Abstract
The cornea is a complex sensory organ that must maintain its transparency for optimal vision. Infections such as with herpes simplex virus can result in blinding immunoinflammatory reactions referred to as herpes stromal keratitis (HSK). In this review we discuss the pathogenesis of HSK referring to work mainly done using animal model systems. We briefly discuss the role of multiple cell types and soluble mediators but focus on the critical role of corneal vascularization (CV) in contributing to corneal damage. We describe how VEGF and other angiogenic molecules are induced following infection and discuss the many ways by which CV can be controlled. Speculations are made regarding future approaches that could improve the management of HSK.
Keywords: Herpes simplex virus, Herpes stromal keratitis, Corneal vascularization, Angiogenesis
1. Introduction
All cells in large multi-cellular organisms require oxygen which is usually acquired from oxygen transporting cells within a blood vascular system. Some tissues lack blood vessels since these would interfere with their normal function. Prime examples are the cornea and lens of the eye, which need to be transparent so as not to interfere with the passage of light to the retina. The eye uses several strategies to limit inflammation, immune responsiveness and neovascularization of the normally avascular cornea. Particularly damaging to vision is the development of corneal vascularization (CV), since new blood vessels can block and diffract light, deposit lipids and proteins into the corneal stroma, and serve as a conduit for inflammatory cells that can both diffract light and damage the structural integrity of the cornea. The fascinating topic of how the eye strives to maintain its transparency by inhibiting immune and inflammatory events is termed immune privilege (Billingham and Boswell, 1953; Medawar, 1948). This topic has received several excellent reviews and will not be further discussed (Niederkorn, 2006; Streilein, 2003).
The term corneal angiogenic privilege was coined to describe numerous mechanisms that act together to prevent the formation of new blood vessels in response to injury (Azar, 2006; Chang et al., 2001). These properties include tight packaging of collagen lamellae due to constant dehydration of the corneal stroma, lower corneal temperature, the presence of soluble and cell membrane bound molecules that inhibit the activity of angiogenic factors in the cornea and low levels of molecules such as metalloproteinases (MMPs) that facilitate CV (Qazi et al., 2010). Unfortunately, neither immune nor angiogenic privilege fully protects the eye when certain injuries occur. These can include development of severe allergic responses and exposure to some infectious agents (Russell et al., 1984). Trachoma is probably the most common infectious cause of corneal damage worldwide, but in developed countries herpes simplex keratitis (HSK) is the most frequent cause of vision loss (Pepose, 1996). In this review, we briefly describe cellular and molecular events that occur in normal as well as pathological angiogenesis and discuss in detail the pathogenesis of a chronic inflammatory lesion that occurs in the corneal stroma in response to herpes simplex virus (HSV-1) infection. Our focus will be on the role of corneal CV during the pathogenesis of HSK and we discuss how CV might be successfully managed.
2. Herpes Simplex virus infection
Infectious keratitis in humans is almost always caused by the type I strain of HSV-1 (Liesegang, 2001). It is usually acquired by close contact with persons with clinical or subclinical infections on the face, oral mucosa, or sometimes the genital mucosa (Liesegang, 2001). On rare occasions, the virus is suspected to be transmitted by corneal transplantation (Remeijer et al., 2001). The initial infection usually involves active viral replication in corneal epithelial cells that die and need to be replaced. Primary ocular infection is probably the result of direct inoculation of the surface of the eye or adnexa, but in atopic and immune compromised can be due to autoinoculation form active HSV-1 infections elsewhere on the body, such as a cold sore. HSK, an immune mediated disease of the relatively acellular collagen matrix of the cornea, rarely occurs during primary ocular HSV-1 infection. Most of the time HSK is triggered by reactivation of latent HSV-1 infection of neurons in the trigeminal ganglion, with subsequent axonal transport of viral proteins and/or infectious virus into the corneal stroma (Pepose, 1996).
Active viral replication in the corneal epithelium proceeds for a few days and the clinical consequences can be reduced by 2–3 days with anti-viral therapy. Therapy does not markedly reduce the duration of viral detection and treatments are only assumed to be effective since untreated controls are rarely available for comparison. Unfortunately, controlling the lesion fails to remove the virus from the body and life-long latency is invariably established in neurons of the trigeminal ganglion. During latency, no replication competent virus is produced and were this situation to remain without interruption, all would be well. Alas, periodically, and perhaps continuously in some cases, a few latently infected neurons are induced by a variety of stress stimuli to reactivate a productive cycle and new virus passes back to peripheral sites where lesions were formerly present (Daheshia et al., 1998). These recurrences may be subclinical or clinically evident, but repeated episodes can result in chronic inflammatory disease in the corneal stroma (Sarangi PP, 2010). About 20% of patients with epithelial keratitis develop the stromal form of HSV-1 corneal disease which is far more likely to compromise vision and more difficult to control therapeutically than epithelial keratitis (Pepose, 1996). Some patients with SK require prolonged, even indefinite treatment with anti-inflammatory drugs and usually the virus is not present in lesions after the initial stages. Indeed, there is a strong suspicion and some evidence that SK can develop into an auto-immune inflammatory process (Panoutsakopoulou et al., 2001). Once CV becomes a prominent feature, HSK lesions are particularly difficult to manage and patients usually require chronic therapy with topical corticosteroids. New blood vessels of pathological angiogenesis are leakier than normal vessels and permit the escape of inflammatory cells into tissues that contribute to vision impairment. In the initial stages of CV, blood vessels can be inhibited with treatments such as anti-VEGF mAb, although ghost vessels remain after treatment and these can become patent again under certain circumstances such as an episode of viral reactivation (Margolis TP, personal communication, 2003).
For ethical and practical reasons it is difficult to manipulate and understand the role of the many events involved in human HSK. In consequence, to assemble the steps involved in pathogenesis it is necessary to use a variety of animal model systems. None of the models are perfect, particularly to mimic the consequences of viral reactivation from latency. The most convenient model to use is primary infection of the mouse cornea with appropriate strains of HSV-1. Inflammatory lesions occur in the stroma and these resemble HSK lesions observed in humans (Biswas and Rouse, 2005). The mouse model is extremely unreliable to achieve lesions following exposure to virus reactivation stimuli (Shimeld et al., 1989; Stuart, 2012). The rabbit model is better for this circumstance although the many disadvantages of working with rabbits to study inflammation and immunology make their use problematic. Most of our understanding of the role of CV in HSK comes from mouse studies.
3. Pathogenesis of stromal keratitis
HSK lesions regularly follow infection of the mouse cornea with appropriate strains of HSV-1. Infecting the scratched corneas of Balb/c or C57BL6 (B6) mice with the RE strain of HSV-1 is the most reproducible system. Some groups favor infection of the non-traumatized cornea with HSV McKrae, but many mice die of encephalitis which usually occurs before SK lesions become fully developed. It is conceivable that pathological events following RE and McKrae could differ since the lesions following RE mainly involve CD4 T cells (Niemialtowski and Rouse, 1992) but with McKrae infection CD8 T cells are far more prominent (Ghiasi et al., 2000). Infection with HSV KOS, the strain for which abundant mutant and recombinant viruses is available, is not a reliable system, particularly in the highly resistant B6 mouse strain. This latter mouse strain is the most useful for pathogenesis studies since there are abundant genetically derived variants. The brief account that follows mainly comes from investigations with the HSV RE strain administered to the scratched B6 or Balb/c cornea.
Following infection, replication commences in epithelial cells and this lasts for 5 to 6 days after which replicating virus is usually no longer detectable in the eye. Normally, little or no replicating virus reaches deeper layers in the eye such as the corneal stroma (Biswas and Rouse, 2005). However, in immunocompromised animals virus does reach deeper tissues and also passes to the brain to cause encephalitis, a usually lethal lesion which in mice might be in part immunopathological (Altmann and Blyth, 1985). Replication invariably occurs too in the trigeminal ganglion where latency is permanently established (Liu et al., 1996).
Virus infection triggers numerous signaling events many of which are driven by pathogen activating molecular pattern (PAMP) properties of the virus. These include at least 3 TLR ligands (for TLR 2, TLR3 and TLR9) all of which activate the innate immune system in some way (Kurt-Jones et al., 2004; Sarangi et al., 2007; Zhang et al., 2007). Curiously, humans without TLR-3 responsiveness show markedly increased susceptibility to HSV-1 infection (Zhang et al., 2007), but ocular consequences have not been studied. It is not clear how and where the virus exerts its TLR ligand stimulating effects. Likely possibilities include virus budding from infected epithelial cells, cell contents released by killed cells, or perhaps extracellular free virus. The consequences of infection are multiple changes in gene expression many of which are involved in innate responses. These include several cytokines and chemokines as well as the increased expression of molecules such as VEGF, a principal angiogenic factor (Zheng et al., 2001). An unresolved issue is the initial source of some of these critical molecules. The virus-infected epithelial cells could be one such source, but this would be minimal and brief since the virus quickly shuts off host cell mRNA and protein synthesis (Kwong and Frenkel, 1989). A few host proteins are stimulated and secreted by infected cells. These include cytokines such as IL-1, IL-6 and perhaps VEGF (Kanangat et al., 1996; Wuest and Carr, 2010).
The most common consequence of infection is that some products generated (such as TLR ligands, HSPs and some cytokines) act in a paracrine fashion to induce uninfected cells to produce and secrete numerous factors that participate in pathogenesis. These include the critical proinflammatory cytokines IL-6, IL-1 and IL-17A (Biswas and Rouse, 2005). Chemokines such as CCL2, CXCL1 and CXCL2 are also produced and these serve to attract inflammatory cells of many types into the corneal stroma from blood vessels present at the ocular limbus (Thomas et al., 1998; Tumpey et al., 1998). Inflammatory cells are evident by 24 hr pi and these include natural killer cells (NK), gamma delta T cells, dendritic cells (DC), macrophages and most prominently neutrophils (Bouley et al., 1996; Jager et al., 1991; Thomas et al., 1997; Tumpey et al., 1996). Notably, there are few if any lymphocytes especially those that are reactive with viral antigens (Gangappa et al., 2000). We remain uncertain as to the role of the various recruited cells, but the NK cells and neutrophils function in some way to abort the infection (Biswas and Rouse, 2005). Thus, removing either cell type prolongs and increases infection levels. Neutrophils also participate in CV since they can be a source of angiogenic factors as well as proteases that facilitate blood vessel growth through the corneal matrix (Suryawanshi et al., 2011a). The DC that invade the eye are likely involved in transporting viral antigen to the draining lymph node and initiating an adaptive immune response. Some DC subtypes and macrophages could be a source of lesion modulating molecules such interferons, transforming growth factor beta (TGFβ) and IL-10. The gamma delta T cells observed as early entrants to the cornea likely participate in initiating angiogenesis since they produce IL- 17A that acts as an inducer of the angiogenic factor VEGF (Suryawanshi et al., 2012).
New blood vessels sprouting from the normal vasculature at the limbus can be seen in the eyes of Balb/c animals in the very early stages of keratitis. These continue to develop in mice and can expand eventually from the entire limbus to almost reach the central cornea. This extent of CV is a rare occurrence in human HSK. As discussed subsequently, the source of stimuli that drives this CV likely changes with time, with non-immune and innate immune events dominating initially and the adaptive immune system critically involved in later stages (Sarangi PP, 2010).
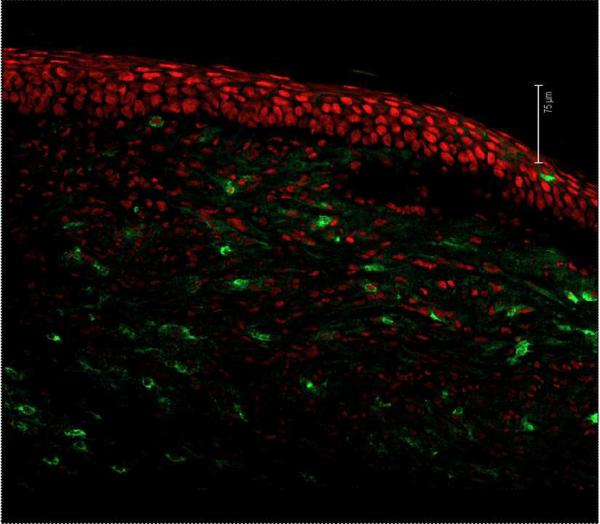
The stromal lesions that occur the eye represent immunopathological events that are orchestrated by T cells. Accordingly, T cells (primarily CD4+) become evident in the stroma by seven days pi and this infiltration peaks around 14–21 days pi (Biswas and Rouse, 2005) (Fig.1) Stromal lesions do not occur in animals that lack T cells, but lesion-producing competence can be restored with adoptive transfers of CD4 T cells, the most disease producing (Mercadal et al., 1993; Russell et al., 1984). It is conceivable that the entrance of T cells, which are presumably corresponding to chemokines such as CCL2, CCL5 and CXCL10 generated in the stroma, is facilitated by the new quite leaky vascular bed that expands from the limbus. A major unresolved issue is to define the antigen specificity of the CD4 T cells involved in orchestrating HSK. Unfortunately, appropriate peptides or tetramer reagents to identify T cell specificity have not been used and are not readily available. Antiviral specificity is suspected to be one of the targets for T cells, but activated T cells of other specificities are present in lesions and these are hypothesized to participate (Deshpande et al., 2001a). Our group, for example, have observed that TCR transgenic mice on a RAG background, where almost 100% of the T cells are reactive to a peptide that has no known cross-reactivity to HSV-1, can be responsible for lesions that appear identical to those seen in normal infected mice (Deshpande et al., 2001b). In fact we have advocated that the environment of the inflamed cornea may serve to stimulate T cells of many specificities providing they gain access to the cornea. We believe that these bystanders can be stimulated to generate pro-inflammatory components that further contribute to HSK. However, these bystander activation ideas are doubted by many traditional immunologists.
Fig. 1.
An alternative concept is that HSK lesions are the consequence of autoantigens being unmasked in the cornea and these drive autoreactive cells to mediate an autoimmune inflammatory reaction (Panoutsakopoulou et al., 2001). An extension of this idea is that the virus itself may act as a molecular mimic for some of these autoantigens (Zhao et al., 1998). This notion has attracted advocates (Zhao et al., 1998), but the concept has never been independently confirmed and has been experimentally refuted (Deshpande et al., 2001b).
It is a curious and unexplained fact that by far the most frequent T cell type in HSK lesions are CD4 T cells. However these T cells can be of many subtypes in terms of function. Early on the major subtypes are Th1 cells along with FoxP3+ regulatory T cells (Niemialtowski and Rouse, 1992; Suvas et al., 2004). In the later stages, Th17 cells become more evident, although these never outnumber the Th1 cells (Suryawanshi et al., 2012). By the time Th17 cells become prominent, CV is quite extensive and it is possible that some cytokines generated from Th17 cells contribute directly or indirectly to the angiogenic process (Suryawanshi et al., 2011b). Murine HSK lesions usually do not resolve spontaneously, particularly if the lesions are severe. However it is conceivable that resolution could be achieved with novel therapies and this is an active area of investigation. One component of successful therapy is to control the CV process. This topic is further discussed in subsequent sections.
3.1. Critical events during HSV-1- induced CV
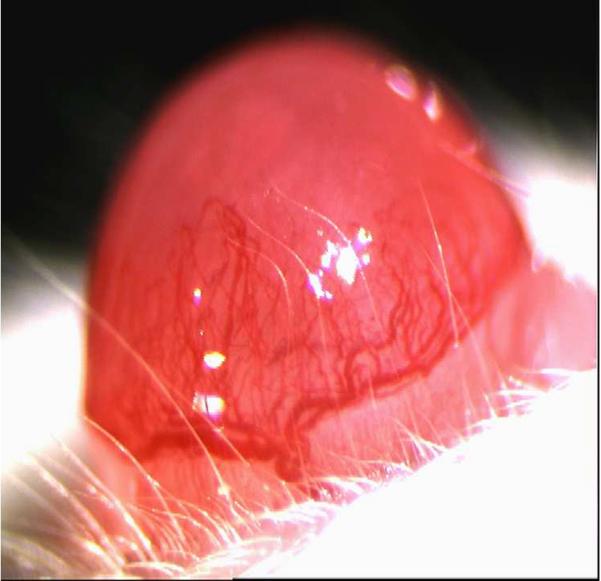
Neovascularization is evident as early as 1 day after corneal infection with the process continuing to advance over a 2 to 3 week period (Zheng et al., 2001). In some animals, almost the whole cornea can be vascularized with the vessels becoming very large and obviously interfering with vision (Fig.2). As mentioned before, fortunately CV in human HSK is usually far less extensive than in mice, but nonetheless it can interfere markedly with vision. In the mouse, CV is likely driven by numerous angiogenic factors and facilitators with such molecules having a variety of cellular sources. Moreover, this source may change during the course of lesion pathogenesis (Suryawanshi et al., 2011a). Most studies on CV have focused on VEGF–A and it is evident that a variety of approaches which impair the production or response to VEGF-A can markedly inhibit the extent of CV (Kim et al., 2004). Some have advocated that a significant source of the VEGF could be the virus infected epithelial cells (Wuest and Carr, 2010). Such infected cells can produce VEGF, at least for a brief time, but the virus rapidly shuts off host cell mRNA and protein synthesis making it doubtful if the infected cell represents a relevant source of the VEGF responsible even for initial angiogenesis.
Fig. 2.
Instead, two additional sources are likely to be more consequential for driving CV. The first is a physiological source present in uninfected eyes of all species. The molecule is present, but is prevented from causing angiogenesis since it is bound to a soluble form of one of its receptors, sVEGFR-1 (Ambati et al., 2006). The virus infection also inhibits the synthesis of sVEGFR-1 more than VEGF itself (Suryawanshi et al., 2011a). This will change the balance of the two molecules and will release some VEGF to cause angiogenesis. Moreover, early inflammatory entrants, such as neutrophils, contain proteases that readily cause sVEGFR-1, but not VEGF, to lose its function (Suryawanshi et al., 2011a). The outcome is also more VEGF to mediate angiogenesis.
The second additional source of the VEGF could be even more instrumental in driving pathological angiogenesis. Thus, our group noted some time ago that IL-6, initially produced by infected cells, could cause nearby cells to produce VEGF (Kanangat et al., 1996). Similarly, viral DNA because of its TLR-9 ligand activity could also cause uninfected cells to produce VEGF (Zheng et al., 2002). More recently, the cytokine IL-17A, produced initially by gamma delta T cells that are rapidly recruited to the cornea in response to HSV-1 infection, was also shown to drive VEGF-A production from uninfected cells (Suryawanshi et al., 2011c). These amplifying paracrine effects of infection, rather than direct effects of virus replication, would seem to be far more relevant as inducers of the VEGF and perhaps additional angiogenic factors (eg fibroblast growth factor and Angiopoietin 1) that drive CV. Such ideas however need to be formally proven.
Replication of HSV-1 is a relatively short event in the mouse yet pathological angiogenesis is usually a progressive process proceeding well beyond the time when virus has been removed from the eye. The explanation for the prolonged CV remains unresolved but a viable hypothesis is that the lesion orchestrating proinflammatory T cells contribute indirectly once they arrive in the cornea. Such cells release a variety of cytokines and chemokines that together act as direct or indirect stimulators of angiogenic factor production. For example, release of the chemokines CXCL1 and CXCL2 recruits neutrophils to the stroma that themselves contain and release VEGF-A (Lee et al., 2002). The neutrophils also produce a number of proteases that can act to degrade the corneal matrix so facilitating angiogenesis (Lee et al., 2002). The same molecules can further breakdown any residual sVEGFR-1 releasing any bound to VEGF to mediate angiogenesis (Suryawanshi et al., 2011a). In fact, MMP inhibitors can be used to diminish the levels of CV induced by HSV-1 infection (Suryawanshi et al., 2011a). Additionally, HSV-1 infected MMP knockout mice show diminished CV compared to wild type animals (Lee et al., 2002).
4. Physiological versus pathological angiogenesis
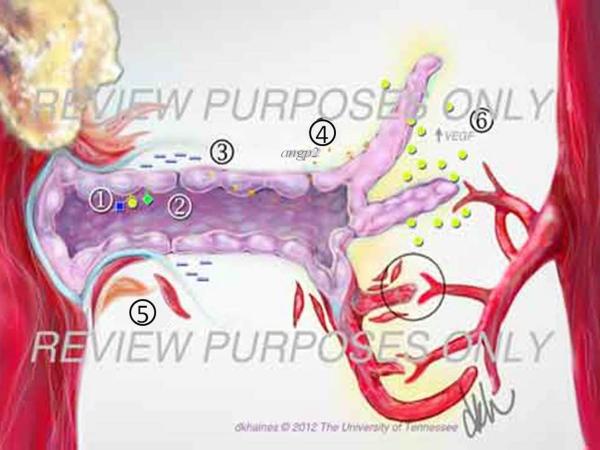
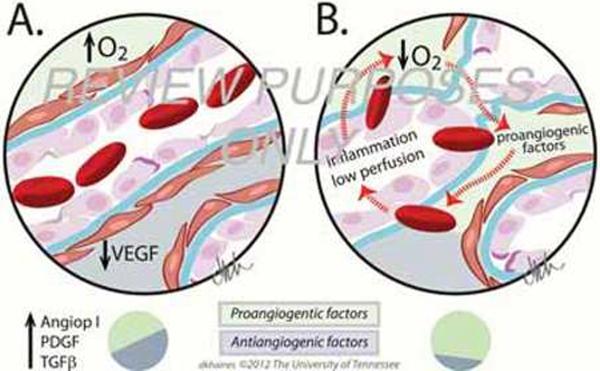
Angiogenesis is the process of formation of new blood vessels in the postnatal period. It occurs in response to physiological needs with the new vessels being derived from pre-existing ones (Folkman, 2006). Physiological angiogenesis occurs during vertebrate development and biological processes such as muscle growth, tissue healing or reproduction. The main triggers of physiological angiogenesis are the production of proangiogenic factors released from hypoxic, inflammatory or tumor cells (Chung and Ferrara, 2011). These factors include members of the VEGF family, basic fibroblast growth factor (bFGF) and several chemokines (Carmeliet and Jain, 2011). When the proangiogenic factors bind to their respective receptors on vascular endothelial cells, a cascade of events occur that include: (i) disruption of endothelial cell junctions by the activation of non-receptor Src-family kinases and the increased expression of integrins, (Potter et al., 2005) (ii) endothelial cell proliferation through the initiation of mitogen activated protein kinase and phosphoinositide 3' kinase (Klemke et al., 1997); (iii) disruption of basal membrane and detachment of pericytes due to the secretion of metalloproteinasas (MMP) by endothelial cells and (Carmeliet and Jain, 2011) (iv) release of angiopoietin 2 (ANG2) from endothelial cell granules promoting blood vessel destabilization (Augustin et al., 2009). Through the coordinated interactions of all these many molecules, endothelial cells proliferate, migrate and start sprouting (Carmeliet and Jain, 2011). VEGF gradients play a key role guiding the sprouting of the blood vessels (Ferrara et al., 2003) and it is mediated by signaling molecules of the Rho family of small GTPases, which include Rho, Cdc42 and RAC (Hoang et al., 2004). Additionally, other receptors such as Roundabouts (ROBO), UNC5B, Plexin-D, Neuropilin and Ephrin are also expressed (Carmeliet and Tessier-Lavigne, 2005). When receptor ligand binding occurs, the growing blood vessels help to guide the angiogenic sprouting until growing vessels encounter the tips of other sprouting vessels or already existing capillaries. This causes a cessation of endothelial cell motility, junctions with endothelial neighboring cells occur and the basal membrane is reformed. The latter process is favored by the secretion of protease inhibitors of MMPs by cells such as endothelial cells and pericytes among others (Adams and Alitalo, 2007). Ultimately endothelial cells change their shape and form a lumen. Once blood starts to flow through the vessel, the increased oxygen concentration results in decreased VEGF-A expression and endothelial cells become quiescent (Adams and Alitalo, 2007). Finally, the deposition of an extracellular matrix occurs and pericytes are recruited to surround the newly formed blood vessels. Such events result in blood vessel maturation and stabilization. Transforming growth factor β (TGF-β), endothelial platelet derived growth factor β and angiopoietin 1 (ANG1) all play roles in this complex process (Jain, 2003). While TGF-β stimulates the induction and differentiation of pericytes and extracellular matrix (Pardali et al., 2010), PDGF̣ promotes the recruitment of pericytes (Hellberg et al., 2010). Additionally ANG1 stabilizes blood vessels forming homomeric Tie2 complexes between endothelial cell junctions which serve to effect cell adhesion (Saharinen et al., 2008). At the end, the final result of physiological angiogenesis is the formation of a new normal blood vessel and the angiogenic process ends (Fig 3 and Fig4A).
Fig. 3.
Fig. 4.
In pathological angiogenesis, the mechanisms used to form new blood vessels resemble the physiological process. However, usually the consequence of pathological angiogenesis may differ with the process often ongoing and resolution usually not occurring. Some of the major differences between physiological and pathological angiogenesis are an excess of proangiogenic factors, which favors hyperactive vessel growth. Abnormal blood vessels are characterized by a disorganized architecture as a result of loosely connected endothelial cells covered by few and abnormal, or dysfunctional, pericytes (Carmeliet, 2005). All such pathological events are the consequence of the lack of proper blood vessel maturation and stabilization at the end of the angiogenic process. The causes of abnormal pericyte behavior are not fully understood, but reduced recruitment or improper signaling between pericytes and endothelial cells are likely possibilities (Bergers and Song, 2005). As the process does not reach resolution, ANG 2 is upregulated, continuously present and responsible for the reduced pericyte recruitment. In addition, ANG 2 counteracts the effects of its related molecule ANG 1, favoring the abnormal endothelial junctions that characterize pathological angiogenesis (Augustin et al., 2009). The final result is the formation of tortuous, wide, and leaky blood vessels which deliver oxygen improperly and create a hypoxic and hostile environment that induces the formation of new abnormal blood vessels (Nagy et al., 2007). Accordingly, whereas in physiological angiogenesis the source of angiogenic factors is diminished once the blood vessel bed is reestablished, in pathological angiogenesis the source of angiogenic factors may continue (Carmeliet, 2005). This occurs with cancers as well as with the chronic inflammatory situation that typifies SK. In the latter situation, the new formed blood vessels derive from the limbal vessels resulting in neovascularization of the normally avascular cornea. It is important to emphasize that during HSV-1-1 infection, inflammation and angiogenesis trigger each other. Infiltration of inflammatory cells and the newly formed leaky blood vessel, due to the lack of pericytes and separated endothelial cells, results in the release of inflammatory cells and cytokines into the corneal stroma (Fig. 3 and Fig. 4B). This causes corneal opacity and haze that impairs normal vision and can result ultimately in blindness (Azar, 2006) (Fig. 5).
Fig. 5.
5. Preventing and controlling CV
As described previously, CV plays a critical role during HSK pathogenesis and its inhibition can help control disease severity. Numerous approaches to limit the extent of CV have been explored most of which so far have targeted the production, availability or signaling of VEGF. Most often CV can be markedly reduced if the anti-VEGF strategy is begun early after infection, but the therapies are much less successful when commenced after CV is well established. Currently, we know of no means of removing well established pathological blood vessels from the cornea. This is a common problem too with pathological angiogenesis in the human cornea. Vessels can be reduced in size when keratitis is successfully treated, but ghost vessels remain that can become patent again under a variety of circumstances. Ophthalmologists usually attempt to solve this problem surgically using lasers to cause photo coagulation and vessel removal (Nirankari, 1992). This topic will not be further discussed. The description that follows briefly discusses the several previous and ongoing approaches evaluated so far to control CV in the mouse.
5.1. Limiting availability of VEGF
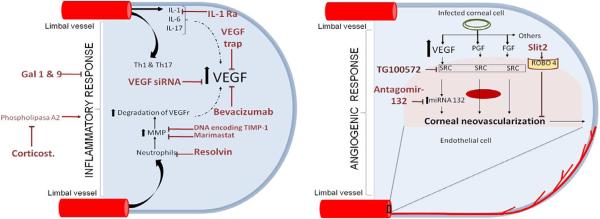
Different approaches can limit the availability of VEGF during pathological angiogenesis. One way is to use a mAb to VEGF (Avastin) that was developed to inhibit angiogenesis in some human tumors (Ferrara et al., 2004). Curiously, avastin (and its more purified product lucensis) appears to be more effective to control aberrant angiogenesis in the human retina (wet macular degeneration) than it is against tumor angiogenesis in the cancers it was developed to control. Avastin works weakly against mouse CV (Sharma et al., 2011) (Table 1) (Fig. 6), but the more appropriate reagent that is specific to mouse VEGF has yet to be evaluated.
Table 1.
Drugs to reduce corneal vascularization (CV) in the murine model
| Drug | Route & frequency of administration | Beginning of therapy | Reference | |
|---|---|---|---|---|
| Drugs that limit the availability of VEGF | siRNA | i.v. twice S/c twice |
1&3 d. p.i. 1&3 d. p.i. |
(Kim et al., 2004) |
| Bevacizumab (avastin) | i.p. daily | 6 d. p.i. | (Sharma et al., 2011) | |
| VEGF-trap | S/c. every other d. | 1 d. p.i. 5 d. p.i. 7 d. p.i. |
(Suryawanshi et al., 2011a) | |
| Drugs inhibiting the signal delivered by VEGF | Src Kinase inhibitor TG100801 | i.p. daily i.p. daily Topical drop Topical drop |
1 d. p.i. 6 d. p.i. 1 d. p.i. 6 d. p.i. |
(Sharma et al., 2011) |
| Recombinant SLIT-2 | S/c every other d. | 1 d. p.i. 7 d. p.i. |
(Mulik et al., 2011) | |
| Antagomir-miR132 | S/c every other d. | 1 d. p.i. 7 d. p.i. 10 d p.i. |
(Mulik et al., 2012) | |
| Aditional approches | Plasmid DNA encoding murine TIMP-1 | I/s twice | 3&6 d. p.i. | (Lee et al., 2002) |
| (rh) IL-1 ra | Combination of infusion pump implantation and S/c every other d. | Infusion pump: 1 d. p.i. & S/c every other day | (Biswas et al., 2004) | |
| Marimastat | S/c every other d. | 1 d. p.i. 5 d. p.i |
(Suryawanshi et al., 2011a) | |
| Galectin-9 | S/c every other d. | 3 d. p.i. 8 d. p.i. |
(Sehrawat et al., 2009) | |
| Resolvin E1 | i.p. daily | 6 d. p.i. 1 d. p.i. |
(Rajasagi et al., 2011) | |
| Galectin-1 | S/c every other d. | 3 d. p.i. 6 d. p.i. |
(Rajasagi et al., 2012) | |
i.v.: intravenous; S/c: subconjunctival; i.p.: intraperitoneal; p.i.: post-infection; b.i.: before infection
Fig. 6.
A second approach to limit VEGF availability is to use recombinant soluble VEGF-R1, a fusion protein also called the VEGF trap (Table 1) (Fig. 6). This reagent is used clinically to counteract aberrant neovascularization in the retina (Saishin et al., 2003) and our group has demonstrated that murine sVEGFR-1 can be used to inhibit CV induced by HSV-1 infection (Suryawanshi et al., 2011a). Accordingly, the local administration of recombinant mouse sVEGFR-1 to infected eyes resulted in significantly reduced levels of CV, although frequent treatment was necessary. Of interest, as mentioned later, procedures that inhibit CV such as neutralizing IL-17A acts in part by changing the balance between VEGF and VEGFR-1 emphasizing the latter (Suryawanshi et al., 2012) (Table 1) (Fig. 6).
5.2. Inhibiting the signals delivered by VEGF
Of the 5 VEGF family members, those mainly involved in CV is VEGF-A. This molecule signals vascular endothelial cells in blood vessels at the limbus by binding to specific receptors, VEGFR1 and VEGFR2, with the last most important (Ferrara et al., 2003). Interrupting VEGF interaction with its receptors using a gene silencing approach (siRNA) given topically was shown to be an effective way of inhibiting CV, at least when used early after HSV-1 infection (Kim et al., 2004) (Table 1) (Fig. 6). Other approaches to counteract VEGF/VEGFR2 signaling are either more convenient to use or more effective especially when used when the CV process is already ongoing. One such approach is to counteract downstream signaling effects of receptor binding. SRC kinase inhibitor drugs achieve that objective (Sharma et al., 2011) (Table 1) (Fig. 6). The approach can also simultaneously inhibit the signaling by several other angiogenic factor receptors. A disadvantage of the approach is that more than once daily administration is needed at least with drugs tested so far (Sharma et al., 2011).
A potentially more powerful anti-angiogenic approach is to modulate the expression of miRNAs involved in the angiogenic activity of VEGF. One such target for evaluation may be miR132 recently shown by the Cheresh group as an activator of pathological angiogenesis in some tumors (Anand et al., 2010). Recently, we assessed the role of miR132 in the CV caused by HSV-1 infection (Mulik et al., 2012) (Table 1) (Fig. 6). We showed that HSV-1 infection caused the upregulation of miR-132 expression. This effect was initially the consequence of IL-6 and IL-17A driven upregulation of miR132 in vascular endothelial cells (Mulik et al., 2012). In later stages, the miR132 upregulation may result from the products released from invading inflammatory cells (Mulik et al., 2012). The upregulated miR132 acted to modulate the signaling response to VEGF/VEGFR2 interaction on vascular endothelial cells. The outcome was a blunting of RAS Gap which normally restrains the cells from proliferating and participating in angiogenesis (Anand et al., 2010). Apparently inhibiting miR132 also acts to restrain retinal angiogenesis and this effect does not result in the compensatory angiogenic mechanisms that usually substitute for VEGF signaling when VEGF is inhibited (Bergers and Hanahan, 2008). MiRNA modulation represents a promising therapy because treatment can be administered infrequently. Moreover our preliminary results show that miR132 modulation in the later phases of CV is a more effective means of controlling the extent of CV than other approaches used so far.
An additional ways to counteract CV could be to enhance blood vessel endothelial cell signals which negatively regulate VEGF signaling (Jones et al., 2008). Our lab recently reported one such regulatory molecules, Slit2 that binds to its endothelial Robo4 receptor and neutralizes VEGF signaling in eyes after HSV-1 infection (Mulik et al., 2011) (Table 1) (Fig. 6). We observed that the majority of the blood vessel endothelial cells isolated from infected corneas expressed the Robo4 receptor while levels of Slit2 were minimal during SK. Accordingly, provision of additional Slit2 protein in infected mice reduced CV and this inhibitory effect was mediated by reduced VEGF signaling downstream products Arf 6 and Rac 1. It is now evident that Robo4 protein occurs in two forms; the transmembrane receptor (ligated by Slit2) and the soluble form (ligand for endothelial UNC5b receptor) (Koch et al., 2011). The latter Robo4 form negatively feedbacks on VEGF signaling (Koch et al., 2011). We are currently analyzing the role of soluble Robo4/UNC5b signaling in CV and we expect that potentiating both arms of Robo4 signaling (Slit2/Robo4 and soluble Robo4/UNC5b) might lead to more effective control of CV.
5.3. Additional approach that could prevent CV
Another means to reduce CV is to target inflammation since many products generated directly or indirectly influence CV. For example, some inflammatory cells produce angiogenic chemokines, or cytokines that act as angiogenic factor inducers or proteases that facilitate angiogenesis. Accordingly inhibiting inflammation often results in diminished CV. We have observed this situation when using drugs derived from polyunsaturated omega-3 fatty acids as well as when using host derived counterinflammatory proteins such as galectin 1 and 9 (Rajasagi et al., 2012; Sehrawat et al., 2009) (Table 1) (Fig. 6). An inhibitory effect on CV can also be achieved by modulating metalloproteinase activity (Lee et al., 2002) (Table 1) (Fig. 6). These molecules act to facilitate angiogenesis by degrading the corneal matrix tissues. However, they may also have additional effects such as changing the balance between VEGF and the sVEGFR-1 molecule with the latter more subject to degradation (Suryawanshi et al., 2011a). Using a corneal micropocket system we could also show that MMP9 may have direct inhibitory effects on VEGF induced CV (Lee et al., 2002). Other additional approaches reported to modulate CV via affects on inflammatory products generated during SK are included in Table1.
6. Conclusion and future directions
Stromal keratitis is a multifactorial disease and its control will likely require a combination of therapies to manage it successfully. Actually mild cases are managed well by current therapies. These usually consist of a combination of antiviral and steriodal anti-inflammatory drugs. More severe cases are mainly the ones that could benefit from additional therapeutic strategies. Suppressing CV could help in such situations especially using drugs that could stop and preferably reverse CV. Recent research to target CV has largely focused on VEGF but we know that additional molecules are involved in CV and co-targeting some of these could improve the efficacy of therapy.
Another issue of relevance is that our success with diminishing CV has largely been accomplished with approaches that are begun early after infection and then administered frequently. This is a far from an ideal situation in clinical circumstances. We need to switch off and preferably reverse CV once it is underway. Apart from approaches such as laser surgery we do not know how to remove pathological blood vessels from the cornea and typically inactive vessels remain as ghosts they can be resurrected. Our laboratory is currently experimenting with approaches that can cause apoptosis in vascular endothelial cells with the hope that this might result in vessel removal, especially when combined with therapies that shut off steps that contribute to HSK lesion production. More studies are needed before we can close the chapter on SK pathogenesis research.
Acknowledgements
This study was supported by National Institute of Allergy and Infectious Diseases Grant AI 06335 and National Institutes of Health Grant EY 005093. We thank Sachin Mulik for his assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Altmann DM, Blyth WA. Protection from herpes simplex virus-induced neuropathology in mice showing delayed hypersensitivity tolerance. J Gen Virol. 1985;66(Pt 6):1297–1303. doi: 10.1099/0022-1317-66-6-1297. [DOI] [PubMed] [Google Scholar]
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S, Majeti BK, Acevedo LM, Murphy EA, Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN, Lapinski PE, King PD, Weis SM, Cheresh DA. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham RE, Boswell T. Studies on the problem of corneal homografts. Proc R Soc Lond B Biol Sci. 1953;141:392–406. doi: 10.1098/rspb.1953.0049. [DOI] [PubMed] [Google Scholar]
- Biswas PS, Banerjee K, Zheng M, Rouse BT. Counteracting corneal immunoinflammatory lesion with interleukin-1 receptor antagonist protein. J Leukoc Biol. 2004;76:868–875. doi: 10.1189/jlb.0504280. [DOI] [PubMed] [Google Scholar]
- Biswas PS, Rouse BT. Early events in HSV keratitis--setting the stage for a blinding disease. Microbes Infect. 2005;7:799–810. doi: 10.1016/j.micinf.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Bouley DM, Kanangat S, Rouse BT. The role of the innate immune system in the reconstituted SCID mouse model of herpetic stromal keratitis. Clin Immunol Immunopathol. 1996;80:23–30. doi: 10.1006/clin.1996.0090. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- Daheshia M, Feldman LT, Rouse BT. Herpes simplex virus latency and the immune response. Curr Opin Microbiol. 1998;1:430–435. doi: 10.1016/s1369-5274(98)80061-1. [DOI] [PubMed] [Google Scholar]
- Deshpande S, Zheng M, Lee S, Banerjee K, Gangappa S, Kumaraguru U, Rouse BT. Bystander activation involving T lymphocytes in herpetic stromal keratitis. J Immunol. 2001a;167:2902–2910. doi: 10.4049/jimmunol.167.5.2902. [DOI] [PubMed] [Google Scholar]
- Deshpande SP, Lee S, Zheng M, Song B, Knipe D, Kapp JA, Rouse BT. Herpes simplex virus-induced keratitis: evaluation of the role of molecular mimicry in lesion pathogenesis. J Virol. 2001b;75:3077–3088. doi: 10.1128/JVI.75.7.3077-3088.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- Gangappa S, Deshpande SP, Rouse BT. Bystander activation of CD4+ T cells accounts for herpetic ocular lesions. Invest Ophthalmol Vis Sci. 2000;41:453–459. [PubMed] [Google Scholar]
- Ghiasi H, Cai S, Perng GC, Nesburn AB, Wechsler SL. Both CD4+ and CD8+ T cells are involved in protection against HSV-1 induced corneal scarring. Br J Ophthalmol. 2000;84:408–412. doi: 10.1136/bjo.84.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res. 2010;180:103–114. doi: 10.1007/978-3-540-78281-0_7. [DOI] [PubMed] [Google Scholar]
- Hoang MV, Whelan MC, Senger DR. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc Natl Acad Sci U S A. 2004;101:1874–1879. doi: 10.1073/pnas.0308525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager MJ, Atherton S, Bradley D, Streilein JW. Herpetic stromal keratitis in mice: less reversibility in the presence of Langerhans cells in the central cornea. Curr Eye Res. 1991;10(Suppl):69–73. doi: 10.3109/02713689109020360. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F, Mukouyama YS, Lindblom P, Seth P, Frias A, Nishiya N, Ginsberg MH, Gerhardt H, Zhang K, Li DY. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanangat S, Babu JS, Knipe DM, Rouse BT. HSV-1-mediated modulation of cytokine gene expression in a permissive cell line: selective upregulation of IL-6 gene expression. Virology. 1996;219:295–300. doi: 10.1006/viro.1996.0250. [DOI] [PubMed] [Google Scholar]
- Kim B, Tang Q, Biswas PS, Xu J, Schiffelers RM, Xie FY, Ansari AM, Scaria PV, Woodle MC, Lu P, Rouse BT. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol. 2004;165:2177–2185. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AW, Mathivet T, Larrivée B, Tong RK, Kowalski J, Pibouin-Fragner L, Bouvrée K, Stawicki S, Nicholes K, Rathore N, Scales SJ, Luis E, del Toro R, Freitas C, Bréant C, Michaud A, Corvol P, Thomas JL, Wu Y, Peale F, Watts RJ, Tessier-Lavigne M, Bagri A, Eichmann A. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev Cell. 2011;20:33–46. doi: 10.1016/j.devcel.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong AD, Frenkel N. The herpes simplex virus virion host shutoff function. J Virol. 1989;63:4834–4839. doi: 10.1128/jvi.63.11.4834-4839.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Invest. 2002;110:1105–1111. doi: 10.1172/JCI15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- Mercadal CM, Bouley DM, DeStephano D, Rouse BT. Herpetic stromal keratitis in the reconstituted scid mouse model. J Virol. 1993;67:3404–3408. doi: 10.1128/jvi.67.6.3404-3408.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulik S, Sharma S, Suryawanshi A, Veiga-Parga T, Reddy PB, Rajasagi NK, Rouse BT. Activation of endothelial roundabout receptor 4 reduces the severity of virus-induced keratitis. J Immunol. 2011;186:7195–7204. doi: 10.4049/jimmunol.1100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulik S, Xu J, Reddy PB, Rajasagi NK, Gimenez F, Sharma S, Lu PY, Rouse BT. Role of miR-132 in Angiogenesis after Ocular Infection with Herpes Simplex Virus. Am J Pathol. 2012;181:525–534. doi: 10.1016/j.ajpath.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–275. doi: 10.1146/annurev.pathol.2.010506.134925. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- Niemialtowski MG, Rouse BT. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992;149:3035–3039. [PubMed] [Google Scholar]
- Nirankari VS. Laser photocoagulation for corneal stromal vascularization. Trans Am Ophthalmol Soc. 1992;90:595–669. [PMC free article] [PubMed] [Google Scholar]
- Panoutsakopoulou V, Sanchirico ME, Huster KM, Jansson M, Granucci F, Shim DJ, Wucherpfennig KW, Cantor H. Analysis of the relationship between viral infection and autoimmune disease. Immunity. 2001;15:137–147. doi: 10.1016/s1074-7613(01)00172-8. [DOI] [PubMed] [Google Scholar]
- Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20:556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Pepose JD. Herpes symplex virus diseases: Anterior segment of the eye. In: Pepose JS, Holland GN, Wilhelmus KR, editors. Ocular infection and immunity. St. Louis: 1996. pp. 905–936. [Google Scholar]
- Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280:31906–31912. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- Qazi Y, Wong G, Monson B, Stringham J, Ambati BK. Corneal transparency: genesis, maintenance and dysfunction. Brain Res Bull. 2010;81:198–210. doi: 10.1016/j.brainresbull.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasagi NK, Reddy PB, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J Immunol. 2011;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasagi NK, Suryawanshi A, Sehrawat S, Reddy PB, Mulik S, Hirashima M, Rouse BT. Galectin-1 reduces the severity of herpes simplex virus-induced ocular immunopathological lesions. J Immunol. 2012;188:4631–4643. doi: 10.4049/jimmunol.1103063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remeijer L, Maertzdorf J, Doornenbal P, Verjans GM, Osterhaus AD. Herpes simplex virus 1 transmission through corneal transplantation. Lancet. 2001;357:442. doi: 10.1016/S0140-6736(00)04011-3. [DOI] [PubMed] [Google Scholar]
- Russell RG, Nasisse MP, Larsen HS, Rouse BT. Role of T-lymphocytes in the pathogenesis of herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 1984;25:938–944. [PubMed] [Google Scholar]
- Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, Olsen BR, Alitalo K. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- Saishin Y, Takahashi K, Lima e Silva R, Hylton D, Rudge JS, Wiegand SJ, Campochiaro PA. VEGF-TRAP(R1R2) suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:241–248. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]
- Sarangi PP, Kim B, Kurt-Jones E, Rouse BT. Innate recognition network driving herpes simplex virus-induced corneal immunopathology: role of the toll pathway in early inflammatory events in stromal keratitis. J Virol. 2007;81:11128–11138. doi: 10.1128/JVI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangi PP, R.B. Herpetic keratitis. In: Levin L, DM A, editors. Ocular disease. Mechanisms and management; 2010. pp. 92–97. [Google Scholar]
- Sehrawat S, Suryawanshi A, Hirashima M, Rouse BT. Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: shifting the balance toward regulators. J Immunol. 2009;182:3191–3201. doi: 10.4049/jimmunol.0803673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Mulik S, Kumar N, Suryawanshi A, Rouse BT. An anti-inflammatory role of VEGFR2/Src kinase inhibitor in herpes simplex virus 1-induced immunopathology. J Virol. 2011;85:5995–6007. doi: 10.1128/JVI.00034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld C, Hill T, Blyth B, Easty D. An improved model of recurrent herpetic eye disease in mice. Curr Eye Res. 1989;8:1193–1205. doi: 10.3109/02713688909000044. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- Stuart PM, Keadle TL. Recurrent herpetic stromal keratitis in mice: a model for studying human hsk. 2012:1–10. doi: 10.1155/2012/728480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi A, Mulik S, Sharma S, Reddy PB, Sehrawat S, Rouse BT. Ocular neovascularization caused by herpes simplex virus type 1 infection results from breakdown of binding between vascular endothelial growth factor A and its soluble receptor. J Immunol. 2011a;186:3653–3665. doi: 10.4049/jimmunol.1003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi A, Mulik S, Sharma S, Reddy PB, Sehrawat S, Rouse BT. Ocular neovascularization caused by herpes simplex virus type 1 infection results from breakdown of binding between vascular endothelial growth factor A and its soluble receptor. J Immunol. 2011b;186:3653–3665. doi: 10.4049/jimmunol.1003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi A, Veiga-Parga T, Rajasagi NK, Reddy PB, Sehrawat S, Sharma S, Rouse BT. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011c;187:1919–1930. doi: 10.4049/jimmunol.1100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi A, Veiga-Parga T, Reddy PB, Rajasagi NK, Rouse BT. IL-17A differentially regulates corneal vascular endothelial growth factor (VEGF)-A and soluble VEGF receptor 1 expression and promotes corneal angiogenesis after herpes simplex virus infection. J Immunol. 2012;188:3434–3446. doi: 10.4049/jimmunol.1102602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- Thomas J, Kanangat S, Rouse BT. Herpes simplex virus replication-induced expression of chemokines and proinflammatory cytokines in the eye: implications in herpetic stromal keratitis. J Interferon Cytokine Res. 1998;18:681–690. doi: 10.1089/jir.1998.18.681. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Chen SH, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey TM, Cheng H, Yan XT, Oakes JE, Lausch RN. Chemokine synthesis in the HSV-1-infected cornea and its suppression by interleukin-10. J Leukoc Biol. 1998;63:486–492. doi: 10.1002/jlb.63.4.486. [DOI] [PubMed] [Google Scholar]
- Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2010;207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Héron B, Vallée L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]
- Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol. 2001;75:9828–9835. doi: 10.1128/JVI.75.20.9828-9835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Klinman DM, Gierynska M, Rouse BT. DNA containing CpG motifs induces angiogenesis. Proc Natl Acad Sci U S A. 2002;99:8944–8949. doi: 10.1073/pnas.132605599. [DOI] [PMC free article] [PubMed] [Google Scholar]