Abstract
Background
Viral upper respiratory infections have been implicated as a major cause of asthma exacerbations among school age children. Regular hand washing is the most effective method to prevent the spread of viral respiratory infections but, effective hand washing practices are difficult to establish in schools.
Objectives
This randomized controlled trial evaluated whether a standardized regimen of hand washing plus alcohol-based hand sanitizer could reduce asthma exacerbations more than schools’ usual hand hygiene practices.
Methods
This was a two year, community-based, randomized controlled crossover trial. Schools were randomized to usual care then intervention (Sequence 1) or intervention then usual care (Sequence 2). Intervention schools were provided with alcohol-based hand sanitizer, hand soap, and hand hygiene education. The primary outcome was the proportion of students experiencing an asthma exacerbation each month. Generalized estimating equations were used to model the difference in the marginal rate of exacerbations between sequences while controlling for individual demographic factors and the correlation within each student and between students within each school.
Results
527 students with asthma were enrolled among 31 schools. The hand hygiene intervention did not reduce the number of asthma exacerbations as compared to the schools’ usual hand hygiene practices (p=0.132). There was a strong temporal trend as both sequences experienced fewer exacerbations during Year 2 as compared to Year 1 (p<0.001).
Conclusions
While the intervention was not found to be effective, the results were confounded by the H1N1 influenza pandemic that resulted in substantially increased hand hygiene behaviors and resources in usual care schools. Therefore, these results should be viewed cautiously.
Keywords: asthma, schools, children, hand hygiene, hand sanitizer
Introduction
Poor asthma control among children is a well documented public health problem.1, 2 It causes respiratory symptoms, limits physical activity, and leads to missed school days and parental work absenteeism.3, 4 As a result of poor control, many children experience frequent asthma exacerbations that lead to urgent care visits, emergency department visits and hospitalizations.1, 2 Among school-age children, the direct and indirect costs attributable to these exacerbation-related outcomes exceed two billion dollars annually.5
Exacerbations have a clear seasonal pattern with most occurring shortly after the summer break from school.6–9 Viral upper respiratory infections have been implicated as the primary cause for this striking seasonal pattern.9–14 Regular hand washing is the most effective method to prevent the spread of viral respiratory infections;15, 16 unfortunately, effective hand washing practices are difficult to establish in schools.17, 18 Barriers include inadequate time, insufficient soap or paper towels and inconveniently located sinks.19–21 A 1998 report of mid-Atlantic elementary school restrooms observed that 66% of soap dispensers were nonfunctional or insufficiently filled and 33% of automatic hand dryers were inoperable.22 A 2009 study of primary and secondary school restrooms in New Mexico reported that soap and hand drying materials were available in 90% of restrooms, but hand sanitizer was reported in fewer than 2%.23
To overcome these barriers, some schools have adopted antimicrobial rinse-free hand sanitizers.18, 24–26 Several studies suggest that hand sanitizer use reduces overall infection-related absenteeism among elementary school students by 20–50%, 18, 19, 25–27 respiratory illnesses by 30–50%,18, 25, 27 and teacher absenteeism by 10%.26 When used in the home, hand sanitizer has been shown to reduce asthma exacerbations in children and respiratory illnesses among family members.17, 28 Despite these findings, a recent Cochrane review did not find evidence to support the incremental effectiveness of hand sanitizer use over that of hand washing alone.15 Despite prior studies of school-based hand sanitizer use suggesting a beneficial effect, a systematic review by Meadows and Le Saux (2004) recommended interpreting the results cautiously as significant limitations in study design were present. 29
To determine if a standardized regimen of hand washing plus alcohol-based hand sanitizer use could reduce asthma exacerbations more than schools’ usual hand hygiene practices, we conducted a randomized controlled trial comparing the two in a large county school system in Birmingham, Alabama.
Methods
This study was a community-based, randomized crossover trial that compared a standardized two-step hand hygiene program (intervention) with schools’ typical hand hygiene practices (usual care). The study was conducted in a single county school district comprising 31 elementary schools and 17,000 students. Approximately, 70% of the district’s enrolled students were white and 30% were black; 33% of students were eligible for free or reduced lunches. The intervention occurred at the school level and was therefore delivered to all 17,000 children. However, study participants on whom data were collected was limited to children with asthma.
After being matched on size and percent of students eligible for free and reduced lunch, individual schools were randomized to receive usual care then intervention (Sequence 1) or intervention then usual care (Sequence 2). The project statistician generated the allocation sequence and assigned schools to sequence. The sequence was concealed until the intervention group was assigned. Children and school employees were not blinded to group assignment but investigators and study staff conducting phone interviews were. The study took place over two school years (10 month August through May period) with the cross-over occurring during the summer break between the first and second school year. Advantages of the crossover design included the ability to control for the seasonal pattern of respiratory illnesses and the ability to avoid assigning some schools to the non-intervention control for the duration of the study. Although the crossover design introduced the potential for carryover effects, the 10 week summer break was thought to be of sufficient length to minimize this possibility as many studies indicate compliance with hand hygiene is low without continued reinforcement.30–32
The two-step hand hygiene intervention included regular hand washing with soap and water supplemented by hand sanitizer use. Intervention schools were provided with alcohol-based hand sanitizer, hand soap, and refills manufactured by GOJO® at no cost. The soap was a fragrance- and dye-free foam solution and the hand sanitizer was a 62% ethyl alcohol foam solution. Study personnel installed hand soap dispensers in the schools’ restrooms and provided disposable hand sanitizer bottles for use in all restrooms, health rooms, and classrooms. The study protocol called for the removal of all hand sanitizer bottles as well as the hand soap dispensers from Sequence 2 schools after the intervention but, we agreed to leave the dispensers in the schools at the request of school officials. However, no hand soap refills were provided for these dispensers during the usual care period. Hand hygiene education was provided using The Centers for Disease Control and Prevention’s School Network for Absenteeism Prevention (SNAP) program.33 Hand washing with soap and water was promoted after using the restroom and when visible dirt was present on the hands.34 Hand sanitizer use was promoted upon arrival in the classroom, before lunch, after using the restroom, and after sneezing or coughing. Hand hygiene education was provided to intervention schools at the start of the school year and was reinforced monthly. The study did not provide usual care schools with hand hygiene education or supplies. Usual care practices were of differing quality and frequency; most usual care schools included hand sanitizer as a personal item on the students’ supply list.
Only students with asthma were enrolled as study participants. These students were enrolled by the study coordinator prior to the determination of the schools’ sequence assignment. Students were recruited by school nurses and referred to the study coordinator if they (1) attended one of the participating schools, (2) had physician diagnosed asthma, and (3) were capable of using a peak flow meter. Written informed consent was obtained from parents and written assent was obtained from the students. The study was approved and monitored by the Institutional Review Boards at the University of Alabama at Birmingham and the University of Arizona. A Data Safety and Monitoring Board monitored participant safety and adverse events.
The primary outcome was the proportion of students experiencing an asthma exacerbation each month as defined as one or more of the following: (1) a red (<50% of personal best) or yellow (50–70% of personal best) peak flow meter reading, (2) increased use of quick relief medication from baseline (≥ 4 puffs), or (3) a respiratory-related school absence.35 A web-based monitoring system (Asthma Agents) developed in collaboration with Blue Cross and Blue Shield of Alabama was used to collect daily data.35, 36 Peak flow meter readings and school absences were recorded daily by students and verified by teachers and/or school nurses. Quick relief medication (Proventil® HFA) for in-school use was provided at no cost to all children enrolled in the study by Schering–Plough, a company now owned by Merck and Co, Inc. A Doser™ was attached to each student’s inhaler to record actuations; actuations were documented every two weeks by study staff. Other variables of interest including age, gender, race, asthma severity, quality of life, asthma control and household smoking exposure were collected during bi-annual phone interviews with parents. The study physician determined asthma severity.
The number of hand soap and hand sanitizer refills was predicted based on the amount dispensed per actuation, the recommended hand hygiene schedule, the school enrollment, and the school environment (number of bathroom and classroom sinks). The total predicted number of bottles of sanitizer per school was calculated as the number of recommended uses per day (5) times the total number of children and staff times the total number of school days (180) divided by the number of uses per bottle (669). The total predicted number of bottles of hand soap was calculated as the number of recommended uses per day (4) times the total number of children and staff times the total number of school days (180) divided by the number of uses per bottle (5000). Refills were stored in the housekeeping office at each school and custodial staff recorded refill dates. The total amount of product provided to each school was also monitored. Monthly assessments inventoried the type of hand hygiene supplies that were available at each school, recorded the hand drying mechanism, monitored the hand hygiene instructions, evaluated general cleanliness, and assessed structural conditions.
Data from the Asthma Agents system and the Doser™ were used to calculate the proportion of students in each group who experienced an exacerbation. Generalized estimating equations were used to determine whether the frequency of exacerbations and marginal rates of exacerbations, defined as the proportion of students within each school who experienced at least one asthma exacerbation each month, differed between the sequences, while allowing for correlation between observations within each student and between students within each school. Adjustment for individual factors such as the student’s age, race, gender and asthma severity were made and a sensitivity analysis was conducted by adjusting for baseline controller therapy. A negative binomial distribution was assumed for assessing the difference in asthma exacerbation frequency while the binomial distribution was assumed for testing the difference in the proportion of children having at least one exacerbation. All analyses were done using SAS Version 9.2.
Power was calculated based on the frequency of exacerbations EPACs observed among the children in our previous school-based study.35 We fixed the sample size and examined a range of magnitudes for the decrease in EPACs due to the intervention, assuming a varying number of children in each school and a varying decrease in the frequency of EPACs due to the intervention. We determined that we would have moderate power (74%) to detect a decrease of 7.5%, even with our smallest projected sample size (average of 14 children per school). If the decrease was as large as 10%, we would have over 90% power to detect this difference. If a carryover effect was observed, we would have slightly over 70% power each year to detect a decrease of 10%, and over 80% power to detect a decrease of 12.5% in each year. We had an average of 17 children per school.
Results
Students with asthma were recruited from January through May of 2009. Schools were randomized in June of 2009 and the intervention began for Sequence 1 schools in August of 2009 and continued through May of 2010. Crossover occurred during the summer break and the intervention began for Sequence 2 schools in August of 2010 and continued through May 2011. Study recruitment, enrollment and drop-out are presented in Figure 1. 527 students with asthma were enrolled among 16 schools randomized to Sequence 1 and 15 schools randomized to Sequence 2. The students’ mean age was 8.9 years (1.8 SD). Sixty percent of students were male, 50% were white, 94% were non-Hispanic, 45% reported baseline daily inhaled corticosteroid use, and 50% qualified for free or reduced lunch. (Table 1) Sequence 1 schools (61.8%) had a higher percentage of white students than Sequence 2 schools (39.8%, p<0.01); otherwise, all measured characteristics were similar across the 2 sequences.
Figure 1.
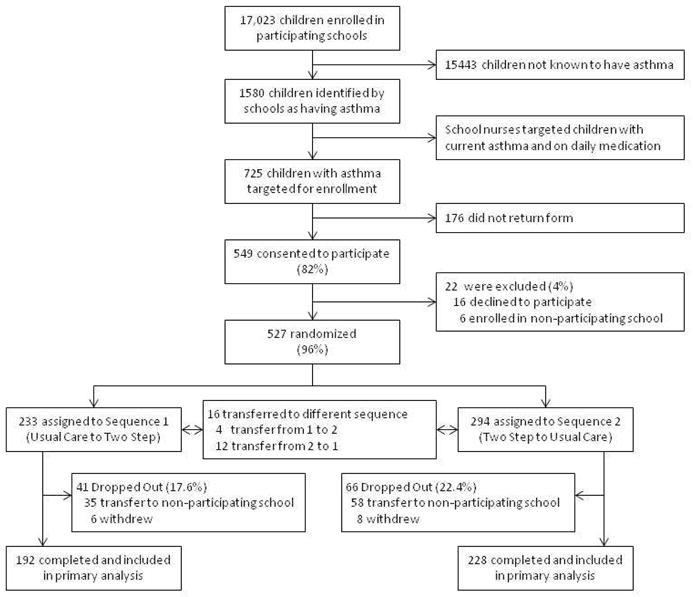
CONSORT diagram.
Table 1.
Baseline Participant Characteristics Overall and by Sequence Assignment
| Overall (N=420) | Sequence 1 (UC to Two Step) (n=192) | Sequence 2 (Two Step to UC) (n=228) | p Value | |
|---|---|---|---|---|
| Schools, (n) | 31 | 16 | 15 | - |
| Nurse Type, (n) | ||||
| Full Time Roving | 5 | 2 | 3 | |
| Full Time | 17 | 11 | 6 | |
| None | 9 | 3 | 6 | 0.30 |
| Enrollment, (n) | 527 | 233 | 294 | - |
| Age, yrs (SD) | 8.9 (1.8) | 8.9 (2.0) | 9.0 (1.7) | 0.92 |
| Gender (male) | 316 (60.0) | 134 (57.5) | 182 (61.9) | 0.31 |
| Race, n (%) | ||||
| White | 261 (49.5) | 144 (61.8) | 117 (39.8) | |
| African-American | 253 (48.0) | 84 (36.1) | 169 (57.5) | <0.01 |
| Mixed | 2 (0.4) | 1 (0.4) | 1 (0.3) | |
| Other | 11 (2.1) | 4 (1.7) | 4 (2.4) | |
| Ethnicity, n (%) | ||||
| Hispanic | 4 (0.8) | 2 (0.9) | 2 (0.7) | 0.31 |
| Non-Hispanic | 495 (93.9) | 217 (93.1) | 278 (94.6) | |
| Missing | 28 (5.3) | 14 (6.0) | 14 (4.8) | |
| Baseline ICS use, n (%) | 236 (44.8) | 104 (44.6) | 132 (44.9) | 0.95 |
| Free/Reduced lunch, n (%) | 232 (50.4) | 100 (47.4) | 132 (53.0) | 0.23 |
ICS, inhaled corticosteroid; UC, usual care
Transfer to a non-participating school accounted for the majority of study withdrawal: 35 of 41 (85%) students in Sequence 1 and 58 of 66 (88%) students in Sequence 2. Fewer students withdrew from Sequence 1 than Sequence 2, but this was not statistically significant (p=0.06). A higher percentage of white students (61%) dropped out of Sequence 1 as compared to Sequence 2 (39%, p=0.03) which was likely attributable to the higher proportion of white students in Sequence 1. Otherwise, the characteristics of the students who dropped-out did not differ by sequence assignment. Sixteen students transferred to a school with a different sequence assignment; these children were analyzed according to their initial assignment. The analytic sample comprised the 420 students who completed the study: 192 of 233 (82%) assigned to Sequence 1 and 228 of 294 (78%) assigned to Sequence 2.
Of the 126,419 expected daily diary reports, 15% were missing the student’s asthma symptoms and 4% were missing the student’s peak flow meter reading. None of the reports for the student’s albuterol use were missing. (Table 2) Of the 7,326 reported absences, 3% were missing the reason for the absence. Sequence 1 students were more likely to have missing symptoms, peak flow meter readings, and absence reasons than Sequence 2 students (all p values <0.0001); however, the absolute magnitude of these differences was small.
Table 2.
Availability of Primary Outcome Data Overall and by Sequence Assignment
| Overall N (%) |
Sequence 1 (UC to Two Step) n (%) |
Sequence 2 (Two Step to UC) n (%) |
p Value | |
|---|---|---|---|---|
| Teacher Reports | 126,419 (100) | 58,715 (100) | 67,704 (100) | - |
| Missing symptoms | 19,311 (15.3) | 10,129 (17.3) | 9,182 (13.6) | <0.0001 |
| Missing PFM value | 5,153 (4.1) | 3,679 (6.3) | 1,474 (2.2) | <0.0001 |
| Missing albuterol use | 0 | 0 | 0 | - |
| School Absences | 7,326 (100) | 3,419 (100) | 3,907 (100) | - |
| Missing reason | 224 (3.1) | 165 (4.8) | 59 (1.5) | <0.0001 |
PFM, peak flow meter; UC, usual care
Overall, schools requested 63% (9,319/25,187) fewer hand sanitizer refills than predicted; however, they requested 77% (4,913/2,782) more hand soap refills. The ratio of observed versus predicted hand sanitizer refills was lower for Sequence 1 schools as compared to Sequence 2 schools (p=0.03; 34% of predicted versus 44% of predicted), but the ratio of observed versus predicted hand soap refills was similar between them (p=0.31; 184% of predicted versus 221% of predicted). Hand soap dispensers were more likely to be operational during intervention years in both sequences (both p<0.001). (Table 3) Bathroom hand soap was more likely to be available during the intervention year in Sequence 1 (p<0.001), but not in Sequence 2 (p=0.65). Classroom hand soap was more likely to be available during intervention years in both sequences (both p<0.001). Hand sanitizer was similarly available during intervention and usual care years in Sequence 1 (p=0.613), but it was more likely to be available during the intervention year in Sequence 2 (p<0.001). After Sequence 1 schools transitioned from usual care to the two-step intervention, more hand soap dispensers were operational (p<0.001) and more hand soap and hand sanitizer were available (all p ≤0.001) during the second year. (Table 3) After Sequence 2 schools transitioned from the two-step intervention to usual care, there were fewer functional dispensers (p<0.001) and less hand soap in the classrooms (p<0.001), but equal amounts of hand soap in the bathrooms (p=0.931) and hand sanitizer in the classrooms (p=0.089) during the second year. No adverse events related to hand sanitizer use were reported during the study.
Table 3.
Operational Status of Hand Soap Dispensers and Availability of Hand Soap and Hand Sanitizer by Year and Sequence Assignment
| Year 1 UC vs. Two Step |
Year 2 UC vs. Two Step |
Sequence 1 Yr 1 vs. Yr 2 |
Sequence 2 Yr 1 vs. Yr 2 |
|
|---|---|---|---|---|
| Operational hand soap dispensers | 74.1% vs. 88.2% p<0.001 |
67.8% vs. 94.8% p<0.0001 |
74.1% vs. 94.8% p<0.0001 |
88.2% vs. 67.8% p<0.0001 |
| Hand soap, bathrooms | 96.3% vs.99.7% p=0.001 |
99.7% vs. 100% p=0.646 |
96.3% vs. 100% p=0.0002 |
99.7% vs. 99.7% p=0.931 |
| Hand soap, classrooms | 77.6% vs.97.4% p<0.0001 |
85.0% vs. 98.9% p<0.0001 |
77.6% vs. 98.9% p<0.0001 |
97.4% vs. 85.0% p<0.0001 |
| Hand sanitizer, classrooms | 80.6% vs.82.1% p=0.613 |
76.9% vs. 90.6% p<0.001 |
80.6% vs. 90.6% p=0.0001 |
82.1% vs. 76.9% p=0.089 |
Chi-square tests showed that the number of monthly asthma exacerbations were similar for students assigned to the two-step intervention and usual care during Years 1 and 2 except for a single month in November 2009 (Year 1) where a greater percent of students in the two-step intervention (36%) had exacerbations than students in usual care (27%; p=0.03). (Table 4) During this month, a greater percentage of students in the two-step intervention experienced a respiratory absence (15% vs. 9%, p=0.04) and a greater percentage had elevated albuterol use (13% vs. 4%, p=0.001); however, the percent with a red/yellow peak flow meter reading were similar (23% vs. 19%, p=0.29). (Table 5).
Table 4.
Number (%) of Monthly Asthma Exacerbations by Year and Sequence Assignment.
| Year 1 | Sep 2009 | Oct 2009 | Nov 2009 | Dec 2009 | Jan 2010 | Feb 2010 | Mar 2010 | Apr 2010 | May 2010 |
|---|---|---|---|---|---|---|---|---|---|
| Sequence 1 (Usual Care) | 75 (32) | 95 (41) | 63 (27) | 49 (21) | 62 (27) | 73 (31) | 66 (28) | 80 (34) | 47 (20) |
| Sequence 2 (Two Step) | 97 (33) | 121 (41) | 106 (36) | 75 (26) | 86 (29) | 101 (34) | 77 (26) | 101 (34) | 57 (19) |
| p-value | 0.84 | 0.93 | 0.03 | 0.23 | 0.50 | 0.46 | 0.58 | 0.99 | 0.82 |
|
| |||||||||
| Year 2 | Sep 2010 | Oct 2010 | Nov 2010 | Dec 2010 | Jan 2011 | Feb 2011 | Mar 2011 | Apr 2011 | May 2011 |
| Sequence 1 (Two Step) | 40 (17) | 59 (25) | 61 (26) | 47 (20) | 47 (20) | 60 (26) | 38 (16) | 55 (24) | 28 (12) |
| Sequence 2 (Usual Care) | 50 (17) | 75 (26) | 88 (30) | 43 (15) | 41 (14) | 73 (25) | 54 (18) | 58 (20) | 25 (9) |
| p-value | 0.96 | 0.96 | 0.34 | 0.09 | 0.06 | 0.81 | 0.54 | 0.28 | 0.18 |
Table 5.
Number (%) of Monthly Asthma Exacerbations by Exacerbation Component, Year and Sequence Assignment.
| ≥1 Red or Yellow Peak Flow Meter Reading | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Year 1 | Sep 2009 | Oct 2009 | Nov 2009 | Dec 2009 | Jan 2010 | Feb 2010 | Mar 2010 | Apr 2010 | May 2010 |
| Sequence 1 (Usual Care) | 47 (20) | 46 (20) | 45 (19) | 28 (12) | 46 (20) | 53 (23) | 52 (22) | 64 (27) | 32 (14) |
| Sequence 2 (Two Step) | 66 (22) | 82 (28) | 68 (23) | 50 (17) | 47 (16) | 65 (22) | 42 (14) | 62 (21) | 39 (13) |
| p-value | 0.53 | 0.03 | 0.29 | 0.11 | 0.26 | 0.86 | 0.02 | 0.09 | 0.88 |
| Year 2 | Sep 2010 | Oct 2010 | Nov 2010 | Dec 2010 | Jan 2011 | Feb 2011 | Mar 2011 | Apr 2011 | May 2011 |
| Sequence 1 (Two Step) | 36 (15) | 49 (21) | 40 (17) | 29 (12) | 27 (12) | 39 (17) | 27 (12) | 27 (12) | 18 (8) |
| Sequence 2 (Usual Care) | 39 (13) | 55 (19) | 55 (19) | 25 (9) | 22 (7) | 48 (16) | 37 (13) | 34 (12) | 11 (4) |
| p-value | 0.48 | 0.51 | 0.65 | 0.14 | 0.11 | 0.90 | 0.73 | 0.99 | 0.05 |
|
|
|||||||||
|
≥1 Respiratory Absence
|
|||||||||
| Year 1 | Sep 2009 | Oct 2009 | Nov 2009 | Dec 2009 | Jan 2010 | Feb 2010 | Mar 2010 | Apr 2010 | May 2010 |
| Sequence 1 (Usual Care) | 35 (15) | 64 (27) | 22 (9) | 20 (9) | 23 (10) | 27 (12) | 25 (11) | 33 (14) | 19 (9) |
| Sequence 2 (Two Step) | 34 (12) | 65 (22) | 45 (15) | 27 (9) | 37 (13) | 40 (14) | 36 (12) | 38 (13) | 16 (5) |
| p-value | 0.24 | 0.16 | 0.04 | 0.81 | 0.33 | 0.49 | 0.59 | 0.68 | 0.21 |
| Year 2 | Sep 2010 | Oct 2010 | Nov 2010 | Dec 2010 | Jan 2011 | Feb 2011 | Mar 2011 | Apr 2011 | May 2011 |
| Sequence 1 (Two Step) | 6 (3) | 11 (5) | 28 (12) | 18 (8) | 22 (9) | 26 (11) | 10 (4) | 21 (9) | 6 (3) |
| Sequence 2 (Usual Care) | 7 (2) | 24 (8) | 39 (13) | 16 (5) | 16 (5) | 39 (13) | 20 (7) | 25 (9) | 6 (2) |
| p-value | 0.89 | 0.12 | 0.67 | 0.29 | 0.08 | 0.47 | 0.22 | 0.84 | 0.68 |
|
|
|||||||||
|
Albuterol Use Greater than Baseline Use
|
|||||||||
| Year 1 | Sep 2009 | Oct 2009 | Nov 2009 | Dec 2009 | Jan 2010 | Feb 2010 | Mar 2010 | Apr 2010 | May 2010 |
| Sequence 1 (Usual Care) | 7 (3) | 8 (4) | 9 (4) | 8 (4) | 6 (3) | 5 (2) | 6 (3) | 8 (4) | 5 (2) |
| Sequence 2 (Two Step) | 18 (7) | 27 (11) | 34 (13) | 14 (5) | 18 (7) | 23 (9) | 14 (5) | 27 (11) | 14 (5) |
| p-value | 0.06 | 0.004 | 0.001 | 0.34 | 0.03 | 0.002 | 0.13 | 0.004 | 0.07 |
| Year 2 | Sep 2010 | Oct 2010 | Nov 2010 | Dec 2010 | Jan 2011 | Feb 2011 | Mar 2011 | Apr 2011 | May 2011 |
| Sequence 1 (Two Step) | 6 (3) | 6 (3) | 6 (3) | 6 (3) | 7 (3) | 6 (3) | 11 (5) | 16 (7) | 8 (4) |
| Sequence 2 (Usual Care) | 12 (5) | 9 (4) | 13 (5) | 11 (4) | 5 (2) | 13 (5) | 11 (4) | 15 (5) | 11 (4) |
| p-value | 0.30 | 0.62 | 0.19 | 0.35 | 0.40 | 0.19 | 0.58 | 0.39 | 0.85 |
Overall, the two-step hand hygiene intervention did not reduce the number of asthma exacerbations as compared to the schools’ usual hand hygiene practices (p=0.132). There was a strong temporal trend as both sequences experienced fewer exacerbations during Year 2 as compared to Year 1 (p<0.001). (Figure 2) Given a non-significant intervention and year interaction term (p=0.551), no evidence of a carryover effect between Year 1 and Year 2 was observed. We also examined the data for year two separately and observed no treatment effect (p=0.82) and adjusting for controller medication at baseline did not change any of the results.
Figure 2.
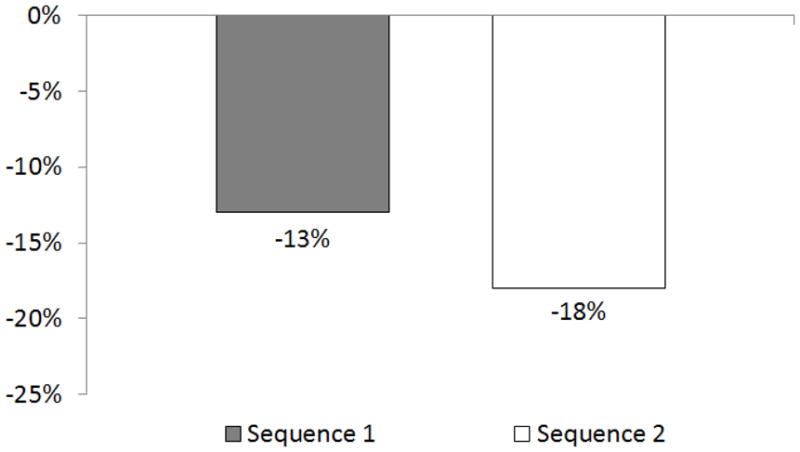
Change in percent of children experiencing an episode of poor asthma control from year 1 to year 2 by treatment group.
Discussion
Providing hand hygiene education plus hand washing and hand sanitizer supplies to elementary schools did not reduce exacerbations among students with asthma more than usual care practices. While the intervention increased the proportion of operational hand soap dispensers in bathrooms and the presence of hand soap and hand sanitizer in classrooms, it did not increase the presence of hand soap in bathrooms. It is not surprising that the intervention did not consistently increase bathroom hand soap presence as it was highly prevalent within usual care schools, greater than 95% of all observations. While the intervention increased the availability of hand sanitizer in classrooms, the increase was modest, 77–81% of usual care observations versus 82–90% of intervention observations. All hand sanitizer present within usual care classrooms was purchased by parents and teachers and reflected their belief in its importance. Although the intervention was not found to be effective, the results were confounded by unusual external events.
Prior to the start of the trial in the spring of 2009, the United States was in the initial stages of an influenza A (H1N1) pandemic. By October of 2009, the CDC declared that H1N1 was widespread in 46 of 50 states, including Alabama.37 Shortly thereafter, President Obama declared a national emergency. The H1N1 pandemic was likely responsible for the temporal trend where higher percentage of students experienced an exacerbation during Year 1 (2009–2010) as compared to Year 2 (2010–2011) in both sequences. For example, 41% of enrolled children experienced an asthma exacerbation in October 2009 as compared to 25% in October 2010. School age children were particularly susceptible during this H1N1 outbreak.38 An investigation of an elementary school outbreak in Pennsylvania noted that children 5–10 years of age were most likely to report an influenza-like illness (24.5%); this percentage was 4.6 times higher than that of adults 19–54 years of age.39 In Chicago, children 5–14 years of age were noted to have the highest influenza-like illness attack rate (147 per 100,00); this rate was 14 times higher than that of adults 60 years of age and older.40
During the H1N1 outbreak, the US public substantially increased its hand hygiene behaviors including more frequent hand washing and hand sanitizer use.38, 41–43 An internet-based survey with 70% of responding adults residing in the US indicated that 80% of adults reported more frequent hand washing and 35% reported more frequent alcohol-based hand sanitizer use.42 A survey of a large US public university revealed that 96% of college students, faculty, and staff reported more frequent hand washing and 79% of students and 66% of faculty and staff reported more frequent hand sanitizer use.41 The H1NI outbreak served as a strong external intervention that increased hand hygiene behaviors in usual care schools thereby diminishing the relative effectiveness of the intervention.
The public’s increased hand hygiene behaviors were reflected in US hand sanitizer sales. As compared to the 3rd quarter of 2008, the 3rd quarter of 2009 saw a three-fold increase in the amount of hand sanitizer shipped in the US, 1 million versus 3 million kilograms, respectively.44 Sales of Purell® brand hand sanitizer were 50% higher in August 2009 as compared to August 2008.45 In the 24 weeks ending October 3rd 2009, dollar sales of hand sanitizer were 71% ($118.4 million) higher than the same 24 week period in 2008.46 These data provide strong evidence that the amount of hand sanitizer present in usual care schools, particularly in 2009, was probably much higher than ever before.
Our trial is not the only one that has failed to establish a clear incremental benefit from hand sanitizer use. Despite encouraging early data,19, 26 subsequent school-based trials have been unable to demonstrate the benefits of adding an alcohol-based hand sanitizer to a school’s typical hand washing practices.28, 47 Two recent reviews question whether hand sanitizer adds any benefit over hand washing alone when attempting to reduce respiratory illnesses in general15 or influenza in particular.48 Both reviews noted that design limitations associated with the early, more favorable studies could have led to confounding. Two school-based studies that have used a non-alcohol hand sanitizer (benzalkonium chloride) have reported 25–40% reductions in illness-related absences among elementary students.27, 49 However, these studies also suffer from similar limitations, particularly the lack of a placebo control.
Our original study design utilized a benzalkonium chloride containing hand sanitizer and accompanying placebo; however, we subsequently adopted an alcohol-based product because of several concerns.50 Several laboratory studies published after the grant award suggested several safety concerns regarding benzalkonium chloride use.51–54 Because we were unable to obtain a suitable placebo for our alcohol-based hand sanitizer, we abandoned the placebo control for a usual care control with. Ultimately, this design change in conjunction with the increased hand hygiene behaviors associated with the H1N1 outbreak created contamination between the intervention and control schools thereby limiting our ability to detect an incremental benefit from hand sanitizer use.
Given H1N1’s impact on our study, it is important to consider evidence supporting hand hygiene as a mechanism to reduce influenza transmission.55–57 In Hong Kong, frequent hand washing plus hand sanitizer use failed to reduce secondary influenza transmission among 407 households containing a confirmed influenza case.55 During the 2009–2010 pandemic in Germany, a similar pattern was observed among 84 households where frequent hand washing plus hand sanitizer use failed to reduce influenza transmission.56. However, both studies demonstrated that hand washing, hand sanitizer, plus facemask use could reduce secondary transmission by 67–85% if instituted within 36 hours of the diagnosis. A cluster randomized trial of Michigan college students during the 2007–2008 influenza season failed to demonstrate a reduction in influenza cases among students assigned to either hand washing plus hand sanitizer use or hand washing, hand sanitizer, plus facemask use.57
Despite repeated attempts, it has been difficult to demonstrate that hand sanitizer when added to a regular hand washing reduces respiratory infections. Given the substantial economic and non-economic costs associated with respiratory illnesses, it is important to know if expending resources on hand sanitizer is beneficial. The ability to answer this question using a community-based trial is hampered by a myriad of threats to internal validity including non-adherence, non-response, cross-over, drop-out, and inadequate blinding.29 Unfortunately, we were unable to adequately account for these challenges in this study. Based on these limitations, our study results should be viewed cautiously.
Clinical Implications.
It is unclear whether hand sanitizer, when added to hand washing, reduces respiratory infections and asthma exacerbations among children.
Acknowledgments
Funding/Support: This work was supported by a grant from the National Heart, Lung, and Blood Institute 1 R01 HL086972. Quick relief medication for in-school use was donated by Schering-Plough, now owned by Merck and Co., Inc. and data system support was provided by BlueCross and BlueShield of Alabama. Registered at clinicaltrials.gov NCT00528814
Jodi Atchison, Katie D’alessandro, Sue Erwin, Terry Hooks, Shayla Knight, Mary Lou Lackey, Joan Mangan, Kenya Mathews, Marianne McLaughlin, Dominga Toner, and the following Jefferson County schools: Bagley, Brighton Middle, Brookville Elementary, Bryan Elementary, Center Point Elementary, Chalkville Elementary, Clay Elementary, Clay-Chalkville Middle, Corner, Crumly Chapel Elementary, Erwin Elementary, Fultondale Elementary, Gardendale Elementary, Grantswood Community, Gresham Elementary, Hillview Elementary, Hueytown Elementary, Hueytown Middle, Johnson Elementary, Lispcomb Elementary, McAdory Elementary, Minor Community, Mount Olive Elementary, North Highland Elementary, Oak Grove Elementary, Pinson Elementary, Pleasant Grove Elementary, Snow Rogers Elementary, Warrior Elementary, West Jefferson Elementary.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lynn B. Gerald, Email: lgerald@email.arizona.edu, Division of Health Promotion Sciences, Mel and Enid Zuckerman College of Public Health, Arizona Respiratory Center, University of Arizona, 1295 N Martin Avenue, PO Box 245163, Tucson, AZ 85724-5163, (520)626-3243, (520)626-6093 FAX.
Joe K. Gerald, Email: geraldj@email.arizona.edu, Division of Community, Environment, and Policy, Mel and Enid Zuckerman College of Public Health, University of Arizona.
Bin Zhang, Email: Bin.Zhang@cchmc.org, Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center.
Leslie A. McClure, Email: LMcClure@ms.soph.uab.edu, Department of Biostatistics, University of Alabama at Birmingham.
William C. Bailey, Email: wcbailey@uab.edu, Lung Health Center School Of Medicine, University of Alabama at Birmingham.
Kathy F. Harrington, Email: Kathy.Harrington@ccc.uab.edu, Lung Health Center, Division of Pulmonary, Allergy and Critical Care Medicine, University of Alabama at Birmingham.
References
- 1.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110(2 Pt 1):315–322. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami L. The state of childhood asthma, United States, 1980–2005. Adv Data. 2006;381:1–24. [PubMed] [Google Scholar]
- 3.Schmier JK, Manjunath R, Halpern MT, Jones ML, Thompson K, Diette GB. The impact of inadequately controlled asthma in urban children on quality of life and productivity. Ann Allergy Asthma Immunol. 2007;98(3):245–251. doi: 10.1016/S1081-1206(10)60713-2. [DOI] [PubMed] [Google Scholar]
- 4.Bateman ED, Frith LF, Braunstein GL. Achieving guideline-based asthma control: does the patient benefit? Eur Respir J. 2002;20(3):588–595. doi: 10.1183/09031936.02.00294702. [DOI] [PubMed] [Google Scholar]
- 5.Wang LY, Zhong Y, Wheeler L. Direct and indirect costs of asthma in school-age children. Prev Chronic Dis. 2005;2(1):A11. [PMC free article] [PubMed] [Google Scholar]
- 6.Dales RE, Schweitzer I, Toogood JH, Drouin M, Yang W, Dolovich J, et al. Respiratory infections and the autumn increase in asthma morbidity. European Respiratory Journal. 1996;9:72–77. doi: 10.1183/09031936.96.09010072. [DOI] [PubMed] [Google Scholar]
- 7.Silverman RA, Ito K, Stevenson L, Hastings HM. The relationship of fall school opening and emergency department asthma visits in a large metropolitan area. Arch Pediatrics and Adolescent Medicine. 2005;159:818–823. doi: 10.1001/archpedi.159.9.818. [DOI] [PubMed] [Google Scholar]
- 8.Blaisdell CJ, Weiss SR, Kimes DS, Levine ER, Myers M, Timmins S, et al. Using seasonal variations in asthma hospitalizations in children to predict hospitalization frequency. J Asthma. 2002;39(7):567–575. doi: 10.1081/jas-120014921. [DOI] [PubMed] [Google Scholar]
- 9.Johnston NW, Johnston SL, Ducan JM, Greene JM, Kebadze T, Keith PKRM, et al. The September epidemic of asthma exacerbations in children: A search for etiology. J Allergy and Clin Immunology. 2005;115:132–138. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freymuth F, Vabret A, Brouard J, Toutain F, Verdon R, Petitjean J, et al. Detection of viral, Chlamydia pneumoniae and Mycoplasma pneumoniae infections in exacerbations of asthma in children. J Clin Virol. 1999;13(3):131–139. doi: 10.1016/S1386-6532(99)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310(6989):1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monto AS. The seasonality of rhinovirus infections and its implications for clinical recognition. Clin Ther. 2002;24(12):1987–1997. doi: 10.1016/S0149-2918(02)80093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159(3):785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 14.Thumerelle C, Deschildre A, Bouquillon C, Santos C, Sardet A, Scalbert M, et al. Role of viruses and atypical bacteria in exacerbations of asthma in hospitalized children: a prospective study in the Nord-Pas de Calais region (France) Pediatr Pulmonol. 2003;35(2):75–82. doi: 10.1002/ppul.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jefferson T, Del Mar CB, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. (7):CD006207. doi: 10.1002/14651858.CD006207.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and P. Guideline for Hand Hygiene in Health-Care Settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR. 2002;51(RR-16):1–44. [PubMed] [Google Scholar]
- 17.Lee G, Salomon J, Friedman J, Hibberd P, Ross-Degnan D, Zasloff E, et al. Illness transmission in the home: A possible role for alcohol-based hand gels. Pediatrics. 2005;115(4):852–860. doi: 10.1542/peds.2004-0856. [DOI] [PubMed] [Google Scholar]
- 18.White CG, Shinder FS, Shinder AL, Dyer DL. Reduction of illness absenteeism in elementary schools using an alcohol-free instant hand sanitizer. J Sch Nurs. 2001;17(5):258–265. [PubMed] [Google Scholar]
- 19.Guinan M, McGuckin M, Ali Y. The effect of a comprehensive handwashing program on absenteeism in elementary schools. Am J Infect Control. 2002;30(4):217–220. doi: 10.1067/mic.2002.120366. [DOI] [PubMed] [Google Scholar]
- 20.Robinson J. The changing role of the school nurse: a partner in infection control and disease prevention. J Sch Nurs. 2002;(Suppl):12–14. doi: 10.1177/105984050201800403. [DOI] [PubMed] [Google Scholar]
- 21.Vessey JA, Sherwood JJ, Warner D, Clark D. Comparing hand washing to hand sanitizers in reducing elementary school students’ absenteeism. Pediatr Nurs. 2007;33(4):368–372. [PubMed] [Google Scholar]
- 22.Early E, Battle K, Cantwall E, English J, Lavin A, Larson E. Effect of several interventions on the frequency of handwashing among elementary public school children. Am J Infect Control. 1998;26(3):263–269. doi: 10.1016/s0196-6553(98)80011-4. [DOI] [PubMed] [Google Scholar]
- 23.Ramos MM, Blea M, Trujillo R, Greenberg C. Inspections of Hand Washing Supplies and Hand Sanitizer in Public Schools. J Sch Nurs. 2010 doi: 10.1177/1059840510370328. [DOI] [PubMed] [Google Scholar]
- 24.White C, Kolble R, Carlson R, Lipson N, Dolan M, Ali Y, et al. The effect of hand hygiene on illness rate among students in university residence halls. Am J Infect Control. 2003;31(6):364–370. doi: 10.1016/s0196-6553(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 25.Morton J, Schulta A. Healthy hands: Use of alcohol gel as an adjunct to handwashing in elementary school children. The Journal of School Nursing. 2004;20(3):161–167. doi: 10.1177/10598405040200030601. [DOI] [PubMed] [Google Scholar]
- 26.Hammond B, Ali Y, Fendler E, Dolan M, Donovan S. Effect of hand sanitizer use on elementary school absenteeism. Am J Infect Control. 2000;28(5):340–346. doi: 10.1067/mic.2000.107276. [DOI] [PubMed] [Google Scholar]
- 27.Dyer DL, Shinder A, Shinder F. Alcohol-free instant hand sanitizer reduces elementary school illness absenteeism. Fam Med. 2000;32(9):633–638. [PubMed] [Google Scholar]
- 28.Sandora T, Taveras E, Shih M, Resnick E, Lee G, Ross-Degnan D, et al. A randomized, controlled trial of a multifaceted intervention including alcohol-based hand sanitizer and hand-hygiene education to reduce illness transmission in the home. Pediatrics. 2005;116(3):587–594. doi: 10.1542/peds.2005-0199. [DOI] [PubMed] [Google Scholar]
- 29.Meadows E, LeSaux N. A systematic review of the effectiveness of antimicrobial rinse-free hand sanitizers for prevention of illness-related absenteeism in elementary school children. BMC Public Health. 2004;4:50. doi: 10.1186/1471-2458-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larson E, Kretzer EK. Compliance with handwashing and barrier precautions. Journal of Hospital Infection. 1995;30(Supplement):88–106. doi: 10.1016/0195-6701(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 31.Maskerine C, Loeb M. Improving adherence to hand hygiene among health care workers. Journal of Continuing Education in the Health Professions. 2006;26:244–251. doi: 10.1002/chp.77. [DOI] [PubMed] [Google Scholar]
- 32.Pittet D. Promotion of Hand Hygiene? Magic, Hype or Scientific Challenge? Infection Control and Hospital Epidemiology. 2002;23(3):118–119. doi: 10.1086/502019. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. [Accessed June 27, 2012];Healthy Schools, Healthy People School Network for Absenteeism Prevention. http://www.itsasnap.org/index.asp.
- 34.Centers for Disease Control and P. [Accessed July 10, 2010];Clean Hands Saves Lives! http://www.cdc.gov/cleanhands. Published 2010.
- 35.Gerald LB, McClure LA, Mangan JM, Harrington KF, Gibson L, Erwin S, et al. Increasing adherence to inhaled steroid therapy among schoolchildren: randomized, controlled trial of school-based supervised asthma therapy. Pediatrics. 2009;123(2):466–474. doi: 10.1542/peds.2008-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClure LA, Harrington K, Graham H, Gerald LB. Internet-based monitoring of asthma symptoms, peak flow meter readings, and absence data in a school-based clinical trial. Clinical Trials. 2008;5:31–37. doi: 10.1177/1740774507086647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zengerie P. [Accessed June 20, 2012];Obama declares swine flu a national emergency. http://www.reuters.com/article/2009/10/24/us-flu-usa-obama-idUSTRE59N19E20091024. Published 2009.
- 38.Iuliano AD, Dawood FS, Silk BJ, Bhattarai A, Copeland D, Doshi S, et al. Investigating 2009 pandemic influenza A (H1N1) in US schools: what have we learned? Clin Infect Dis. 52(Suppl 1):S161–167. doi: 10.1093/cid/ciq032. [DOI] [PubMed] [Google Scholar]
- 39.Marchbanks TL, Bhattarai A, Fagan RP, Ostroff S, Sodha SV, Moll ME, et al. An outbreak of 2009 pandemic influenza A (H1N1) virus infection in an elementary school in Pennsylvania. Clin Infect Dis. 52(Suppl 1):S154–160. doi: 10.1093/cid/ciq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.2009 pandemic influenza A (H1N1) virus infections - Chicago, Illinois, April-July 2009. MMWR Morb Mortal Wkly Rep. 2009;58(33):913–918. [PubMed] [Google Scholar]
- 41.Mitchell T, Dee DL, Phares CR, Lipman HB, Gould LH, Kutty P, et al. Non-pharmaceutical interventions during an outbreak of 2009 pandemic influenza A (H1N1) virus infection at a large public university, April–May 2009. Clin Infect Dis. 52(Suppl 1):S138–145. doi: 10.1093/cid/ciq056. [DOI] [PubMed] [Google Scholar]
- 42.Jones JH, Salathe M. Early assessment of anxiety and behavioral response to novel swine-origin influenza A(H1N1) PLoS One. 2009;4(12):e8032. doi: 10.1371/journal.pone.0008032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubin GJ, Amlot R, Page L, Wessely S. Pulbic perceptions, anxiety, and behavior change in relation to the swine flu outbreak: Cross-sectional telephone survey. BMJ. 2009:339. doi: 10.1136/bmj.b2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rooney B. [Accessed June 22, 2011];Hand sanitizer in short supply as swine flu hits. http://money.cnn.com/2009/10/22/news/economy/hand_sanitizer/. Published 2009.
- 45.Crowe A. [Accessed June 22, 2012];Swine flu leading to Purell shortage. http://www.dailyfinance.com/2009/10/15/swine-flu-leading-to-purell-shortage/. Published 2009.
- 46. [Accessed June 22, 2012];Hand sanitizer sales clean up amid flu concerns. http://blog.nielsen.com/nielsenwire/consumer/hand-sanitizer-sales-clean-up-amid-flu-concerns/. Published October 30, 2009.
- 47.Stebbins S, Cummings DA, Stark JH, Vukotich C, Mitruka K, Thompson W, et al. Reduction in the incidence of influenza A but not influenza B associated with use of hand sanitizer and cough hygiene in schools: a randomized controlled trial. Pediatr Infect Dis J. 30(11):921–926. doi: 10.1097/INF.0b013e3182218656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aledort JE, Lurie N, Wasserman J, Bozzette SA. Non-pharmaceutical public health interventions for pandemic influenza: an evaluation of the evidence base. BMC Public Health. 2007;7:208. doi: 10.1186/1471-2458-7-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lau CH, Springston EE, Sohn MW, Mason I, Gadola E, Damitz M, et al. Hand hygiene instruction decreases illness-related absenteeism in elementary schools: a prospective cohort study. BMC Pediatr. 12(1):52. doi: 10.1186/1471-2431-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerald LB, Gerald JK, McClure LA, Harrington KF, Erwin S, Bailey WC. Redesigning a Large School-Based Clinical Trial in Response to Changes in Community Practice. Clinical Trials. 2011;8:311–319. doi: 10.1177/1740774511403513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferk F, Misik M, Hoelzl C, Uhl M, Fuerhacker M, Grillitsch B, et al. Benzalkonium chloride (BAC) and dimethyldioctadecyl-ammonium bromide (DDAB), two common quaternary ammonium compounds, cause genotoxic effects in mammalian and plant cells at environmentally relevant concentrations. Mutagenesis. 2007;22(6):363–370. doi: 10.1093/mutage/gem027. [DOI] [PubMed] [Google Scholar]
- 52.Varani J, Perone P, Spahlinger DM, Singer LM, Diegel KL, Bobrowski WF, et al. Human skin in organ culture and human skin cells (keratinocytes and fibroblasts) in monolayer culture for assessment of chemically induced skin damage. Toxicol Pathol. 2007;35(5):693–701. doi: 10.1080/01926230701481907. [DOI] [PubMed] [Google Scholar]
- 53.Houari A, Di Martino P. Effect of chlorhexidine and benzalkonium chloride on bacterial biofilm formation. Lett Appl Microbiol. 2007;45(6):652–656. doi: 10.1111/j.1472-765X.2007.02249.x. [DOI] [PubMed] [Google Scholar]
- 54.Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157(4):795–798. doi: 10.1111/j.1365-2133.2007.08134.x. [DOI] [PubMed] [Google Scholar]
- 55.Cowling BJ, Chan KH, Fang VJ, Cheng CK, Fung RO, Wai W, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;151(7):437–446. doi: 10.7326/0003-4819-151-7-200910060-00142. [DOI] [PubMed] [Google Scholar]
- 56.Suess T, Remschmidt C, Schink SB, Schweiger B, Nitsche A, Schroeder K, et al. The role of facemasks and hand hygiene in the prevention of influenza transmission in households: results from a cluster randomised trial; Berlin, Germany, 2009–2011. BMC Infect Dis. 12:26. doi: 10.1186/1471-2334-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aiello AE, Perez V, Coulborn RM, Davis BM, Uddin M, Monto AS. Facemasks, hand hygiene, and influenza among young adults: a randomized intervention trial. PLoS One. 7(1):e29744. doi: 10.1371/journal.pone.0029744. [DOI] [PMC free article] [PubMed] [Google Scholar]


