Abstract
PURPOSE
To compare corneal endothelial cell density (ECD) and morphology 5 years after laser in situ keratomileusis (LASIK) between flap creation with a femtosecond laser and flap creation with a mechanical microkeratome.
SETTING
Mayo Clinic, Rochester, Minnesota, USA.
DESIGN
Prospective randomized masked paired-eye study.
METHODS
In this study of LASIK for myopia or myopic astigmatism, fellow eyes were randomized by ocular dominance to flap creation by a femtosecond laser or by a mechanical microkeratome. Central endothelial images were analyzed before and 3 years and 5 years after LASIK; endothelial cell variables were compared between treatments at each examination. Relationships between endothelial cell loss and contact lens wear, residual bed thickness, and preoperative refractive error were evaluated.
RESULTS
There were no differences in the ECD, percentage of hexagonal cells, or coefficient of variation of cell area between treatments at any examination (all P = .99); the smallest detectable differences were 120 cells/mm2, 5%, and 2%, respectively. The mean annual rate of corneal endothelial cell loss was −0.1% ± 1.2% (SD) and −0.1% ± 1.0% for the femtosecond laser and the mechanical microkeratome, respectively. Endothelial cell loss was not associated with contact lens wear, residual bed thickness, or preoperative refractive error.
CONCLUSIONS
The energy delivered to the cornea during femtosecond laser flap creation did not affect the corneal endothelium 5 years after LASIK when compared with flap creation with a mechanical microkeratome. Corneas that have had either method of flap creation could be accepted as donor tissue for endothelial keratoplasty from the standpoint of endothelial health.
Laser in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK) are photoablative procedures of the corneal stroma that treat refractive errors and are the most common types of corneal surgery. Corneal clarity and function depend on an intact and healthy corneal endothelium, and surgical procedures of the cornea should not adversely affect this nonregenerative cell layer. Although an early study1 suggested that endothelial cell loss 12 months after LASIK was as high as 8%, we recently found that the rate of cell loss during the first 9 years after LASIK did not differ from age-related physiologic cell loss in unoperated corneas.2 In our previous study,2 LASIK flaps were created with a mechanical microkeratome; we were unable to extrapolate our results to LASIK with flaps created using a femtosecond laser, which imparts energy to the cornea to photodisrupt tissue.3,4 The plasma and shock waves created by femtosecond laser photodisruption followed by excimer laser ablation could affect the corneal cells, including the endothelium, more than excimer ablation alone.
In this randomized paired-eye study, we compared endothelial cell changes 5 years after LASIK with the flap created by a femtosecond laser in 1 eye and LASIK with the flap created by a mechanical microkeratome in the other eye.A We also evaluated relationships between endothelial cell loss and contact lens wear, residual bed thickness, and preoperative refractive error.
PATIENTS AND METHODS
Patients with myopia or myopic astigmatism were recruited from the refractive surgery service at Mayo Clinic, Rochester, Minnesota, USA. All patients were at least 21 years of age and were deemed suitable for LASIK after an extensive preoperative evaluation. Exclusion criteria included corneal abnormality; a history of ocular disease, trauma, or surgery; diabetes mellitus or other systemic disease known to affect the eye; use of any ocular medication; and use of systemic medication known to affect the cornea or anterior segment. The study complied with the U.S. Health Insurance Portability and Accountability Act and was prospectively approved by the Mayo Clinic Institutional Review Board. After receiving a complete explanation of the nature and possible outcomes of the study, all patients provided informed consent.
Randomization
This randomized paired-eye trial was designed to compare visual outcomes and postoperative ocular preference between LASIK with the flap created by a femtosecond laser and LASIK with the flap created by a mechanical microkeratome. One eye of each patient was randomized by ocular dominance to LASIK with the flap created by a femtosecond laser and the other eye to LASIK with the flap created by a mechanical microkeratome. Ocular dominance was determined by asking the patient to frame a distant object with both hands while an observer recorded which eye the patient used to view the object. Treatments were randomized to consecutive participants before participant recruitment.
Surgical Technique
The LASIK procedure has been described in detail.5,6 Briefly, bladeless flaps were created using a 15 kHz femtosecond laser (Intralase FS, Intralase Corp.). All flaps had a superior hinge and intended thickness of 120 μm. Raster line and spot separation were 9 μm and 11 μm, respectively. The raster energy was 2.3 μJ, and the side-cut energy was 2.5 μJ. Flaps created by the mechanical microkeratome (Hansatome, Bausch & Lomb) had a superior hinge and an intended thickness of 180 μm. Ablation of the stromal bed was performed with a Star S4 excimer laser (Visx, Inc.). Emmetropia was attempted in all eyes by using an ablation zone ranging from 6.5 mm × 6.5 mm for spherical corrections to 6.5 mm × 5.0 mm for astigmatic corrections. Postoperative topical regimens were identical for each eye and consisted of ciprofloxacin ophthalmic solution 4 times per day for 5 days and fluorometholone 0.1% 4 to 8 times daily with a taper over 3 weeks.
Endothelial Cell Analysis
The corneal endothelium in each eye was examined before LASIK and 3 years and 5 years after LASIK using in vivo confocal microscopy (Confoscan 3 or Confoscan 4, Nidek Technologies). The confocal examination technique has been described.7 Briefly, topical proparacaine hydrochloride 0.5% was instilled into the eye. An observer aligned the ×40 contact objective lens, coated with an optical coupling medium (Genteal Gel, Novartis Pharmaceuticals Corp.), with the center of the cornea. Digital images of the corneal endothelium were recorded. Original images were 768 pixels × 576 pixels (428 μm × 325 μm) and were rescaled to 640 pixels × 480 pixels for analysis. Magnification of the images in both microscopes was calibrated using the image of a scale with lines at 10 μm intervals etched into a glass slide. The calibration was verified periodically throughout the study, and the same observer analyzed all images in a masked manner.
Images were transferred to an image-analysis system (KSS-400, Konan Medical) and were analyzed using a centers method, in which the center of each endothelial cell was digitized by a masked observer.8 At least 100 cells were counted in each endothelial photograph. Outcome measures were endothelial cell density (ECD), endothelial cell loss from preoperative cell density, the percentage of hexagonal cells, and the coefficient of variation (CoV) of the cell area (standard deviation divided by the mean).
Residual Bed Thickness
Corneas were also examined with a tandem scanning confocal microscope (Tandem Scanning Corp.) 1 month after LASIK to determine residual bed thickness. The technique has been described in detail.9,10 Briefly, proparacaine hydrochloride 0.5% was instilled into the eye. A drop of optical coupling medium was placed on the tip of the objective, and the objective was advanced and aligned with the central cornea. Full-thickness scans through the cornea were acquired as the optical section was advanced at known speed from anterior to the epithelium to posterior to the endothelium. The best scan with no axial movement of the cornea relative to the objective was selected for analysis. Residual bed thickness was the distance between the LASIK interface and the endothelium.9 Because the Confoscan 3 confocal microscope was not equipped with a Z-ring adapter like the Confoscan 4 confocal microscope,11 residual bed thickness could not be measured with the Confoscan 3 confocal microscope, which was used to examine 14 patients 1 month after LASIK.
Statistical Analysis
The primary outcome in this study was visual acuity (reported previously5,6), and a priori power analysis was performed to determine the sample size for the study based on visual acuity. Because the endothelial cell analysis was a secondary outcome, post hoc power analyses were performed for nonsignificant comparisons by determining the minimum detectable differences. Endothelial cell density, endothelial cell loss, the percentage of hexagonal cells, and the CoV of the cell area were compared between treatments at each examination using 2-tailed paired t tests if the data were distributed normally and signed-rank tests if the data were not distributed normally. A P value of 0.05 or less was considered significant, and all P values were adjusted for 3 comparisons by the Bonferroni method. Differences in the same parameters between the preoperative and postoperative periods were also assessed using paired t tests or signed-rank tests, but with Bonferroni correction for 2 comparisons. Minimum detectable differences were calculated for nonsignificant comparisons (α = 0.05/3 or 0.05/2, β = 0.20, paired analyses).
Correlations between endothelial cell loss and residual bed thickness or preoperative spherical equivalent in all eyes were determined using Pearson correlation coefficients. The significance of these correlations was determined using generalized estimating equation models,12 which account for possible correlation between fellow eyes of the same patient. Differences in the endothelial cell variables between preoperatively and 5 years postoperatively for contact lens wearers and noncontact lens wearers were assessed using generalized estimating equation models.
RESULTS
Twenty-one patients were enrolled and examined before LASIK; 11 patients were habitually wearing contact lenses before surgery. The mean patient age at surgery was 38 years ± 10 (SD) (range 22 to 54 years). At 3 years and 5 years, 1 eye was not examined because of recurrent corneal erosions and 1 patient declined to participate in confocal examinations. At 5 years, confocal microscopy data were not available in 1 additional patient and data from another patient were excluded because of interim cataract surgery. Thus, endothelial cell data were available for 39 eyes (20 patients) at 3 years and for 35 eyes (18 patients) at 5 years.
Endothelial cell density did not differ between fellow eyes before LASIK (P = .99) or between treatments (femtosecond and mechanical microkeratome) at 3 years (P = .99) or 5 years (P = .99) (Table 1). Endothelial cell density 5 years after LASIK did not differ from the preoperative density in either treatment group (P = .99).
Table 1.
Endothelial cell variables before and after LASIK.
| Parameter | Mean ± SD | |||
|---|---|---|---|---|
| Before LASIK (21 Paired Eyes) | 3 Y Postop (19 Paired Eyes) | 5 Y Postop (17 Paired Eyes) | MDD* (%) | |
| ECD (cells/mm2) | 120 | |||
| Femtosecond laser | 2758 ± 406 | 2760 ± 383 | 2829 ± 317 | |
| Mechanical microkeratome | 2773 ± 399 | 2796 ± 398 | 2853 ± 355 | |
| Hexagonal cells (%) | 5 | |||
| Femtosecond laser | 59 ± 8 | 57 ± 8 | 57 ± 7 | |
| Mechanical microkeratome | 59 ± 6 | 58 ± 6 | 57 ± 6 | |
| CoV of cell area (%) | 2 | |||
| Femtosecond laser | 32 ± 6 | 32 ± 4 | 31 ± 4 | |
| Mechanical microkeratome | 32 ± 6 | 32 ± 3 | 32 ± 3 | |
CoV = coefficient of variation; ECD = endothelial cell density; MDD = mean minimum detectable difference
Determined because there were no significant differences in any variable at any examination between treatments (P = .99)
The percentage of hexagonal cells did not differ between the 2 treatments at any examination (P = .99) and remained stable 5 years after LASIK (P ≥ .30) (Table 1). The CoV of cell area did not differ between treatments (P = .99) and also remained stable 5 years after LASIK (P ≥ .62) (Table 1).
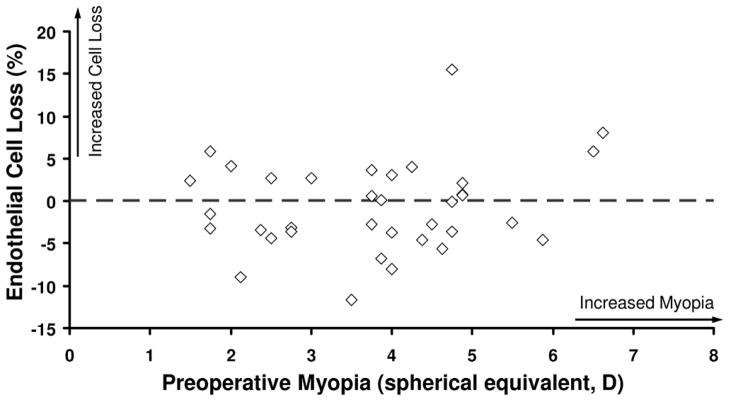
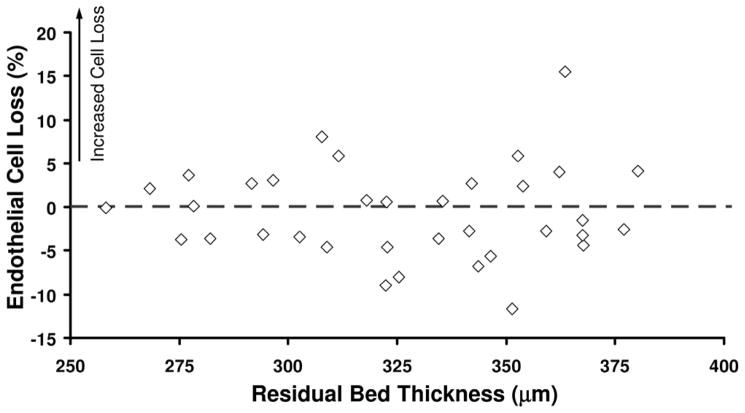
At 5 years, the mean endothelial cell loss from before LASIK was −0.8% ± 5.8% for femtosecond laser treatments and −0.4% ± 5.0% for mechanical microkeratome treatments (P = .85, signed-rank test). The annual rate of endothelial cell loss was −0.1% ± 1.2% and −0.1% ± 1.0%, respectively. When all eyes were combined, the endothelial cell loss at 5 years was not correlated with the preoperative refractive error (r = 0.21, P = .18) (Figure 1) or with the residual bed thickness (r = −0.10, P = .43) (Figure 2).
Figure 1.
Relationship between endothelial cell loss after LASIK and preoperative refractive error (N = 35).
Figure 2.
Relationship between endothelial cell loss and residual bed thickness after LASIK (N = 35).
Five years after LASIK, the ECD, percentage of hexagonal cells, and CoV of the cell area did not differ from preoperatively in contact lens wearers or noncontact lens wearers (Table 2). The minimum detectable difference for ECD at 5 years versus preoperatively was 148 cells/mm2 for contact lens wearers and 72 cells/mm2 for noncontact lens wearers.
Table 2.
Endothelial cell changes after LASIK by preoperative contact lens status.
| Parameter | Before LASIK | 5 Y Postop | P Value* | ||
|---|---|---|---|---|---|
| Eyes (n) | Mean ± SD | Eyes (n) | Mean ± SD | ||
| ECD (cells/mm2) | |||||
| Contact lens wearers | 22 | 2899 ± 307 | 17 | 2972 ± 282 | .94 |
| Non-contact lens wearers | 20 | 2618 ± 440 | 18 | 2733 ± 338 | .15 |
| Hexagonal cells (%) | |||||
| Contact lens wearers | 22 | 58 ± 8 | 17 | 57 ± 8 | .66 |
| Non-contact lens wearers | 20 | 60 ± 7 | 18 | 58 ± 5 | .10 |
| CoV of cell area (%) | |||||
| Contact lens wearers | 22 | 34 ± 6 | 17 | 32 ± 4 | .18 |
| Non-contact lens wearers | 20 | 32 ± 4 | 18 | 31 ± 3 | .63 |
CoV = coefficient of variation; ECD = endothelial cell density
Generalized estimating equation models
DISCUSSION
Corneal endothelial changes 5 years after LASIK with the flap created with a femtosecond laser were similar to those after LASIK with the flap created with a mechanical microkeratome; differences in the mean density, if they exist, are likely less than 120 cells/mm2, or approximately 4% of the mean cell density. This indicates that the additional energy imparted to the cornea during LASIK with a femtosecond laser is not detrimental to the health of the endothelium.
Endothelial cell loss over the 5-year follow-up in this study was close to zero for both treatments, similar to that of normal corneas,13 showing that LASIK surgery did not have an effect. Similarly, with 9 years of follow-up, we previously found no effect of LASIK with a microkeratome on endothelial cell loss above that of age-related physiologic cell loss.2 Kato et al.14 retrospectively analyzed 779 eyes after myopic LASIK with a microkeratome and found that endothelial loss at 5 years was 1.2%, which is within the range of physiologic cell loss.
In recent years, femtosecond lasers have been increasingly used to create LASIK flaps15; however, there are few reports of the long-term effect, if any, on the corneal endothelium. Smith et al.16 found no difference in ECD between fellow eyes receiving LASIK with a femtosecond laser versus PRK; however, their end point was 3 months after surgery, which might not have been long enough to detect small changes. Muñoz et al.17 created flaps with a 15 kHz femtosecond laser; 1 year after LASIK, they found that the ECD increased in previous contact lens wearers and remained stable in noncontact lens wearers. The results in our study with 5 years of follow-up provide further evidence that LASIK with a femtosecond laser is not detrimental to the corneal endothelium. The clinical results are supported by laboratory studies of endothelial keratoplasty tissue preparation with femtosecond lasers,18,19 in which lamellar cuts deeper than those used in LASIK were not associated with endothelial cell damage. Similarly, the depth of ablation in LASIK in this study was not correlated with endothelial cell loss, confirming previous results.2
Contact lens wear is known to induce morphologic changes in the corneal endothelium, although ECD is typically unaffected.20,21 Several studies2,17,22,23 found an improvement in ECD and morphology after photoablative refractive surgery, and this has often been attributed to cessation of contact lens wear. In this study, there was a trend toward increased cell density 5 years after LASIK in contact lens wearers. However, the trend was not statistically significant, although our statistical power prevented us from detecting an increase smaller than 148 cells/mm2 (or 5%). Although we had sufficient statistical power to detect an increase in cell density as low as 2% in noncontact lens wearers, determining the effect of cessation of contact lens wear after refractive surgery would require a larger controlled study.23
The strengths of this study were its randomized paired-eye design and 5-year prospective follow-up with careful calibration and standardization of the endothelial cell analysis. The small sample size was a limitation and was determined based on the primary outcome of the trial, which was visual acuity. Nevertheless, post hoc analyses indicated sufficient power to detect a difference in cell loss as small as 4% at 5 years, if indeed a difference existed between treatments. All femtosecond flaps were created with a 15 kHz femtosecond laser, which was standard at the time the patients were enrolled in the study. Because femtosecond lasers have since improved and now deliver much less energy to the cornea during flap creation, we expect that similar results would be found with the newer lasers. Although we used 2 different confocal microscopes (Confoscan 3 and Confoscan 4) for endothelial imaging in the initial phases of the study, the microscopes were spatially calibrated with the same scale etched into a glass slide and the ECD measured using both microscopes has been validated with a specular microscope.24,25
In summary, the energy delivered to the cornea during femtosecond laser flap creation did not affect the corneal endothelium 5 years after LASIK when compared with flap creation using a mechanical microkeratome. The results support the use of corneas that have had LASIK as acceptable donor tissue for endothelial keratoplasty with respect to endothelial cell health, enabling these corneas to expand the donor pool.2 The latter is contingent on successful preparation of endothelial grafts from donor corneas that have had LASIK and is aided by a complete donor history and careful screening of the tissue.26
WHAT WAS KNOWN
With 9-year follow-up, LASIK with the flap created by a mechanical microkeratome had not been not associated with corneal endothelial cell changes above those associated with age. Similarly, adverse endothelial cell changes have not been found after LASIK with the flap created by a femtosecond laser, although follow-up has been limited to 1 year.
WHAT THIS PAPER ADDS
In a randomized contralateral eye trial, LASIK with the flap created by a femtosecond laser was not associated with adverse effects on the corneal endothelium with 5 years of follow-up. The longer follow-up provides stronger evidence that femtosecond laser flap creation is safe for the corneal endothelium.
Acknowledgments
Supported by National Institutes of Health EY02037, Bethesda, Maryland; Research to Prevent Blindness (unrestricted departmental grant, and SVP as Olga Keith Wiess Special Scholar), New York, New York; and the Mayo Foundation, Rochester, Minnesota, USA.
Footnotes
Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, USA, May 2012.
Financial Disclosure: No author has a financial or proprietary interest in any material or method mentioned.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pallikaris IG, Siganos DS. Excimer laser in situ keratomileusis and photorefractive keratectomy for correction of high myopia. J Refract Corneal Surg. 1994;10:498–510. [PubMed] [Google Scholar]
- 2.Patel SV, Bourne WM. Corneal endothelial cell loss 9 years after excimer laser keratorefractive surgery. [Accessed August 12, 2012];Arch Ophthalmol. 2009 127:1423–1427. doi: 10.1001/archophthalmol.2009.192. Available at: http://archopht.jamanetwork.com/data/Journals/OPHTH/10132/ecs90022_1423_1427.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juhasz T, Kastis GA, Suárez C, Bor Z, Bron WE. Time-resolved observations of shock waves and cavitation bubbles generated by femtosecond laser pulses in corneal tissue and water. Lasers Surg Med. 1996;19:23–31. doi: 10.1002/(SICI)1096-9101(1996)19:1<23::AID-LSM4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Lubatschowski H, Maatz G, Heisterkamp A, Hetzel U, Drommer W, Welling H, Ertmer W. Application of ultrashort laser pulses for intrastromal refractive surgery. Graefes Arch Clin Exp Ophthalmol. 2000;238:33–39. doi: 10.1007/s004170050006. [DOI] [PubMed] [Google Scholar]
- 5.Calvo R, McLaren JW, Hodge DO, Bourne WM, Patel SV. Corneal aberrations and visual acuity after laser in situ keratomileusis: femtosecond laser versus mechanical microkeratome. Am J Ophthalmol. 2010;149:785–793. doi: 10.1016/j.ajo.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SV, Maguire LJ, McLaren JW, Hodge DO, Bourne WM. Femtosecond laser versus mechanical microkeratome for LASIK; a randomized controlled study. Ophthalmology. 2007;114:1482–1490. doi: 10.1016/j.ophtha.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 7.McLaren JW, Bourne WM, Patel SV. Automated assessment of keratocyte density in stromal images from the ConfoScan 4 confocal microscope. [Accessed August 12, 2012];Invest Ophthalmol Vis Sci. 2010 51:1918–1926. doi: 10.1167/iovs.09-4186. Available at: http://www.iovs.org/content/51/4/1918.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel SV, McLaren JW, Bachman LA, Bourne WM. Comparison of flex-center, center, and corner methods of corneal endothelial cell analysis. Cornea. 2010;29:1042–1047. doi: 10.1097/ICO.0b013e3181cc7a60. [DOI] [PubMed] [Google Scholar]
- 9.Patel SV, Erie JC, McLaren JW, Bourne WM. Confocal microscopy changes in epithelial and stromal thickness up to 7 years after LASIK and photorefractive keratectomy for myopia. J Refract Surg. 2007;23:385–392. doi: 10.3928/1081-597X-20070401-11. [DOI] [PubMed] [Google Scholar]
- 10.Patel SV, McLaren JW, Hodge DO, Bourne WM. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. [Accessed August 12, 2012];Invest Ophthalmol Vis Sci. 2001 42:333–339. Available at: http://www.iovs.org/cgi/reprint/42/2/333. [PubMed] [Google Scholar]
- 11.McLaren JW, Nau CB, Patel SV, Bourne WM. Measuring corneal thickness with the ConfoScan 4 and Z-ring adapter. Eye Contact Lens. 2007;33:185–190. doi: 10.1097/ICL.0b013e31802b3114. [DOI] [PubMed] [Google Scholar]
- 12.Zeger SL, Liang K-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 13.Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. [Accessed August 11, 2012];Invest Ophthalmol Vis Sci. 1997 38:779–782. Available at: http://www.iovs.org/cgi/reprint/38/3/779. [PubMed] [Google Scholar]
- 14.Kato N, Toda I, Hori-Komai Y, Sakai C, Tsubota K. Five-year outcome of LASIK for myopia. Ophthalmology. 2008;115:839–844.e2. doi: 10.1016/j.ophtha.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Soong HK, Malta JB. Femtosecond lasers in ophthalmology. Am J Ophthalmol. 2009;147:189–197. doi: 10.1016/j.ajo.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Smith RT, Waring GO, IV, Durrie DS, Stahl JE, Thomas P. Corneal endothelial cell density after femtosecond thin-flap LASIK and PRK for myopia: a contralateral eye study. J Refract Surg. 2009;25:1098–1102. doi: 10.3928/1081597X-20091117-09. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz G, Albarrán-Diego C, Sakla HF, Ferrer-Blasco T, Javaloy J. Effects of LASIK on corneal endothelium using the 15-kHz IntraLase femtosecond laser. J Refract Surg. 2011;27:672–677. doi: 10.3928/1081597X-20110415-02. [DOI] [PubMed] [Google Scholar]
- 18.Cheng YYY, Pels E, Cleutjens JPM, van Suylen RJ, Hendrikse F, Nuijts RMMA. Corneal endothelial viability after femtosecond laser preparation of posterior lamellar discs for Descemet-stripping endothelial keratoplasty. Cornea. 2007;26:1118–1122. doi: 10.1097/ICO.0b013e31814531d1. [DOI] [PubMed] [Google Scholar]
- 19.Mootha VV, Heck E, Verity SM, Petroll WM, Lakshman N, Muftuoglu O, Bowman RW, McCulley JP, Cavanagh HD. Comparative study of Descemet stripping automated endothelial keratoplasty donor preparation by Moria CBm microkeratome, Horizon microkeratome, and Intralase FS60. Cornea. 2011;30:320–324. doi: 10.1097/ICO.0b013e3181f22cc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlson KH, Bourne WM, Brubaker RF. Effect of long-term contact lens wear on corneal endothelial cell morphology and function. [Accessed Augusts 12, 2012];Invest Ophthalmol Vis Sci. 1988 29:185–193. Available at: http://www.iovs.org/content/29/2/185.full.pdf. [PubMed] [Google Scholar]
- 21.Hirst LW, Auer C, Cohn J, Tseng SCG, Khodadoust AA. Specular microscopy of hard contact lens wearers. Ophthalmology. 1984;91:1147–1153. doi: 10.1016/s0161-6420(84)34167-7. [DOI] [PubMed] [Google Scholar]
- 22.Trocmé SD, Mack KA, Gill KS, Gold DH, Milstein BA, Bourne WM. Central and peripheral endothelial cell changes after excimer laser photorefractive keratectomy for myopia. Arch Ophthalmol. 1996;114:925–928. doi: 10.1001/archopht.1996.01100140133003. [DOI] [PubMed] [Google Scholar]
- 23.Woodward MA, Edelhauser HF. Corneal endothelium after refractive surgery. J Cataract Refract Surg. 2011;37:767–777. doi: 10.1016/j.jcrs.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Kitzmann AS, Winter EJ, Nau CB, McLaren JW, Hodge DO, Bourne WM. Comparison of corneal endothelial cell images from a noncontact specular microscope and a scanning confocal microscope. Cornea. 2005;24:980–984. doi: 10.1097/01.ico.0000159737.68048.97. [DOI] [PubMed] [Google Scholar]
- 25.Raecker ME, McLaren JW, Kittleson KM, Patel SV. Endothelial image quality after Descemet stripping with endothelial keratoplasty: a comparison of three microscopy techniques. Eye Contact Lens. 2011;37:6–10. doi: 10.1097/ICL.0b013e318203dc19. [DOI] [PubMed] [Google Scholar]
- 26.Moshirfar M, Khalifa YM, Davis D, Fenzl CR, Espandar L, Chang JC, Mamalis N, Mifflin MD. Descemet stripping automated endothelial keratoplasty using donor corneas with previous laser in situ keratomileusis or photorefractive keratectomy: a case series and donor cap histopathology. Cornea. 2012;31:533–537. doi: 10.1097/ICO.0b013e31820142be. [DOI] [PubMed] [Google Scholar]
Other Cited Material
- A. National Institutes of Health Clinical Trials. [Accessed August 12, 2012];Long-term effects of laser refractive surgery. : NCT00350246. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00350246?term=NCT00350246&rank=1.