Abstract
Context
Overall, end-of-life (EOL) discussions are unrelated to psychological distress and associated with lower rates of aggressive care near death. Nevertheless, patients who report they feel emotionally numb about their illness might encounter difficulties cognitively processing an EOL discussion.
Objectives
We hypothesized that emotional numbness would modify the influence of EOL discussions on the receipt of less aggressive EOL care.
Methods
Data were derived from structured interviews with 290 participants in the federally-funded Coping with Cancer Study, a multisite, prospective cohort study of advanced cancer patients followed through their death. Patients’ reports of EOL discussions with their physician and emotional numbness were assessed a median of 4.6 months before death. Information about aggressive EOL care (i.e., ventilation, resuscitation in the last week of life, death in the Intensive Care Unit) was obtained from postmortem caregiver interviews and medical charts. Main and interactive effects of EOL discussions and emotional numbness on aggressive EOL care, adjusting for potential confounds, were evaluated using multiple logistic regression.
Results
The likelihood of aggressive EOL care associated with having EOL discussions increased by a factor of nine (adjusted odds ratio=9.02, 95% confidence interval 1.37, 59.6, P=0.022) for every unit increase in a patient’s emotional numbness score.
Conclusion
Emotional numbness diminishes a patient’s capacity to benefit from EOL discussions. EOL decision making may be more effective if clinical communications with emotionally numb patients are avoided.
Keywords: Cancer, terminal illness, end-of-life care, communication, numbness, psychological reactions, dissociation
Introduction
Conversations in which patients and physicians discuss the care that patients would want to receive if they were dying are generally associated with patients’ heightened prognostic awareness,1– 3 increased rates of advance care planning1, 3, 4 and hospice use,1, 2 as well as decreased use of expensive aggressive end-of-life (EOL) procedures that impair quality of life without enhancing survival.1– 5 EOL discussions also increase the likelihood that patients will receive EOL care that is consistent with their expressed preferences.3 Nevertheless, conversations about death and dying are difficult to initiate,6, 7 require considerable skill and sensitivity,7 may provide more information than patients actually want,8 and are often simply avoided.6 Despite the likely benefits of EOL discussions, most terminally ill patients report not having EOL conversations with their health care providers.1
A common view is that health care professionals should initiate discussions about EOL issues when patients seem ready to have them,7 an approach that relies heavily on intangible capacities such as clinical intuition, sensitivity, and common sense. Patients who are or who might become overwhelmed or distraught by thoughts of death and dying may not be ready to have an EOL discussion. The threatening nature of a terminal illness or of an EOL discussion may trigger a dissociative response in some patients that manifests itself as emotional numbness.9, 10 The fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM–IV) 11 (p. 519) defines dissociation as “a disruption in the usually integrated function of consciousness, memory, identity, or perception of the environment.” Dissociation and emotional numbness impose psychological barriers to normal human interactions. Gershuny and Thayer assert that “when emotional pain is acute and deemed unbearable by the sufferer, dissociation may be called upon as a means of escape.” 12 (p. 647) In dissociative responses to psychological threats, symptoms of “freezing, analgesia, and emotional numbing” are believed to be elicited automatically.13 (p. 65)
Dissociation and the associated symptom of emotional numbness in patients with terminal illnesses may indicate a lack of patient readiness to engage effectively in EOL discussions. Enlisted as a way to avoid processing shocking and upsetting information, emotional numbness may result in cognitive difficulties such as memory and attention problems.10, 14 Patients with advanced cancer who are emotionally numb may have restricted capacities to process health status and treatment information and not benefit from EOL discussions to the same extent as their less numb counterparts. Consequently, EOL discussions may not reduce the odds that cancer patients who are or who become emotionally numb will receive aggressive care at the EOL when the patient’s ability to weigh the pros and cons of life-prolonging treatment is compromised. In fact, EOL discussions with patients who are numb in their wake may exacerbate their fears and result in an instinctive demand that everything possible be done to keep them alive at all costs.
The present study sought to determine whether and to what extent patients’ emotional numbness modifies the effect of EOL discussions on receipt of aggressive EOL care. We hypothesized that greater emotional numbness would diminish the influence EOL discussions on the receipt of less aggressive EOL care.
Methods
Study Sample
Participants (N=290) came from the Coping with Cancer (CwC) study, an National Cancer Institute- and National Institute of Mental Health-funded multi-institutional investigation of advanced cancer patients and their informal caregivers. Conducted from September 2002 through August 2008, CwC was designed to examine relationships between psychosocial factors and EOL outcomes such as utilization of aggressive medical care near death. Patients were recruited from six outpatient sites: Yale Cancer Center (New Haven, CT), Veterans’ Affairs Connecticut Healthcare System Comprehensive Cancer Clinics (West Haven, CT), Simmons Comprehensive Cancer Care Center (Dallas, TX), Parkland Hospital Palliative Care Service (Dallas, TX), Dana-Farber/Harvard Cancer Center (Dana-Farber Cancer Institute and Massachusetts General Hospital, Boston, MA), and New Hampshire Oncology-Hematology (Hooksett, NH). Eligibility criteria included: 1) diagnosis of an advanced cancer with metastases; 2) disease progression following first-line chemotherapy; 3) age at least 20 years; 4) availability of an informal caregiver (e.g., spouse); 5) adequate stamina to complete the 45 minute interview; and 6) ability to speak either English or Spanish. Patient-caregiver dyads in which either party was significantly cognitively impaired (by neurobehavioral cognitive status examination with more than five errors15) were excluded. Both patients and caregivers provided written informed consent in accordance with protocols approved by the institutional review board of each participating site.
Each week, outpatient clinic lists were reviewed by research and clinical staff to identify eligible participants. Of the 939 eligible patients, 661 (70.4%) participated. The most common reasons for nonparticipation were “not interested” (n=106), “caregiver refuses” (n=32), and “too upset” (n=21). Participants and non-participants did not differ significantly in age, gender, race/ethnicity, or years of education.
Because this report focuses on predictors of EOL care, the sample for the present study was restricted to deceased individuals with postmortem data and complete baseline assessments of EOL discussions and emotional numbness. At the time of the analysis, 385 participants had died. Postmortem data were available for 369 (95.8%) of the deceased patients, and complete EOL discussion and emotional numbness assessments were present for 290 (78.6%) of these. Those with incomplete data did not differ significantly from those with complete data with respect to gender, race, education, and marital status. Subjects with missing data were significantly younger than those with complete data: mean = 57.7 (standard deviation [SD]=12.7) versus 61.6 (SD=10.9) years of age (t=−2.50, df=366; P=0.013).
The final cohort for the present study consisted of 290 patients with advanced cancer who provided a baseline interview (for which they received $25 compensation) and who died a median of 4.6 months following the baseline assessment.
Measures
Baseline Sociodemographic and Health Status Characteristics
Patients’ age, gender, race/ethnicity, years of education, and marital status were reported by the patient. Disease information was obtained from medical charts. Performance status was determined by trained interviewers using the Karnofsky scale.16 Karnofsky performance status ratings included: 100= patient has no symptoms, carries out all normal activities; 50= patient requires medical care and much assistance with self care; 0= patient is dead. Karnofsky scores were significantly associated with the number of days from patients’ baseline interviews until death (Spearman rank correlation rs=0.35, P<0.001).
EOL Discussions
Patients were asked “Have you and your doctor discussed any particular wishes you have about the care you would want to receive if you were dying?” Responses were coded as 1=yes and 2=no.
Patient Emotional Numbness
The Prolonged Grief Disorder scale (PG-12),17 asks patients about their current emotional state in relation to their illness. Patient emotional numbness was assessed at the time of the baseline interview using a single item from the patient version of the PG-12 that asked patients about the extent to which they felt emotionally numb about their illness. Responses were rated: 1= not at all, 2= slightly, 3= somewhat, 4= quite a bit, 5= overwhelmingly. As indications of its construct validity, the numbness item in the present sample was significantly associated with the PG-12 grief summary score (Spearman rank correlation rs= 0.58, P<0.001); with disbelief (rs= 0.33, P<0.001), bitterness (rs= 0.33, P<0.001), and yearning (rs= 0.16, P=0.005). The numbness item also was significantly associated with patients’ feeling traumatized by their initial cancer diagnosis (rs= 0.14, P=0.015), assessed using the Structured Clinical Interview for Diagnostic and Statistical Manual-DSM-IV (SCID) 18,19 screening question for Posttraumatic Stress Disorder as it pertained to the initial cancer diagnosis. Patients were asked “When you received your diagnosis, were you very afraid or did you feel terrified or helpless?” Responses were coded as 1=yes/somewhat and 2=no.
Aggressive Medical Care at the EOL
Patient’s receipt of aggressive, life-prolonging care at the EOL, defined here as the use of mechanical ventilation or resuscitation in the last week of life or occurrence of death in the Intensive Care Unit (ICU), was documented from primary caregiver interviews and verified with medical chart reviews.
Statistical Methods
Bivariate associations between patient characteristics and primary study outcome variables, i.e., EOL discussions, emotional numbness, and receipt of aggressive EOL care, were assessed using logistic (for EOL discussions and aggressive EOL care as outcomes) and ordinal logistic (for emotional numbness as outcome) regression without adjustment for other variables. Multiple logistic regression was used to test the hypothesis that emotional numbness modifies an association between EOL discussions and receipt of aggressive EOL care. Receipt of aggressive medical care at the EOL was regressed on the main and interactive effects of EOL discussions and emotional numbness score (mean=1.70, SD=1.08), adjusting for patient characteristics (age and race) at least marginally significantly associated (P<0.10) with aggressive EOL care. Statistical analyses were performed using SAS Version 9.1 (SAS Institute Inc., Cary, NC).
Results
Table 1 presents descriptive statistics of the patient sample (N=290) at the baseline assessment. Patients were, on average, middle aged (mean=57.7 years, SD=12.7 years), 52.4% male, and racially/ethnically diverse (63.5% White, 19.3% Black, 15.5% Hispanic, and 1.7% Other). Patients’ average performance status (mean=64.3, SD=14.7) was partway between “patient is able to care for self, but is unable to do normal activities or active work” (Karnofsky score=70) and “patient is able to care for self, but requires occasional assistance” (Karnofsky score=60). A substantial minority of patients (35.2%) reported EOL discussions. Small minorities of patients were either “quite a bit” (6.9%) or “overwhelmingly” (2.4%) emotionally numb. A minority of patients (10.7%) received aggressive EOL medical care.
Table 1.
Patient Characteristics and Outcomes (N=290)
| Characteristic | n | % |
|---|---|---|
| Age in years, mean (SD) | 57.7 | (12.7) |
| Gender, male | 152 | (52.4) |
| Race/Ethnicity | ||
| White | 184 | (63.5) |
| Black | 56 | (19.3) |
| Hispanic | 45 | (15.5) |
| Other | 5 | (1.7) |
| Education in years, mean (SD) | 12.4 | (4.1) |
| Marital Status, Married | 154 | (54.0) |
| Cancer Type | ||
| Lung | 56 | (19.7) |
| Colon | 39 | (13.7) |
| Pancreatic | 22 | (7.7) |
| Other Gastrointestinal | 36 | (12.6) |
| Breast | 41 | (14.4) |
| Other | 91 | (31.9) |
| Karnofsky performance status score, mean (SD) | 64.3 | (14.7) |
|
| ||
| Outcome
| ||
| EOL Discussions | 102 | (35.2) |
| Emotional Numbness | ||
| Not at all (score=1) | 185 | (63.8) |
| Slightly (score=2) | 41 | (14.1) |
| Somewhat (score=3) | 37 | (12.8) |
| Quite a bit (score=4) | 20 | (6.9) |
| Overwhelmingly (score=5) | 7 | (2.4) |
| Aggressive EOL Care | 31 | (10.7) |
Missing observations: marital status (n=5), cancer type (n=5), Karnofsky score (n=4)
Table 2 presents odds ratios (ORs) between patient characteristics and EOL discussions, patient emotional numbness, and patient receipt of aggressive EOL care. Karnofsky performance status score was significantly inversely related to EOL discussions (OR=0.71 per 10 unit change in Karnofsky score, P<0.001). Higher performance status was related to lower levels of emotional numbness (OR=0.79 per 10 unit change in Karnofsky score, P=0.004). White as opposed to non-White patient race/ethnicity was inversely related to aggressive medical care near death (OR=0.37, P=0.011).
Table 2.
Odds Ratios Between Patient Characteristics and EOL Discussions, Emotional Numbness, and Aggressive EOL Care (N=290)
| EOL Discussions | Emotional Numbness | Aggressive EOL Care | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Patient Characteristic | OR | P | OR | P | OR | P |
| Age in years | 0.99 | 0.435 | 0.99 | 0.095 | 0.97 | 0.065 |
| Gender, male | 1.17 | 0.532 | 0.85 | 0.485 | 1.75 | 0.157 |
| Race, White | 1.02 | 0.943 | 1.21 | 0.441 | 0.37 | 0.011 |
| Education in years | 1.01 | 0.787 | 0.97 | 0.273 | 0.99 | 0.889 |
| Marital Status, Married | 0.97 | 0.902 | 0.91 | 0.703 | 1.63 | 0.218 |
| Karnofsky score/10 | 0.71 | <0.001 | 0.79 | 0.004 | 1.17 | 0.239 |
Missing observations: marital status (n=5), cancer type (n=5), Karnofsky score (n=4).
Karnofsky score/10 represents the Karnofsky performance status score divided by 10. Odds ratios for Karnofsky score/10 represent the change in the odds of the outcome per 10 unit change in Karnofsky score.
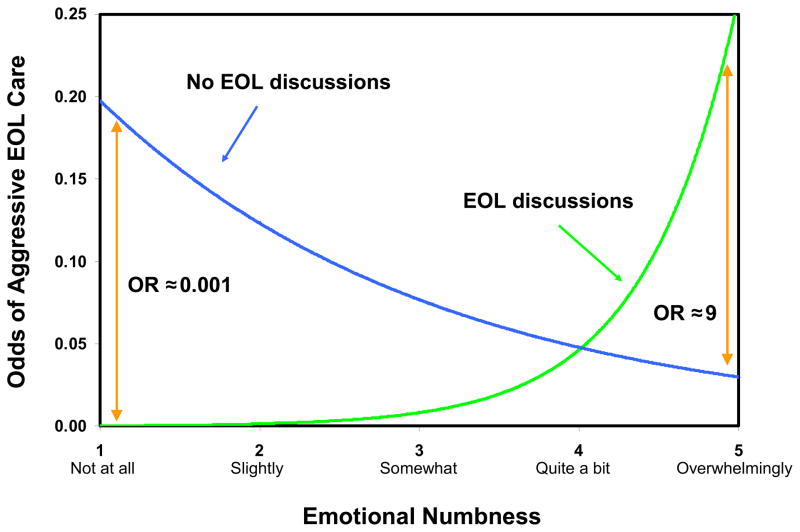
Table 3 shows that there was a significant interaction whereby the adjusted relative odds of receipt of aggressive EOL care associated with having EOL discussions increased by a factor of nine for every unit increase in the patient’s emotional numbness score (interaction adjusted OR =9.02, P=0.022). As illustrated in Fig. 1, patients who were “overwhelmingly” numb, i.e., those with emotional numbness score=5, were predicted to be approximately nine times more likely to received aggressive EOL care if they reported an EOL discussion than if they did not.
Table 3.
Patients’ Emotional Numbness Modifies an Association Between EOL Discussions and Aggressive EOL Care (N=290)
| Aggressive EOL Care | ||
|---|---|---|
|
| ||
| Predictor | AOR a | P |
| EOL Discussions | 0.001 | 0.038 |
| Emotional Numbness Score | 0.62 | 0.101 |
| EOL Discussions × Emotional Numbness Score | 9.02 | 0.022 |
Adjusted odds ratio (AOR): Odds ratio adjusted for age and race (White versus non-White).
Fig. 1.
Patient emotional numbness modifies relationship between EOL discussions and aggressive EOL care (N=290). Predicted odds of aggressive EOL care adjusting for age and race (White versus non-White).
Discussion
The results of the present study demonstrate rather dramatically that patients’ emotional numbness diminishes the effect of EOL discussions on the receipt of less aggressive EOL care. We found that the odds of aggressive EOL care associated with having EOL discussions increased by a factor of nine for every unit increase in a patient’s emotional numbness score. Elsewhere we have shown a relationship between aggressiveness of care received in the last week and worse quality of life in that week.1 For this reason, we consider patients’ receipt of less aggressive EOL care to be a beneficial outcome of EOL discussions. From this perspective, our present findings indicate that EOL discussions are most beneficial to patients who are least emotionally numb. Any steps clinicians might take to reduce a patient’s level of emotional numbness would be expected to enhance the benefit of having EOL discussions by reducing the likelihood that the patient receives aggressive, non-curative EOL care.
There appear to be many missed opportunities for physicians to engage in beneficial EOL discussions with patients diagnosed with terminal illnesses. In the present study, only 35.2% of patients with advanced cancers reported having EOL discussions with their physicians. Based on the present findings, EOL discussions would likely protect the vast majority (90.7%) of patients with advanced cancer who were either not at all, slightly, or somewhat numb from receiving aggressive, non-curative care near death. Still, EOL discussions will not affect all patients similarly; EOL discussions would be expected to be of questionable benefit to a small subset (9.3%) of patients who were either quite a bit or overwhelmingly emotionally numb. Indeed, EOL discussions for a small minority (2.4%) of patients who were overwhelmingly numb may actually heighten rather than protect patients from the risk of receipt of aggressive, expensive, burdensome EOL care.
We found that EOL discussions were more likely to occur in patients with worse performance status, and that worse performance status was linked to greater emotional numbness. This suggests that physicians wait until patients are in a state of severe physical decline (presumably close to death) before engaging in EOL discussions. This practice results in EOL discussions occurring with patients who are both physically and psychologically compromised and lacking the capacity to benefit from these conversations (and who potentially might be harmed by EOL discussions if these patients are or become overwhelming numb). One reason to have EOL discussions earlier in the course of illness would be to reduce the odds of patients being in physically and psychologically fragile states when they occur.
This study was limited by the cross-sectional nature of the assessments of patients’ reports of EOL discussions and present state of emotional numbness. Thus, the causal association and direction between these baseline variables cannot be known with absolute certainty. Nevertheless, the wording and intent of the CwC survey places these two variables in a distinct time sequence, i.e., EOL discussions in the past (implicitly since the time of diagnosis) and degree of emotional numbness presently at the time of the baseline assessment. Additionally, EOL discussions were based on potentially biased patient self-report. Longitudinal studies of patients and their oncology providers that include documentation of EOL discussions, perhaps via audio-taped clinic visits, are needed to confirm the results reported here. Another limitation is that emotional numbness was assessed using a single-item measure. Nevertheless, the single item of numbness was significantly associated with patient grief, other individual grief symptoms, and feelings of fear or helpless at the time of the initial cancer diagnosis. It is worth noting that only patient emotional numbness, and neither other individual grief symptoms nor the grief summary measure (data not shown), modifies the effect of EOL discussions on care received in the last week of life. Evidently, there is something particular to emotional numbness, and not other aspects of grief, that affects the impact of EOL discussions on EOL care. Future studies are needed to refine measurement and advance understanding of emotional numbness and its effects on medical decision making
In conclusion, emotionally numb patients are unlikely to benefit from EOL discussions in terms of receipt of less aggressive EOL care. There also appears to be a need for clinicians to identify and selectively not engage in EOL discussions with patients who display higher levels of emotional numbness or who are likely to become excessively numb in response to them. Finally, there is a need to understand the signs and risks of emotional numbness so that it can be prevented or reduced and thereby enhance the effectiveness of communication at the EOL.
Acknowledgments
This research was supported in part by the following grants to Dr. Prigerson: MH63892 from the National Institute of Mental Health, and CA 106370 and CA 156732 from the National Cancer Institute.
Footnotes
An earlier version of this report entitled “Tailoring End-of-Life Discussions to Advanced Cancer Patients’ State of Grief” was presented as a Clinical Science Symposium at the American Society for Clinical Oncology Annual Meeting in Chicago, May 29, 2009.
Disclosures
Neither author has relationships with any entities having financial interest in this topic.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prigerson HG. Socialization to dying: social determinants of death acknowledgement and treatment among terminally ill geriatric patients. J Health Soc Behav. 1992;33(4):378–395. [PubMed] [Google Scholar]
- 3.Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mack JW, Paulk ME, Viswanath K, Prigerson HG. Racial disparities in the outcomes of communication on medical care received near death. Arch Intern Med. 2010;170(17):1533–1540. doi: 10.1001/archinternmed.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480–488. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quill TE. Perspectives on care at the close of life. Initiating end-of-life discussions with seriously ill patients: addressing the “elephant in the room”. JAMA. 2000;284(19):2502–2507. doi: 10.1001/jama.284.19.2502. [DOI] [PubMed] [Google Scholar]
- 7.Clayton JM, Butow PN, Tattersall MH. When and how to initiate discussion about prognosis and end-of-life issues with terminally ill patients. J Pain Symptom Manage. 2005;30(2):132–144. doi: 10.1016/j.jpainsymman.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Fried TR, Bradley EH, O’Leary J. Prognosis communication in serious illness: perceptions of older patients, caregivers, and clinicians. J Am Geriatr Soc. 2003;51(10):1398–1403. doi: 10.1046/j.1532-5415.2003.51457.x. [DOI] [PubMed] [Google Scholar]
- 9.Nijenhuis ER, Spinhoven P, van Dyck R, van der Hart O, Vanderlinden J. Psychometric characteristics of the somatoform dissociation questionnaire: a replication study. Psychother Psychosom. 1998;67(1):17–23. doi: 10.1159/000012254. [DOI] [PubMed] [Google Scholar]
- 10.Giesbrecht T, Lynn SJ, Lilienfeld SO, Merckelbach H. Cognitive processes in dissociation: An analysis of core theoretical assumptions. Psychol Bull. 2008;134(5):617–647. doi: 10.1037/0033-2909.134.5.617. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 12.Gershuny BS, Thayer JF. Relations among psychological trauma, dissociative phenomena, and trauma-related distress: a review and integration. Clin Psychol Rev. 1999;19(5):631–657. doi: 10.1016/s0272-7358(98)00103-2. [DOI] [PubMed] [Google Scholar]
- 13.Nijenhuis ER, Spinhoven P, Vanderlinden J, van Dyck R, van der Hart O. Somatoform dissociative symptoms as related to animal defensive reactions to predatory imminence and injury. J Abnorm Psychol. 1998;107(1):63–73. doi: 10.1037//0021-843x.107.1.63. [DOI] [PubMed] [Google Scholar]
- 14.Wright DB, Osborne JE. Dissociation, cognitive failures, and working memory. Am J Psychol. 2005;118(1):103–113. [PubMed] [Google Scholar]
- 15.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc Oct. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 16.Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma - with particular reference to bronchogenic carcinoma. Cancer. 1948;1(4):634–656. [Google Scholar]
- 17.Jacobsen JC, Zhang B, Block SD, Maciejewski PK, Prigerson HG. Distinguishing symptoms of grief and depression in a cohort of advanced cancer patients. Death Stud. 2010;34(3):257–273. doi: 10.1080/07481180903559303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for the DSM-IV axis I disorders – Patient edition (SCID-I/P) version 2.0. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 19.Williams JB, Gibbon M, First MB, et al. The structured clinical interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Arch Gen Psychiatry. 1992;49(8):630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]