Abstract
Two mammalian introns, the human growth hormone intron and the Simian virus 40 large T antigen intron, were inserted into the coding sequences of diphtheria toxin fragment A (DT-A) and barnase (Bar), respectively, to disrupt their open-reading frames (ORFs). Expression of these two toxic proteins were totally abolished, which enabled the production of normal levels of recombinant baculoviral and adeno-associated viral (AAV) vectors in insect cells. When these viral vectors were introduced into mammalian cells, the introns were spliced out and the toxic proteins were expressed, which resulted in apoptosis in mammalian cells. This is the first report to show that viral vectors harboring toxin genes can be produced at normal levels by exploiting the intron-splicing mechanism of insect cells. Furthermore, viral vectors carrying the DT-A gene under control of tumor-specific promoters were able to exert tumor-specific cell killing. This novel method to produce viral vectors harboring toxic genes under control of tumor-specific promoter offers a powerful tool for further research, as well as for the development of toxin-based suicide gene therapy drugs.
Keywords: adeno-associated virus (AAV), baculovirus, cancer, gene therapy, toxin
Introduction
Toxic genes and proteins encoded by such genes have been used or proposed for use in disease therapies, such as in cancer therapies involving expression of genes that cause cell death (“suicide gene therapy”).1 Certain proteins encoded by toxic genes can kill a host cell at very low levels of expression. For example, a single molecule of diphtheria toxin fragment A (DT-A) is sufficient to kill a cell.2 As a result, suicide gene therapy has become an attractive strategy for cancer treatment. It relies on the delivery of genes whose products are either toxic or can lead to the production of a toxic product in conjunction with prodrug administration.3 DT is a potent cellular toxin that is believed to poison protein synthesis by catalyzing ADP-ribosylation of elongation factor 2. DT kills primarily by an apoptosis-mediated pathway. Furthermore, DT is believed to kill cells in a cell cycle-independent fashion. Taken together, these attributes make DT an attractive cancer therapeutic candidate, as some malignancies such as prostate cancer tend to have a very low mitotic index.
Previous attempts at limiting toxicity of a toxic gene, such as DT-A by use of an inducible and/or tissue-specific promoter, have led to variable results. For example, Maxwell et al.4 used a truncated form of the metallothionein promoter to demonstrate that basal expression of this promoter, even in the absence of heavy metals, resulted in substantial inhibition of protein synthesis. This inhibition could be augmented by the addition of an immunoglobulin enhancer element, but only minimally by cadmium. The authors were not able to demonstrate true specific cytotoxicity, but rather only a preferential cell susceptibility to DT-A–mediated cell death, presumably as a result of basal expression of this highly toxic gene. Subsequent efforts by this group and others concentrated on introducing an attenuated mutant of DT-A5 or on tightly regulating gene expression using prokaryotic control elements.6,7 In both cases, although preferential cell killing could be demonstrated, complete abolition of nonspecific cell killing was not achieved. Keyvani et al.8 used a tet repressor-based system as they had reported previously with the wild-type DT-A gene with an attenuated DT-A mutant, but were still unable to demonstrate complete abolition of background expression and subsequent cell death. Wang et al.9 described a recombinant baculovirus accommodating the transcriptional regulatory sequence of glial fibrillary acidic acid protein (GFAP) to drive the expression of DT-A gene in glioma cells. Because GFAP promoter is inactive in insect cells, they were able to generate recombinant baculovirus carrying the DT-A toxic gene. However, a cell-type specific promoter such as GFAP displays relatively weak transcriptional activity compared with positive control sequences derived from viruses, e.g., the enhancer/promoter of human cytomegalovirus (CMV) immediate-early gene, and therefore, limits its applications. Li et al.10 reported the generation of a DT-resistant HEK293 packaging cell line that can tolerate the toxin concentration up to 10−7 mol/l, and improved the adenovirus vector production yield. Kohlschütter et al.11 described AAV vectors carrying the DT-A gene in which the TetR repressor system was used to decrease the expression of DT-A. Even though the authors managed to produce some AAV vectors carrying the DT-A gene in HEK293T cells, their AAV yields were generally 2–3 logs lower than AAV vectors that did not carry the DT-A gene, such as vectors carrying the PUMA gene (AAVTetO2-PUMA). The authors attributed the lower titer to residual expression of the DT-A protein. In general, the use of viral vectors, such as recombinant adenovirus, recombinant baculovirus, and AAV, for the delivery of toxic genes to a target cell has been largely hampered by the inherent toxicity of the toxic gene. As a result, viral vectors harboring a toxic gene must be grown under conditions that are not lethal to the host cell.
In the present study, two mammalian introns that are not functional in insect cells were inserted into the coding sequences of DT-A and barnase (Bar) to disrupt their open-reading frames (ORFs), which successfully abolished the expression of toxic proteins in insect cells. This enabled the production of baculoviral and AAV vectors harboring toxin genes at similar levels to those of baculoviral and AAV vectors carrying the green fluorescent protein (GFP) gene. Furthermore, these baculoviral or AAV vectors harboring the toxin genes were able to kill mammalian cells, demonstrating that the mammalian introns were spliced out properly and toxin proteins were expressed. Finally, tumor-specific promoters were cloned to drive the expression of the toxin genes, which resulted in tumor-specific cell killing. These findings, taken together, should pave the way for further research, as well as for the development of toxin-based suicide gene therapy drugs.
Results
Insertion of mammalian introns to disrupt toxin gene ORFs and production of recombinant baculoviruses
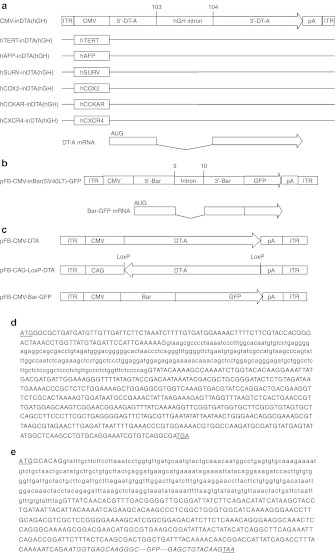
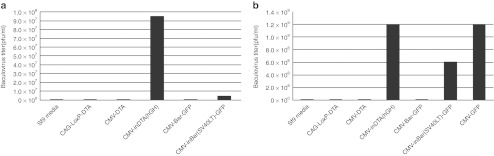
Two mammalian introns and two toxin genes were chosen for these experiments. Since the consensus intron-splicing sequence, 5′-AG/GUAAGU-intron-YNCURAC-YnNAG/G-3′, requires a 5′-AG and a ′'-G nucleotides to flank the intron,12 the human growth hormone (hGH) intron was inserted into the DT-A ORF between nucleotides 103 and 104 (the first letter of start codon ATG is assigned as nucleotide no. 1), which meets this requirement. In the same way, the SV40 large T antigen (SV40LT) intron was inserted into the Bar ORF between nucleotides 9 and 10. The DT-A and Bar ORFs without intron interruption were used as control. All the toxin-coding sequences with or without intron interruption were cloned into the pFastBac shuttle plasmid, and the schematic depiction of the expression cassettes are shown in Figure 1. The resulted pFastBac plasmids were used to transform into DH10Bac-competent bacteria, and bacmid DNAs from white colonies were prepared. The bacmid DNAs were used to produce recombinant baculoviruses as described in Materials and Methods. The results show that no recombinant baculovirus could be generated from the bacmid DNAs containing the DT-A or Bar ORFs without intron insertion, whereas normal levels of recombinant baculoviruses were generated from the bacmid DNAs containing the DT-A or Bar ORFs interrupted by the mammalian introns (Figure 2a). Though the titer of recombinant baculovirus CMV-inBar(SV40LT)-GFP (4.4 × 106 plaque-forming unit (pfu)/ml) is lower than that of CMV-inDTA(hGH) (9.5 × 107 pfu/ml), it is still within the normal range of baculovirus generation from bacmid transfection. After one round of amplification, normal levels of recombinant baculoviruses carrying intron-interrupted toxin genes were produced and compared with control recombinant baculovirus carrying the GFP gene (Figure 2b). The failure to generate recombinant baculovirus with bacmid DNAs containing the toxin genes without intron interruption demonstrates that DT-A or Bar is lethal to insect cells even though the toxin genes were under control of the CMV promoter (not an insect promoter). On the other hand, the successful generation and amplification of recombinant baculoviruses from bacmid DNAs containing intron-interrupted toxin genes indicate that both the hGH and SV40LT introns were not spliced in insect cells, and therefore, no toxins were produced due to the interruption of the DT-A and Bar ORFs. Surprisingly, no recombinant baculovirus was generated or amplified even if the DT-A gene flanked by LoxP sites was placed in an antisense direction to the promoter (Figure 2a,b).
Figure 1.
Schematic depiction of the genetic and transcriptional map of representative recombinant baculoviruses carrying the toxin expression cassettes flanked by AAV ITRs. (a) DT-A gene inserted with the hGH intron and under the control of various tumor-specific promoters. (b) Barnase gene inserted with the SV40LT antigen intron and fused in-frame with the GFP gene. (c) DT-A gene without intron insertion or flanked by LoxP sites and placed in the antisense direction, and barnase-GFP gene without intron insertion. (d) Detailed sequence of the DT-A coding region (upper case letters) comprising the hGH intron (lower case letters). (e) Detailed barnase-GFP coding region (upper case letters) comprising the SV40LT antigen intron (lower case letters). AAV, adeno-associated virus; Bar, barnase; CAG, chicken β-actin promoter; CMV, cytomegalovirus promoter; DT-A, diphtheria toxin fragment A; GFP, green fluorescent protein; hAFP, human alfa-fetoprotein promoter; hCCKAR, human cholecystokinin type A receptor promoter; hCOX2, human cyclooxygenase-2 promoter; hCXCR4, human C-X-C chemokine receptor type 4 promoter; hGH, human growth hormone; hSURV, human surviving promoter; hTERT, human telomerase reverse transcriptase promoter; ITR, inverted terminal repeat; LoxP, specific sequence recognized by Cre recombinase; pA, polyadenylation signal.
Figure 2.
Generation of recombinant baculoviruses with or without intron insertion. (a) Generation of recombinant baculoviruses by transfecting Bacmid DNAs into Sf9 cells. (b) Amplification of recombinant baculoviruses by infecting Sf9 cells with baculovirus stocks generated from a. Bar, barnase; CAG, chicken β-actin promoter; CMV, cytomegalovirus promoter; DT-A, diphtheria toxin fragment A; GFP, green fluorescent protein; hGH, human growth hormone.
Splicing of the introns from the toxin ORFs carried by recombinant baculoviruses and expression of the toxin proteins in mammalian cells
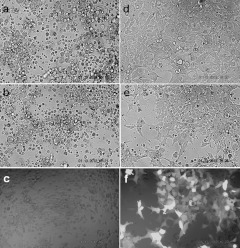
The successful generation and amplification of recombinant baculoviruses containing the intron-interrupted toxin ORFs indicate that the mammalian introns were not spliced in insect cells. In order to determine whether the introns inserted in the toxin ORFs could be spliced out in mammalian cells before moving forward to perform AAV vector production, the recombinant baculoviruses harboring the intron-interrupted DT-A or Bar gene were transduced into HEK293 cells. The cell morphology was examined and photographed 2 days after the transduction. The results show that recombinant baculoviruses carrying either the intron-interrupted DT-A or Bar gene caused fragmentation of HEK293 cells (Figure 3a,b), a typical sign of apoptosis, whereas recombinant baculoviruses carrying the GFP gene did not cause this fragmentation (Figure 3e,f). It is worth noting that in HEK293 cells transduced with baculovirus carrying the Bar-GFP gene, only very faint GFP expression was observed, indicating that most of the protein synthesis was inhibited by the Bar (Figure 3c).
Figure 3.
The killing effects on HEK293 cells by recombinant baculoviruses harboring toxic genes. The cells were seeded in a 24-well plate (1.5 × 105 cells/well) overnight and transduced with baculoviral vectors (5 × 107 pfu/well) for 48 hours. The images were photographed with ×20 lens under (a,b,d,e) tungsten lighting or (c,f) fluorescent lighting. (a) Bac-CMV-inDTA(hGH); (b) Bac-CMV-inBar(SV40LT)-GFP; (c) same field as b; (d) untreated; (e) Bac-CMV-GFP; (f) same field as e. Bar, barnase; CMV, cytomegalovirus promoter; DT-A, diphtheria toxin fragment A; GFP, green fluorescent protein; hGH, human growth hormone; pfu, plaque-forming unit.
Production of AAV vectors harboring the toxin ORFs in insect cells
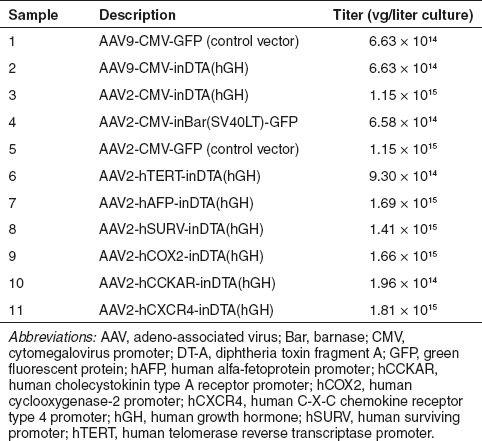
After the confirmation of intron splicing from the toxin ORFs in mammalian cells, AAV vector production was performed in Sf9 cells with the recombinant baculoviruses harboring intron-interrupted toxin ORFs. The recombinant baculoviruses were used to coinfect Sf9 cells with a second recombinant baculovirus expressing AAV Rep and Cap genes for 3 days and AAV vectors were purified and titrated. The results in Table 1 show very similar yields of AAV vectors carrying the intron-interrupted toxin ORFs were produced as compared with AAV vectors carrying just the GFP gene. These results again confirm the previous observation that the mammalian introns were not functional in insect cells, which resulted in the successful production of AAV vectors that carry the intron-interrupted toxin ORFs. In addition, both AAV2 and AAV9 vectors were produced at similar yields, indicating that this AAV vector production was universal and not serotype-specific.
Table 1. Production of AAV vectors carrying toxin genes in Sf9 cells.
Nonspecific cell killing by AAV vectors containing DT-A or Bar gene under control of CMV promoter
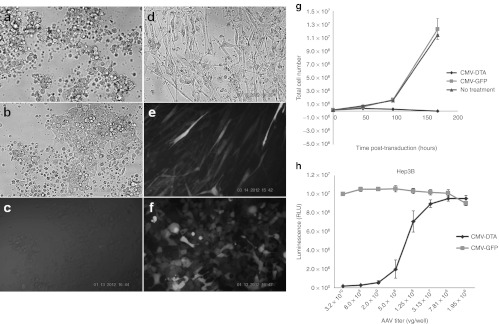
Since recombinant baculoviruses harboring the intron-interrupted toxin genes were able to kill mammalian cells through transduction, it would be interesting to know whether the AAV vectors harboring the toxin genes could also exert the killing effect on HEK293 and WI38 (normal human lung) cells. To test this possibility, both cell lines were plated on 24-well plates overnight and transduced with AAV2-CMV-inDTA(hGH), AAV2-Bar(SV40LT)-GFP, or AAV2-CMV vectors. The reason AAV2 instead of AAV9 vectors was used is because AAV2 vectors work best in in vitro assays. The results show that after transduction for 48 hours, HEK293 cells transduced with AAV2 vectors carrying either the DT-A or Bar gene, and WI38 cells transduced with AAV2 vectors carrying the DT-A gene, displayed fragmented cellular morphology (Figure 4a,b,d), indicating apoptosis. In contrast, there was no sign of apoptosis in the cells transduced with AAV2 vectors carrying the GFP gene (Figure 4e,f). These results clearly demonstrate that the introns were spliced out from the toxin-coding sequences to form mature mRNAs, and the mRNAs were translated into toxin proteins that killed the cells. Furthermore, in HEK293 cells transduced with AAV2 vectors carrying the Bar-GFP fusion coding sequence, only very faint GFP expression was observed (Figure 4c), which again confirms the previous observation that most of the protein synthesis was inhibited by the Bar. Since DT-A is more potent than Bar, AAV2 vectors carrying the DT-A gene was used for further experiments. A cell proliferation assay was performed on HEK293 cells to further confirm the cell-killing effect and the results are shown in Figure 4g. HEK293 cells transduced with AAV2-CMV-inDTA(hGH) were inhibited with no signs of growth, whereas cells transduced with AAV2-CMV-GFP grew as well as the cell in the untreated group. In addition, a cell viability assay was performed to verify the cytotoxicity of AAV2-CMV-inDTA(hGH) on Hep3B cells. The results are shown in Figure 4h, where a nice dose–response curve can be observed. While cell viability increased with the decrease of AAV2 vectors carrying the DT-A, there was essentially no change of cell viability for AAV2-CMV-GFP–treated Hep3B cells.
Figure 4.
The nonspecific killing effect of AAV2 vectors carrying toxin genes on mammalian cells. The cells were seeded in a 24-well plate (1.5 × 105 cells/well) overnight and transduced with AAV2 vectors (1.5 × 1010 vg/well) for 48 hours. Photographs were taken with (a,b,d) tungsten lighting or (c,e,f) fluorescent lighting. (a) HEK293 cells transduced with AAV2-CMV-inDTA(hGH); (b) HEK293 cells transduced with AAV2-CMV-inBar(SV40LT)-GFP; (c) same field as b; (d) WI38 cells transduced with AAV2-CMV-inDTA(hGH); (e) WI38 cells transduced with AAV2-CMV-GFP as control; (f) HEK293 cells transduced with AAV2-CMV-GFP as control. (g) Proliferation of HEK293 cells transduced with AAV2 vectors. The cells (1.5 × 105 cells/well) were seeded in a 24-well plate overnight and transduced with AAV2-CMV-inDTA(hGH), or AAV2-CMV-GFP. The cell numbers were counted at different timepoints. When they reached confluency, the cells were split into a larger culture areas and let grow. (h) The viability of Hep3B cells transduced with AAV2 vectors. Hep3B cells (3.2 × 104 cells/well) were seeded in a 96-well plate overnight and transduced with a fourfold serial dilution of AAV2-CMV-GFP, or AAV2-CMV-inDTA(hGH). The cell viability was determined with CellTiter Glo Luminescent Cell Viability Assay Kit (Promega). AAV, adeno-associated virus; CMV, cytomegalovirus; DT-A, diphtheria toxin fragment A; GFP, green fluorescent protein; hGH, human growth hormone; RLU, relative luminescence unit; vg, vector genome.
Tumor-specific cell killing by AAV vectors carrying DT-A under control of tumor-specific promoters
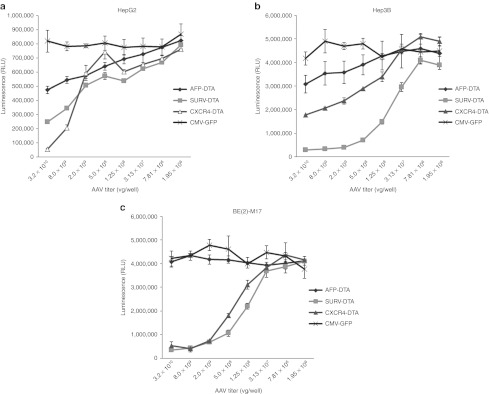
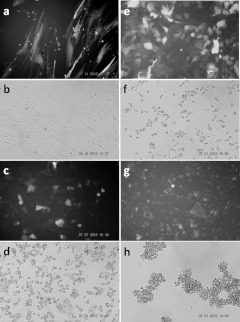
Since AAV vectors cannot distinguish between normal and tumor cells, a tumor-specific promoter is required to direct the expression of toxins in tumor cells. Several tumor-specific promoters and tumor cell lines were used in this study. The cells were seeded on 24-well plates and transduced with AAV2 vectors carrying DT-A under the control of the various tumor-specific promoters. The results from the cell viability assay indicate that HepG2 cells were killed by DT-A under the control of CXCR4, SURV, and AFP promoters, with the CXCR4 promoter showing the strongest killing effect when high titers of AAV2 vectors were used (Figure 5a). Hep3B cells were killed by DT-A under the control of SURV, CXCR4, and AFP promoters, with the SURV promoter showing the strongest killing effect (Figure 5b). Neuroblastoma BE(2)-M17 cells were killed by DT-A under the control of SURV and CXCR4 promoters, but not the AFP promoter (Figure 5c), indicating that the AFP promoter was not active in neuroblastoma cells. A representative result to show the morphologies of AAV2 vector-transduced cells is shown in Figure 6. The normal human lung cell line WI38 was not affected by DT-A under control of the SURV promoter (Figure 6b), whereas the three tumor cell lines, HepG2, Hep3B, and BE(2)-M17 were killed by DT-A under control of the SURV promoter (Figure 6d,f,h). The three tumor cell lines were also tested with DT-A under control of human COX2, CCKAR, and hTERT promoters, but no significant cell-killing effect was observed (data not shown), probably due to the weak promoter activities.
Figure 5.
Cell viability assay of tumor cells transduced with AAV2 vectors carrying DT-A under the control of various human tumor-specific promoters. The cells were seeded on 96-well plates and transduced with AAV2 vectors at fourfold serial dilutions for 4 days, and cell viability was assayed with the CellTiter Glo Luminescent Cell Viability Assay kit. (a) HepG2 cells, (b) Hep3B cells, and (c) BE(2)-M17 were transduced with AAV2 vectors carrying DT-A under control of different tumor-specific promoters. AAV, adeno-associated virus; CMV, cytomegalovirus; DT-A, diphtheria toxin fragment A; GFP, green fluorescent protein; RLU, relative luminescence unit.
Figure 6.
Representative results of tumor-specific cell killing by AAV2 vectors carrying DT-A under the control of the survivin promoter. The cells were seeded in 24-well plate and transduced with (a,c,e,g) AAV2-CMV-GFP or (b,d,f,h) AAV2-hSURV-inDTA(hGH). Photographs were taken 3 days post-transduction. (a,b), WI38; (c,d), HepG2; (e,f), Hep3B, and (g,h), BE(2)-M17 cells. AAV, adeno-associated virus; CMV, cytomegalovirus; DT-A, diphtheria toxin fragment A; GFP, green fluorescent protein; hGH, human growth hormone.
Discussion
Suicide gene therapy has been widely investigated for the treatment of HIV infection, for controlling graft-versus-host disease, and also for the treatment of cancer. While the production of viral vectors carrying suicide genes, such as the herpes simplex virus thymidine kinase or cytosine deaminase are easily achievable, the generation of viral vectors containing toxin genes, such as DT-A, Pseudomonas exotoxin, or Bar has always been a challenge due to the extreme toxicity of these toxins. The current study presents a novel method to circumvent this toxicity issue by exploiting the differences of intron-splicing machineries between mammalian and insect cells. Since some mammalian introns are not functional in insect cells,13,14 two commonly used mammalian introns, hGH and SV40LT introns, were selected and cloned to interrupt the ORFs of DT-A and Bar, respectively. The insertion sites were chosen between 5′-AG and 3′-G nucleotides according to intron-splicing consensus sequence.12 As predicted, the insertion of these mammalian introns in the toxin ORFs totally abolished their expression in insect cells and enabled the production of recombinant baculoviruses carrying these intron-interrupted toxin ORFs at normal levels. In contrast, no recombinant baculovirus was produced when the toxin ORFs were not interrupted by the introns (Figure 2). Furthermore, the recombinant baculoviruses carrying the intron-interrupted toxin ORFs were able to kill mammalian cells once they were introduced into the cells, demonstrating that the mammalian introns were spliced out, which allowed the toxin proteins to be expressed (Figure 3). This is the first report to show that mammalian introns can be used to interrupt the ORFs of toxin genes and abolish their expression in insect cells so that viral vectors (both recombinant baculoviral and AAV vectors) can be produced at normal levels. These viral vectors can then be introduced into mammalian cells for various purposes that require cell killing. This discovery is significant because it can be used to produce viral vectors carrying any toxic genes at normal levels and use them to kill mammalian cells, which should pave the way for further research, as well as for the development of toxin-based suicide gene therapy drugs.
DT-A is extremely toxic to mammalian cells, for as few as one single molecule is enough to kill a cell.2 DT-A is also toxic to insect cells and recombinant baculovirus could only be generated when the DT-A ORF was under control of the GFAP promoter, which is inactive in insect cells.9 This extreme cytotoxicity was confirmed in the current study by the observation that no recombinant baculovirus could be produced from bacmid DNA containing DT-A–coding sequence under control of the CMV promoter (Figure 2a). Though it is not an insect promoter, the CMV promoter has some activity in insect cells and is able to drive low level of hemagglutinin expression.15 This low level of promoter activity could drive some basal expression of DT-A, which kills any insect cells that express the toxin. It was also observed that recombinant baculovirus carrying the DT-A ORF flanked with LoxP sites in an antisense direction to the chicken β-actin promoter (CAG) promoter was not generated, indicating that even cryptic promoter TATA-like sequence of the LoxP site was able to drive trace amounts of DT-A expression that can kill insect cells and inhibit baculovirus production (Figure 2). The same phenomenon was observed for the Bar in which recombinant baculoviruses could be generated only when its ORF was interrupted by the SV40LT antigen intron, indicating that Bar is also toxic to insect cells. On the other hand, the successful generation of recombinant baculoviruses harboring either the intron-interrupted DT-A or the Bar-GFP gene indicated that hGH and SV40LT antigen introns were not spliced in insect cells, and that DT-A and Bar-GFP expressions were totally abolished by this intron interruption.
AAV vector is one of the most promising vectors for human gene therapy and has been used in numerous clinical trials.16 The development of AAV vectors for cancer therapy has focused on antiangiogenic, immunomodulatory, and suicide genes that require a prodrug, or on genes that mediate cell repair.17 It has been reported that AAV vectors carrying the DT-A gene under control of the Tet-repressor system can be produced in HEK293 cells with yields generally 2–3 logs lower than that of control AAV vectors due to the basal level expression of DT-A.11 This demonstrates that the poisonous effect of the toxin on the producer cells even at trace amounts is enough to limit AAV vector production. In the current study, the expression of DT-A or Bar toxins was totally abolished during AAV vector production process in insect cells, and their expression was restored when the AAV vectors were introduced into mammalian cells. This was accomplished by exploiting the differences in intron-splicing mechanisms between insect and mammalian cells. This novel method enables the high production yields of AAV vectors carrying the toxin gene (Table 1), and should provide abundant AAV vectors for further research and the potential development of cancer therapy drugs.
Specificity is paramount in cancer therapy when lethal toxins such as DT-A or Pseudomonas exotoxin are used. Several approaches, such as tumor-specific promoters driving toxic gene expression and engineered AAV capsids to target cancer cells, can be employed to restrict the toxin expression in cancer cells so that unwanted harm can be minimized. It has been reported that tumor-specific promoters can direct gene expression in tumor cells with limited expression in normal cells.17,18,19,20 In the present study, the human TERT, AFP, SURV, COX2, CCKAR, and CXCR4 promoters were cloned to drive the expression of DT-A gene, and their specific cell-killing effects were examined. The normal cell line WI38 was not affected by DT-A under control of any of the above mentioned tumor-specific promoters, but was killed by DT-A under control of the CMV promoter. While TERT, COX2, and CCKAR promoters showed weak activity in HepG2, Hep3B, and BE (2)-M17 cells; SURV and CXCR4 promoters showed strong activity in all three tumor cell lines. The SURV promoter showed the strongest activity in the tumor cells tested, but no apparent activity in WI38 cells, which agrees with the observation that the SURV promoter has extremely low activity in normal cells.17 Further studies with animal models are under way to test the inhibitory effect of DT-A carried by AAV vectors on tumor growth.
In summary, this study presents a novel method for overcoming the toxicity issue of toxin genes during production of viral vectors by exploiting the intron-splicing mechanism of insect cells. Normal titers of both recombinant baculoviral and AAV vectors carrying the toxic DT-A and Bar genes were produced. The introns inserted into the toxic DT-A and Bar genes were spliced out, and toxic proteins were expressed which killed the mammalian cells. This study further demonstrates that AAV2 vectors carrying DT-A under control of tumor-specific promoters killed tumor cells but not normal human cells, which should provide new tools for further developments in toxin-based cancer therapy drugs.
Materials and Methods
Construction of plasmids. Plasmid pFB-CMV-DTA was created by cutting DT-A fragment (GenBank access no. X00703) out with EcoRI and BamHI restriction endonucleases from a plasmid that contains the DT-A–coding sequence and ligating to the EcoRI and BamHI sites of plasmid pFB-AAV-CMV-SV40pA. The same DTA fragment was ligated to the EcoRI and BamHI sites of a pFB-Flex plasmid to create pFB-CAG-LoxP-DTA. To insert the hGH intron into the DT-A gene, a PCR method was employed. First, the upstream junction sequence together with the 5′-DT-A sequence from nucleotide numbers 1 to 103 were amplified using forward primer 5′-GATTATGATCACTAGTCGAG-3′ and reverse primer 5′-GGG CGCTTACCTTTTTGAATGGAATCTACA-3′ (bold typeface indicates hGH intron sequence). The hGH intron was amplified with forward primer 5′-ATTCAAAAAGGTAAGCGCCCCTAAAATCCC-3′ (bold typeface indicates hGH intron sequence) and reverse primer ′'-TTTTGTATACCTGGGGAGAAACCAGA GGGC-3′ (italic typeface indicates restriction site for BstZ17I; bold typeface indicates hGH intron sequence). These two PCR fragments were then joined together through a second PCR amplification with the forward primer for 5′-DT-A and the reverse primer for the hGH intron as described above. The joined PCR fragment was then digested with restriction endonucleases EcoRI and BstZ17I and ligated to the EcoRI and BstZ17I sites of pFB-CMV-DTA to create pFB-CMV-inDTA (hGH). The expression cassette was verified by DNA-sequencing analysis.
The Bar-coding sequence (GenBank access no. M14442) was PCR amplified from plasmid pF1A-T7 (Promega, Madison, WI) with forward primer 5′-CCCGAATTCGCCACCATGGCACAGGTTATCAAC-3′ (italic typeface indicates restriction site for EcoRI) and reverse primer 5′-TGCTCACCATTCTGATTTTTGTAAAGGTCT-3′. The GFP-coding sequence (GenBank access no. U55762) was amplified from pFB-GFP plasmid with forward primer 5′-CAAAAATCAGAATGGTGAGCAAGGGCGAGG-3′ and reverse primer 5′-GGGGGGTACCTCATTACTTGTACAGCTCGTCC-3′ (italic typeface indicates restriction site for KpnI). A second round of PCR was performed to fuse together the Bar-coding sequence in-frame with GFP-coding sequence using the forward primer for 5′-Bar and the reverse primer for the GFP as described above. The fused PCR fragment was digested with restriction endonucleases EcoRI and KpnI and ligated to the EcoRI and KpnI sites of pFB-CMV-inDTA (hGH) to replace the DT-A sequence and create pFB-CMV-Bar-GFP.
In order to insert the SV40 large T-antigen intron into the Bar-coding sequence, the PCR method was employed. The 5′-portion of the Bar-coding sequence together with a full-length SV40 large T-antigen intron was PCR amplified from a SV40 plasmid (pUCSV40-B2E; ATCC, Manassas, VA) with forward primer 5′- CCCCGAATTCGCCACCATGGCACAGG TATTTGCTTCTTCCTTAAA-3′ (italic typeface indicates the EcoRI restriction site; bold typeface indicates SV40 large T-antigen intron sequence) and reverse primer 5′-TGTTGATAACCTAAAATACACAAACAATTA-3′ (bold typeface indicates SV40 large T-antigen intron sequence). The 3′-portion of the Bar-coding sequence together with GFP-coding sequence was PCR amplified with forward primer 5′-TGTATTTTAGGTTATCAACACGTTTGACGG-3′ (bold typeface indicates SV40 large T-antigen intron sequence), and the reverse primer 5′-GGG GGGTACCTCATTACTTGTACAGCTCGTCC-3′ with pFB-CMV-Bar-GFP as template. A second PCR was performed to join these two PCR fragments together with the forward primer for 5′-Bar and the reverse primer for the GFP-coding sequence as described above. The joined PCR fragment was digested with restriction endonucleases EcoRI and KpnI and ligated to the EcoRI and KpnI sites of pFB-CMV-inDTA (hGH) to replace the DTA sequence and create pFB-CMV-inBar (SV40LT)-GFP.
The human telomerase reverse transcriptase (hTERT) promoter sequence was PCR amplified with genomic DNA purified from human embryonic kidney HEK293 cells with forward primer 5′-GCGCACGCGTATCATCAGCTTTTCAAAGAC-3′ (italic typeface indicates restriction site for MluI) and reverse primer 5′- CGCGACCGGTCGCTGCCTGAAACTCGCGCC -3′ (italic typeface indicates restriction site for AgeI). The PCR fragment was digested with MluI and AgeI and ligated to the MluI and AgeI sites of pFB-CMV-inDTA (hGH) to replace the CMV promoter and create pFB-hTERT-inDTA (hGH).
The human AFP enhancer and promoter sequences were both PCR amplified from genomic DNA purified from human embryonic kidney HEK293 cells. The AFP enhancer was amplified with forward primer 5′-CCGCACGCGTCTTAGAAATATGGGGGTAGGGGTGG-3' (italic typeface indicates restriction site for MluI) and reverse primer 5′-CTCAAACTCTAGTGGCCTGGATAAAGCTGAGTG-3′. The AFP promoter was amplified with forward primer 5′-CTTTATCCAGGCCACTAGAGTTTGAGGAGAATATTTG-3′ and reverse primer 5′-ACTTACCTGACCGGTTGCTAGTTATTTTGTTAT-3′. The two PCR fragments were joined together by a second PCR amplification with the forward primer for the AFP enhancer and the reverse primer for the AFP promoter as described above. The final PCR fragment was digested with MluI and AgeI and ligated to the MluI and AgeI sites of pFB-CMV-inDTA (hGH) to replace the CMV promoter and create pFB-hAFP-inDTA (hGH).
The human survivin (SURV) promoter sequence was PCR amplified with genomic DNA purified from human embryonic kidney HEK293 cells with forward primer 5′-GGGGACTAGTCTGGCCATAGAACCAGAGAAGTGA-3′ (italic typeface indicates restriction site for SpeI) and reverse primer 5′-TTTTACCGGTCCACCTCTGCCAACGGGTCCCGCG-3′ (italic typeface indicates restriction site for AgeI). The PCR fragment was digested with SpeI and AgeI and ligated to the SpeI and AgeI sites of pFB-CMV-inDTA (hGH) to replace the CMV promoter and create pFB-hSURV-inDTA (hGH).
The human cyclooxygenase-2 (COX2) promoter was PCR amplified with genomic DNA purified from human embryonic kidney HEK293 cells with forward primer 5′-GCCCACTAGTTGAGGTACCTGGTGTAGTTT-3′ (italic typeface indicates restriction site for SpeI) and reverse primer 5′-ATATACCGGTCAGCGGCGGGCAGGGCGCGG-3′ (italic typeface indicates restriction site for AgeI). The PCR fragment was digested with SpeI and AgeI and ligated to the SpeI and AgeI sites of pFB-CMV-inDTA (hGH) to replace the CMV promoter and create pFB-hCOX2-inDTA (hGH).
The human cholecystokinin type-A receptor (CCKAR) promoter sequence was PCR amplified with genomic DNA purified from human embryonic kidney HEK293 cells with forward primer 5′-GCCCACTAGTACCCAGGTACCTATGTTCAAAAG-3′ (italic typeface indicates restriction site for SpeI) and reverse primer 5′-GCGCACCGGTTTGCCTGCTGCTTTCCACCAAG-3′ (italic typeface indicates restriction site for AgeI). The PCR fragment was digested with SpeI and AgeI and ligated to the SpeI and AgeI sites of pFB-CMV-inDTA (hGH) to replace the CMV promoter and create pFB-hCCKAR-inDTA (hGH).
The human C-X-C chemokine receptor type 4 (CXCR4) promoter sequence was PCR amplified with genomic DNA purified from human embryonic kidney HEK293 cells with forward primer 5′-GCCCACTAGTTACCGACCACCCGCAAACAG-3′ (italic typeface indicates restriction site for SpeI) and reverse primer 5′-GCGCACCGGTGTAACCGCTGGTTCTCCAGA-3′ (italic typeface indicates restriction site for AgeI). The PCR fragment was digested with SpeI and AgeI and ligated to the SpeI and AgeI sites of pFB-CMV-inDTA (hGH) to replace the CMV promoter and create pFB-hCXCR4-inDTA (hGH).
Cell culture. HEK293 cells were maintained at 37 °C in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) containing 100 U/ml of penicillin and 100 mg/ml of streptomycin, and supplemented with 10% fetal calf serum (Sigma-Aldrich, St Louis, MO). HepG2, Hep3B, and WI38 cells were maintained at 37 °C in Eagle's minimal essential medium complete growth medium (ATCC) containing 100 U/ml of penicillin and 100 mg/ml of streptomycin, and supplemented with 10% fetal calf serum. BE (2) M17 cells (ATCC) were maintained in 50% Eagle's minimal essential medium + 50% F12 complete growth media containing 100 U/ml of penicillin and 100 mg/ml of streptomycin, and supplemented with 10% fetal calf serum. Spodoptera frugiperda Sf9 cells (Invitrogen) were maintained at 28 °C in ESF921 serum-free medium (Expression Systems, Woodland, CA) containing 100 U/ml of penicillin and 100 mg/ml of streptomycin (Invitrogen).
Generation and titration of recombinant baculoviruses. Plasmids constructed as described above were used for transforming DH10Bac-competent cells to generate recombinant bacmids. The bacmids containing the target genes were used for generating recombinant baculoviruses in accordance with the manufacturer's protocol (Invitrogen), with minor modifications. Briefly, 2 ng of the plasmid DNA was used for transforming 20 µl of DH10Bac-competent cells. After 4 hours of incubation at 37 °C in 500 µl of Super Optimal broth with Catabolite repression medium, 25 and 2.5 µl of the culture were plated separately in selection plates and incubated for 48 hours to allow white/blue colonies to form. Generally, three white colonies were picked for each construct and miniprep bacmid DNAs were prepared. The bacmid DNAs were used for transfecting Sf9 cells to generate recombinant baculoviruses. The recombinant baculoviruses were amplified once and titers determined with real-time quantitative PCR assay and converted to pfu based on empirical studies in which 20 copies was converted into 1 pfu.
AAV vector production, purification, and titration. The methods for AAV vector production in Sf9 cells, purification, and titration have been described previously.21,22 Briefly, Sf9 cells were grown at 28 °C to ~1 × 107 cells/ml in ESF921 serum-free medium containing 100 U/ml of penicillin and 100 µg/ml of streptomycin, and diluted to ~5 × 106 cells/ml before infection. Double infection was employed to produce AAV vectors. Five multiplicity of infection of Bac-AAV-transgene, and 10 multiplicity of infection of Bac-inCap-inRep were used to infect Sf9 cells at 28 °C for 3 days to produce AAV vectors. After 3 days of infection, cell pellets were collected by centrifugation at 3,000 rpm for 15 minutes. The cell pellets were lysed in a modified Sf9 lysis buffer (50 mmol/l Tris-HCl, pH 8.0, 1% sarkosyl, 1% Triton X-100, 2 mmol/l MgCl2) and cellular nucleic acids (DNA and RNA) were digested using benzonase (Sigma-Aldrich). The cell lysates were cleared by centrifugation at 8,000 rpm for 20 minutes and supernatants were subjected to two rounds of cesium chloride ultracentrifugation to purify the AAV vectors. The AAV vectors were buffer-exchanged into phosphate-buffered saline containing 0.001% pluronic F-68 (Sigma-Aldrich) with 2 PD-10 desalting columns (GE HealthCare Bio-Science, Piscataway, NJ). After purification, the AAV vectors were titrated with real-time quantitative PCR method.
Cell proliferation assay. HEK293 cells were grown in 24-well plate (1.5 × 105 cells/well) overnight and transduced with AAV2 vectors (1.5 × 109 vector genome/well) for 48 hours. The cells were then trypsinized and cell number counted. The cells were further cultivated for another 48 hours until reaching confluency and trypsinized, cell number counted and then expanded into larger vessels. After another 72-hour cultivation, the cells were trypsinized and counted. Viable cells were counted with trypan blue staining.
Cell viability assay. The CellTiter Glo Luminescent Cell Viability Assay kit was used to test the cell viability according to manufacturer's protocol (Promega). Briefly, the cells (WI38, HepG2, Hep3B, and BE (2) M17) were seeded on 96-well plates at 3.2 × 104 cells/well overnight and transduced with AAV2 vectors at fourfold serial dilutions for 4 days. The reagent was reconstituted and added to the cells. After mixing on an orbital shaker for 2 minutes to lyse the cells, the plate was incubated at room temperature for 10 minutes and then the luminescent signals were recorded.
References
- Rodriguez R., and, Simons JW. Urologic applications of gene therapy. Urology. 1999;54:401–406. doi: 10.1016/s0090-4295(99)00171-5. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M, Mekada E, Uchida T., and, Okada Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 1978;15:245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]
- Denning C., and, Pitts JD. Bystander effects of different enzyme-prodrug systems for cancer gene therapy depend on different pathways for intercellular transfer of toxic metabolites, a factor that will govern clinical choice of appropriate regimes. Hum Gene Ther. 1997;8:1825–1835. doi: 10.1089/hum.1997.8.15-1825. [DOI] [PubMed] [Google Scholar]
- Maxwell IH, Maxwell F., and, Glode LM. Regulated expression of a diphtheria toxin A-chain gene transfected into human cells: possible strategy for inducing cancer cell suicide. Cancer Res. 1986;46:4660–4664. [PubMed] [Google Scholar]
- Maxwell F, Maxwell IH., and, Glode LM. Cloning, sequence determination, and expression in transfected cells of the coding sequence for the tox 176 attenuated diphtheria toxin A chain. Mol Cell Biol. 1987;7:1576–1579. doi: 10.1128/mcb.7.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DF., and, Maxwell IH. Suppression of single and double nonsense mutations introduced into the diphtheria toxin A-chain gene: a potential binary system for toxin gene therapy. Hum Gene Ther. 1995;6:137–143. doi: 10.1089/hum.1995.6.2-137. [DOI] [PubMed] [Google Scholar]
- Paulus W, Baur I, Oberer DM, Breakefield XO., and, Reeves SA. Regulated expression of the diphtheria toxin A gene in human glioma cells using prokaryotic transcriptional control elements. J Neurosurg. 1997;87:89–95. doi: 10.3171/jns.1997.87.1.0089. [DOI] [PubMed] [Google Scholar]
- Keyvani K, Baur I., and, Paulus W. Tetracycline-controlled expression but not toxicity of an attenuated diphtheria toxin mutant. Life Sci. 1999;64:1719–1724. doi: 10.1016/s0024-3205(99)00109-5. [DOI] [PubMed] [Google Scholar]
- Wang CY, Li F, Yang Y, Guo HY, Wu CX., and, Wang S. Recombinant baculovirus containing the diphtheria toxin A gene for malignant glioma therapy. Cancer Res. 2006;66:5798–5806. doi: 10.1158/0008-5472.CAN-05-4514. [DOI] [PubMed] [Google Scholar]
- Li Y, McCadden J, Ferrer F, Kruszewski M, Carducci M, Simons J.et al. (2002Prostate-specific expression of the diphtheria toxin A chain (DT-A): studies of inducibility and specificity of expression of prostate-specific antigen promoter-driven DT-A adenoviral-mediated gene transfer Cancer Res 622576–2582. [PubMed] [Google Scholar]
- Kohlschütter J, Michelfelder S., and, Trepel M. Novel cytotoxic vectors based on adeno-associated virus. Toxins (Basel) 2010;2:2754–2768. doi: 10.3390/toxins2122754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver RF. McGraw-Hill: Boston; 2002. Molecular Biology. [Google Scholar]
- Urabe M, Ding C., and, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- Jeang KT, Holmgren-Konig M., and, Khoury G. A baculovirus vector can express intron-containing genes. J Virol. 1987;61:1761–1764. doi: 10.1128/jvi.61.5.1761-1764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Ho Y, Yu L., and, Kwang J. WSSV ie1 promoter is more efficient than CMV promoter to express H5 hemagglutinin from influenza virus in baculovirus as a chicken vaccine. BMC Microbiol. 2008;8:238. doi: 10.1186/1471-2180-8-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High KA., and, Aubourg P. rAAV human trial experience. Methods Mol Biol. 2011;807:429–457. doi: 10.1007/978-1-61779-370-7_18. [DOI] [PubMed] [Google Scholar]
- Zhu ZB, Makhija SK, Lu B, Wang M, Kaliberova L, Liu B.et al. (2004Transcriptional targeting of tumors with a novel tumor-specific survivin promoter Cancer Gene Ther 11256–262. [DOI] [PubMed] [Google Scholar]
- Zhu ZB, Makhija SK, Lu B, Wang M, Kaliberova L, Liu B.et al. (2004Transcriptional targeting of adenoviral vector through the CXCR4 tumor-specific promoter Gene Ther 11645–648. [DOI] [PubMed] [Google Scholar]
- Hallenbeck PL, Chang YN, Hay C, Golightly D, Stewart D, Lin J.et al. (1999A novel tumor-specific replication-restricted adenoviral vector for gene therapy of hepatocellular carcinoma Hum Gene Ther 101721–1733. [DOI] [PubMed] [Google Scholar]
- Gu J, Andreeff M, Roth JA., and, Fang B. hTERT promoter induces tumor-specific Bax gene expression and cell killing in syngenic mouse tumor model and prevents systemic toxicity. Gene Ther. 2002;9:30–37. doi: 10.1038/sj.gt.3301619. [DOI] [PubMed] [Google Scholar]
- Chen H. Intron splicing-mediated expression of AAV Rep and Cap genes and production of AAV vectors in insect cells. Mol Ther. 2008;16:924–930. doi: 10.1038/mt.2008.35. [DOI] [PubMed] [Google Scholar]
- Merten O-W., and, Al-Rubeai M. Humana Press: New York; Viral Vectors for Gene Therapy: Methods and Protocols. [Google Scholar]