reveal spatiotemporal profiles of word processing. Neuroimage. 2010;53:707–717. doi: 10.1016/j.neuroimage.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JD, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social, Cognitive, and Affective Neuroscience. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. Journal of Neurochemistry. 2008a;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Mennes M, Vega Potler N, Kelly C, Di Martino A, Castellanos FX, Milham MP. Resting state functional connectivity correlates of inhibitory control in children with attention-deficit/hyperactivity disorder. Frontiers in Psychiatry. 2011;2:83. doi: 10.3389/fpsyt.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. The evolution of complexity in human brain development: an EEG study. Electroencephalography and Clinical Neurophysiology. 1996;99:405–411. doi: 10.1016/s0013-4694(96)95699-0. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC. Diffusion weighting by the trace of the diffusion tensor within a single scan. Magn Reson Med. 1995;33:41–52. doi: 10.1002/mrm.1910330107. [DOI] [PubMed] [Google Scholar]
- Morr ML, Shafer VL, Kreuzer JA, Kurtzberg D. Maturation of mismatch negativity in typically developing infants and preschool children. Ear and Hearing. 2002;23:118–136. doi: 10.1097/00003446-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am. 2006;16:19–43. doi: 10.1016/j.nic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Murphy K, Garavan H. An empirical investigation into the number of subjects required for an event-related fMRI study. Neuroimage. 2004a;22:879–885. doi: 10.1016/j.neuroimage.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Murphy K, Garavan H. Artifactual fMRI group and condition differences driven by performance confounds. Neuroimage. 2004b;21:219–228. doi: 10.1016/j.neuroimage.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Alho K. Generators of electrical and magnetic mismatch responses in humans. Brain Topography. 1995a;7:315–320. doi: 10.1007/BF01195257. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Alho K. Mismatch negativity--a unique measure of sensory processing in audition. International Journal of Neuroscience. 1995b;80:317–337. doi: 10.3109/00207459508986107. [DOI] [PubMed] [Google Scholar]
- Nelson CA, 3rd, McCleery JP. Use of event-related potentials in the study of typical and atypical development. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1252–1261. doi: 10.1097/CHI.0b013e318185a6d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwit CL. Functional neuroanatomy of spatial working memory in children. Developmental Psychology. 2000;36:109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- Nunez PL. Electric fields of the brain. New York: Oxford University Press; 1981. [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. Journal of Neuroscience. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oun W, Numenmaa A, Hamalainen M, Golland P. Multimodal functional imaging using fMRI-informed regional EEG/MEG source estimation. Information Processing in Medical Imaging. 2009;21:88–100. [PMC free article] [PubMed] [Google Scholar]
- Palmer ED, Brown TT, Petersen SE, Schlaggar BL. Investigation of the functional neuroanatomy of single word reading and its development. Scientific Studies of Reading. 2004;8:203–223. [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Biscay-Lirio R. Spatial resolution of neuronal generators based on EEG and MEG measurements. International Journal of Neuroscience. 1993;68:93–105. doi: 10.3109/00207459308994264. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Pearce JW, Crowell DH, Tokioka A, Pacheco GP. Childhood developmental changes in the auditory P300. Journal of Child Neurology. 1989;4:100–106. doi: 10.1177/088307388900400204. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Development. 2009;80:1016–1038. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps ME, Mazziotta JC. Positron emission tomography: Human brain function and biochemistry. Science. 1985;228:799–809. doi: 10.1126/science.2860723. [DOI] [PubMed] [Google Scholar]
- Pihko E, Kujala T, Mickos A, Antell H, Alku P, Byring R, Korkman M. Magnetic fields evoked by speech sounds in preschool children. Clinical Neurophysiology. 2005;116:112–119. doi: 10.1016/j.clinph.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Plunkett K, Karmiloff-Smith A, Bates E, Elman JL, Johnson MH. Connectionism and developmental psychology. Journal of Child Psycholology and Psychiatry. 1997;38:53–80. doi: 10.1111/j.1469-7610.1997.tb01505.x. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Interpreting developmental changes in neuroimaging signals. Human Brain Mapping. 2010;31:872–878. doi: 10.1002/hbm.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. Cognitive Brain Potentials. Current Directions in Psychological Science. 1993;2:175–179. [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2011;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartz SR, Sejnowski TJ. The neural basis of cognitive development: a constructivist manifesto. Behavioral and Brain Sciences. 1997;20:537–556. doi: 10.1017/s0140525x97001581. discussion 556–596. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL. Brain development, gender and IQ in children: A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Remijn GB, Kikuchi M, Yoshimura Y, Shitamichi K, Ueno S, Nagao K, Munesue T, Kojima H, Minabe Y. Hemodynamic responses to visual stimuli in cortex of adults and 3- to 4-year-old children. Brain Research. 2011;1383:242–251. doi: 10.1016/j.brainres.2011.01.090. [DOI] [PubMed] [Google Scholar]
- Ressel V, Wilke M, Lidzba K, Lutzenberger W, Krageloh-Mann I. Increases in language lateralization in normal children as observed using magnetoencephalography. Brain and Language. 2008;106:167–176. doi: 10.1016/j.bandl.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Rossion B, Kung CC, Tarr MJ. Visual expertise with nonface objects leads to competition with the early perceptual processing of faces in the human occipitotemporal cortex. Proceedings of the National Academy of Sciences. 2004;101:14521–14526. doi: 10.1073/pnas.0405613101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schneider JF, Il’yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46:258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The resting brain: unconstrained yet reliable. Cerebral Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Snyder AZ, Raichle ME. A brief history of the resting state: The Washington University perspective. Neuroimage. 2012;62:902–910. doi: 10.1016/j.neuroimage.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2010;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999a;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999b;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine and Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Stanfield BB, O’Leary DD. The transient corticospinal projection from the occipital cortex during the postnatal development of the rat. Journal of Comparative Neurology. 1985:238. doi: 10.1002/cne.902380210. [DOI] [PubMed] [Google Scholar]
- Stanfield BB, O’Leary DD, Fricks C. Selective collateral elimination in early postnatal development restricts cortical distribution of rat pyramidal tract neurones. Nature. 1982:298. doi: 10.1038/298371a0. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. Journal of Neurophysiology. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Rohlfing T, Baker FC, Padilla ML, Colrain IM. Developmental change in regional brain structure over 7 months in early adolescence: comparison of approaches for longitudinal atlas-based parcellation. Neuroimage. 2011;57:214–224. doi: 10.1016/j.neuroimage.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Sutton S, Tueting P, Zubin J, John ER. Information delivery and the sensory evoked potential. Science. 1967;155:1436–1439. doi: 10.1126/science.155.3768.1436. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Matsuzawa H, Kwee IL, Nakada T. Absolute eigenvalue diffusion tensor analysis for human brain maturation. NMR Biomed. 2003;16:257–260. doi: 10.1002/nbm.848. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Batty M, Itier RJ. The faces of development: a review of early face processing over childhood. Journal of Cognitive Neuroscience. 2004;16:1426–1442. doi: 10.1162/0898929042304732. [DOI] [PubMed] [Google Scholar]
- Thatcher R. Cyclic cortical reorganization during early childhood. Brain and Cognition. 1992;20:24–50. doi: 10.1016/0278-2626(92)90060-y. [DOI] [PubMed] [Google Scholar]
- Thatcher R, Walker R, Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236:1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Hunt RH, Vizueta N, Sommer T, Durston S, Yang Y, Worden MS. Evidence of developmental differences in implicit sequence learning: an fMRI study of children and adults. Journal of Cognitive Neuroscience. 2004;16:1339–1351. doi: 10.1162/0898929042304688. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis KE, Leonard MK, Brown TT, Hagler DJ, Jr, Curran M, Dale AM, Elman JL, Halgren E. Spatiotemporal neural dynamics of word understanding in 12- to 18-month-old-infants. Cerebral Cortex. 2011;21:1832–1839. doi: 10.1093/cercor/bhq259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cerebral Cortex. 2010a;20:2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Frontiers in Systems Neuroscience. 2010b;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. Journal of Neuroscience. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Foss-Feig J, Shook D, Kaplan L, Kenworthy L, Gaillard WD. Controlling attention to gaze and arrows in childhood: an fMRI study of typical development and Autism Spectrum Disorders. Developmental Science. 2011;14:911–924. doi: 10.1111/j.1467-7687.2011.01041.x. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2011;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Baym CL, Gazzaley A, Bunge SA. Neural indices of improved attentional modulation over middle childhood. Developmental Cognitive Neuroscience. 2011a;1:175–186. doi: 10.1016/j.dcn.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, O’Hare ED, Whitaker KJ, Ferrer E, Bunge SA. Increased functional selectivity over development in rostrolateral prefrontal cortex. Journal of Neuroscience. 2011b;31:17260–17268. doi: 10.1523/JNEUROSCI.1193-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N, Roddey C, Shankaranarayanan A, Han E, Rettmann D, Santos J, Kuperman J, Dale A. PROMO: Real-time prospective motion correction in MRI using image-based tracking. Magnetic Resonance in Medicine. 2010a;63:91–105. doi: 10.1002/mrm.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Schmidt M, Kim DI, Calhoun VD. Disrupted functional brain connectivity during verbal working memory in children and adolescents with schizophrenia. Cerebral Cortex. 2010b;21:510–518. doi: 10.1093/cercor/bhq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I, Karmos G, Naatanen R. Adaptive modeling of the unattended acoustic environment reflected in the mismatch negativity event-related potential. Brain Research. 1996;742:239–252. doi: 10.1016/s0006-8993(96)01008-6. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Muetzel RL, Bell CJ, Hoecker HL, Nelson ML, Chang PN, Lim KO. Inter-hemispheric functional connectivity disruption in children with prenatal alcohol exposure. Alcohol: Clinical and Experimental Research. 2011;35:849–861. doi: 10.1111/j.1530-0277.2010.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford, UK: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]
- Yamaguchi S, Knight RT. P300 generation by novel somatosensory stimuli. Electroencephalography and Clinical Neurophysiology. 1991;78:50–55. doi: 10.1016/0013-4694(91)90018-y. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res. 1989;50:11–32. doi: 10.1016/0165-3806(89)90124-7. [DOI] [PubMed] [Google Scholar]