Introduction
There are now multiple effective strategies for the activation and expansion, or direct selection of virus-specific T cells that may be able to eliminate a range of virus infections in the immunocompromised host. In this mini-review, we explain how the stage is set for their rigorous evaluation in large-scale clinical trials.
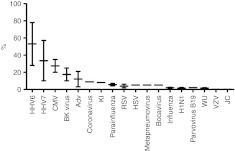
Virus infections are the cause of 30% or more of transplant-related deaths in recipients of T-cells depleted, allogeneic hematopoietic stem cell transplants (HSCT).1 Epstein-Barr virus (EBV), cytomegalovirus (CMV), and adenoviruses (AdV) are the most common culprits, but other common viruses like parainfluenza virus, respiratory syncytial virus, influenza viruses, polyomaviruses, and human herpesvirus 6 together contribute significant morbidity and mortality (Figure 1).2,3,4 Small molecule therapies are often ineffectual, always costly and frequently produce significant adverse effects.
Figure 1.
Relative frequency of viral infections after HSCT. Adv, adenovirus; BK, JC, KI, and WU (polyomaviruses); CMV, cytomegalovirus; H1N1, influenza strain Hemagglutinnin 1 Neuraminidase 1); HHV, human herpes virus; HSCT, hematopoietic stem cell transplant; HSV, herpes simplex virus; RSV, respiratory syncytial virus; VZV, varicella zoster virus.
Virus-specific T-cells derived from stem cell donors can prevent and treat post-transplant viral infections, in the recipients for whom they were intended, and also in partially human leukocyte antigen (HLA)-matched, third party recipients.5,6,7 The low toxicity and long-term protection provided by virus-specific T-cells compares favorably with the significant toxicities and short-term effects of most antivirals.5,6,8,9,10 Is it time, therefore, to begin the transfer of T-cell manufacturing from academic phase I/II clinical trials into hospital or industry-supported facilities so that virus-specific T-cells can be made available to all high-risk HSCT recipients?
Several barriers prevent the broader use of virus-specific T-cell therapies after stem cell transplantation. While T-cell therapies for EBV, CMV, and AdV have clearly demonstrated their safety and efficacy both as prophylaxis and as therapy, for many other viruses, the antigens that induce protective T-cells have yet to be identified. Moreover, these infections may occur in <5% of patients making it difficult to perform the rigorous comparative effectiveness studies that will be required to show lower overall cost, fewer adverse effects, and equivalent or superior efficacy. Before any of these barriers can be breached effectively by the academic institutions who are the major developers of these T-cells therapeutics, we must select and optimize manufacturing strategies that are robust and scalable and have the lowest possible cost. Finally, the leap to late phase trials cannot be accomplished by academic institutions alone, but requires partnership with industry. This article will largely deal with selection and optimization of virus-specific T-cell manufacturing strategies.
Choice of Protective Viral Antigens
The mere existence of circulating virus-specific T-cells does not mean they are protective, since viral antigens may be cross-presented by professional antigen-presenting cells even when absent or ineffectively presented by the infected cells themselves. Protective antigens are often virion proteins, like hexon and penton of adenovirus and pp65 of CMV, or immediate early proteins that are presented by newly infected cells before they produce infectious virus or express their ubiquitous immune evasion genes.5,11,12,13 T-cells specific for immediate early antigens, like CMV-IE, should also eliminate cells in which viruses reactivate from latency. For EBV, T-cells must recognize and kill proliferating B cells expressing latent cycle proteins but must also kill productively infected cells before their release of infectious virus. EBV-transformed B-cell line (EBV-LCL)-activated T-cells are protective but recognize a broad range of latent and early lytic cycle proteins, which tend to vary depending on the donor's haplotype, and single antigens that provide protective immunity are yet to be identified. Our group is testing whether T-cells specific for the latency proteins, EBNA1 and LMP2, and the immediate early ZEBRA protein, are able to protect against EBV.14 For other viruses, protective antigens may be predicted, and tested in animal models if available, but they can be validated only in human clinical trials.
Problems with Current Manufacturing Strategies
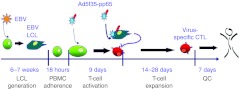
Several strategies for the manufacture of virus-specific T-cells have been described. The first studies used T-cells that had been activated and expanded in vitro to remove alloreactive T-cells that could cause graft-versus-host disease, while increasing virus-specific T-cell number and effector functions to increase their speed of action. This T-cell activation and expansion used cells infected with live viruses, namely CMV-infected fibroblasts and EBV-LCLs, as antigen-presenting cells.5,8 Subsequently, EBV-LCLs were used as antigen-presenting cells to expand T cells specific for other viruses, since LCLs can be made from almost any donor, are available in unlimited numbers, and can be transduced with viral vectors to express and present heterologous viral antigens such as CMV-pp65 (see Figure 2).7 This approach, while effective, introduced undesirable pathogens (EBV) into the manufacturing process, which itself was extremely prolonged. The production time for EBV-LCLs is about 6 weeks, while the cytotoxicT-cell lines required an additional 4 weeks of culture followed by 7–14 days for quality control testing. This timing is feasible for prophylaxis of viral infections, but inappropriate for reactive therapy. Finally, the approach was hard to scale; cytotoxicT-cell lines needed to be grown in 24-well plates, which introduces a risk of contamination and does not satisfy good manufacturing practice requirements. Fortunately, strategies to remove live virus and viral vectors, shorten manufacturing time, and scale the culture conditions are now available.
Figure 2.
Time line for original (complex) manufacture of T-cells specific for cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenoviruses (AdV). Establishment of the lymphoblastoid cell line (LCL) used as the antigen-presenting cell takes about 6 weeks. Once the LCL is established, the cytotoxic T-cell line (CTL) is initiated. Peripheral blood mononuclear cells (PBMCs) are cultured overnight to activate monocytes that are then transduced with an adenovirus vector expressing the pp65 protein from CMV (Ad5f35-pp65).They are then cultured for 9 days during which time, T-cells specific for virion proteins of the AdV and its CMV-pp65 transgene are activated. On day 9 and weekly thereafter, the T cells are restimulated with the autologous EBV-LCL transduced with Ad5f35-pp65. Interleukin (IL)-2 is added twice weekly from day 14. Sufficient EBV-specific T cells remain in the culture on day 9 to be reactivated by the EBV-LCL. After the third or fourth stimulation, sufficient T-cells specific for CMV, EBV, and AdV are cryopreserved for infusion and tested for sterility, identity by HLA typing, phenotype, lack of alloreactivity by Cr51 release assay, and specificity by γ-IFN ELIspot assay (a further 7–10 days). HLA, human leukocyte antigen; QC, quality control.
Reducing the Time For T-Cell Manufacture and Eliminating Viral Vectors
Investigators have extracted antigen-specific T-cells directly from blood using HLA-peptide multimers,11,15 or by capturing cells that secrete γ-interferon in response to overnight antigen stimulation (γ-catch).16 Multimers select T-cells that recognize peptide epitopes in association with specific HLA alleles, an approach that is limited by their availability for a small number of HLA class I alleles. A phase 2 trial of streptamer-selected CMV-specific T cells (Cytovir-CMV) is currently being sponsored by Cell Medica (London, UK). The γ-capture technique is more universal and can capture both CD4+ and CD8+ T-cells specific for multiple epitopes in any antigen, regardless of HLA phenotype. Both techniques are rapid and clinically effective, since small numbers of T-cells undergo massive expansion after infusion and can reconstitute immunity to CMV, AdV, and EBV.17,18,19,20 However, their broader use may be limited by the large blood volumes required to rapidly select sufficient numbers of virus-specific T-cells from unrelated donors, and by the low numbers of circulating T-cells specific for nonpersistent viruses.
Rapid Expansion of Virus-Specific T-Cells
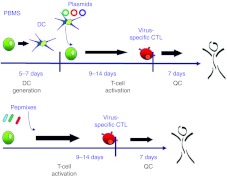
Virus-specific memory T-cells undergo 2–3 logs of expansion after a single in vitro stimulation. Thus, as little as 20 ml of blood containing 105 virus-specific T-cells could yield 107 or 108 virus-specific T-cells after 8–10 days, given appropriate stimulation and the right culture conditions. This expansion has been achieved by stimulation of peripheral blood mononuclear cells with autologous dendritic cells nucleofected with plasmids expressing antigens for four different viruses (CMV, EBV, BK, and AdV),14 and expansion in highly scalable, gas permeable G-Rex culture devices (Wilson Wolf Manufacturing, Minneapolis, MN) (see Figure 3).21,22 Sufficient virus-specific non-alloreactive T-cells could be infused within 10 days of culture initiation. This strategy is currently under clinical evaluation in an National Heart, Lung, and Blood Institute (NHLBI)-Production Assistance for Cellular Therapies (PACT)-sponsored study. In a further simplification, virus-specific T-cells could be expanded by stimulation of peripheral blood mononuclear cells with overlapping peptide libraries representing the entire protein sequences of specific viral antigens (Figure 3).23 Similar fold expansion was achieved without a requirement for dendritic cell manufacture, shortening the procedure by 7 days.22 The small volumes of blood required for this manufacturing strategy could readily be stored from stem cell donors at the time of transplant and virus-specific T-cells could be manufactured prophylactically or at the first indication of virus reactivation or infection.
Figure 3.
Time line for simplified manufacture of antigen-specific T cells using plasmids or pepmixes. In the upper panel, peripheral blood mononuclear cells (PBMCs) are stimulated with autologous, mature dendritic cells nucleofected (Amaxa) with DNA plasmids expressing viral antigens.14 In the lower panel, PBMCs are directly stimulated with pepmixes (peptide libraries containing 15mers overlapping by 11 amino acids). In both cases, cells are stimulated in the presence of interleukin (IL)-4 and IL-7 in G-Rex culture devices, and expanded for 9–14 days. The T cells are then cryopreserved and tested for sterility, identity by HLA typing, phenotype, lack of alloreactivity by Cr51 release assay, and specificity by γ-IFN ELIspot assay. CTL, cytotoxic T-cell line; DC, dendritic cell; HLA, human leukocyte antigen; QC, quality control.
“Off the Shelf” Virus-Specific T-Cells
Haque and colleagues showed that partly HLA-matched third party EBV-specific T-cells produced a 65% response rate in post-transplant lymphoproliferative disease after solid organ transplantation, with a 42% complete response rate.24,25,26 Similarly, O'Reilly and colleagues have used partially HLA-matched EBV-targeted T-cells as treatment for EBV lymphoproliferative disease in allogeneic HSCT recipients and achieved complete or partial remissions in 68%.27,28 In a second NHLBI-PACT–sponsored study, we used banks of third party T-cells specific for EBV, CMV, and AdVs in HSCT recipients and observed high response rates for all three viruses, which were achieved even when only a single HLA antigen was matched between the virus-specific line and third party recipient. Hence, these cells provide a stand alone off the shelf product that may also serve to gain time for the rapid manufacture of a patient-specific product.
Ancillary Reagents For T-Cell Manufacture
The availability of clinical grade ancillary reagents, such as tissue culture medium, serum, cytokines, and peptides, for the manufacture of T-cells to be used in late phase trials may also pose a barrier. Research grade reagents can be used for phase 1 trials, provided they adhere to specific manufacturing and testing requirements as listed in the U.S. Pharmacopeia, Chapter 1043. For example, cytokines made in animal cells must undergo two viral exclusion tests. For phase 3 studies, however, the ability of the exclusion systems to eliminate virus must be documented. This holds true for other potential contaminants, such as solvents (see the International Conference on Harmonization document “Q3C (R4): Impurities: Guideline for Residual Solvents”). Optimal growth of T-cells requires culture in serum, typically fetal calf serum, from certified US herds, or human serum. These can be obtained, but are generally not marketed for clinical use. Manufacturers are beginning to step up to the plate. For example good manufacturing practice-grade cytokines are marketed by CellGenix (Freiburg, Germany) and Miltenyi and cell culture medium for good manufacturing practice is available from CellGenix and Lonza (Basel, Switzerland).
Acceptance of Cell Therapies As a Standard Treatment Option
Resistance to the acceptance of patient-specific, complex biologics as standard of care by physicians, hospitals, and national and private payors must be overcome before they can be considered as serious options for patients. Among other considerations, this will require cost-effectiveness studies that compare the economics of short- and long-term toxicities and quality of life issues associated with standard chemoradiotherapies with those of cellular therapies. As innovative cellular therapies with lower toxicity and greater efficacy become available, there will be a marked shift towards these agents, driven as much by patient knowledge and demand (in an ever more internet-connected environment) as by physician preference. Biotech is now taking serious notice of cell therapies as evidenced by the recent licensing of Provenge by Dendreon and increasing interest and investment by other companies.
Acknowledgments
We would like to thank Malcolm Brenner for reading the manuscript and acknowledge grants from National Institutes of Health-National Heart, Lung, and Blood Institute-Production Assistance for Cellular Therapies (NIH-NHLBI-PACT) N01-HB-10-03, NIH-NHLBI – U54 HL081007, the National Marrow Donor Program through funding from the Amy Strelzer Manasevit Research Program, and an infrastructure grant from Alex's Lemonade Stand that have allowed us to pursue cell therapies. C.R. is a member of the Cell Medica scientific advisory board. Adapted from an article in the Production Assistance for Cell Therapy (PACT) quarterly newsletter, Volume 9, by the same authors. The other author declared no conflict of interest.
References
- Kennedy-Nasser AA, Bollard CM, Myers GD, Leung KS, Gottschalk S, Zhang Y.et al. (2008Comparable outcome of alternative donor and matched sibling donor hematopoietic stem cell transplant for children with acute lymphoblastic leukemia in first or second remission using alemtuzumab in a myeloablative conditioning regimen Biol Blood Marrow Transplant 141245–1252. [DOI] [PubMed] [Google Scholar]
- Renaud C., and, Campbell AP. Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Curr Opin Infect Dis. 2011;24:333–343. doi: 10.1097/QCO.0b013e3283480440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönberger S, Meisel R, Adams O, Pufal Y, Laws HJ, Enczmann J.et al. (2010Prospective, comprehensive, and effective viral monitoring in children undergoing allogeneic hematopoietic stem cell transplantation Biol Blood Marrow Transplant 161428–1435. [DOI] [PubMed] [Google Scholar]
- Leen AM, Tripic T., and, Rooney CM. Challenges of T cell therapies for virus-associated diseases after hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2010;10:337–351. doi: 10.1517/14712590903456003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED.et al. (1995Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor N Engl J Med 3331038–1044. [DOI] [PubMed] [Google Scholar]
- Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA.et al. (2010Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients Blood 115925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS.et al. (2006Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals Nat Med 121160–1166. [DOI] [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA.et al. (1995Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation Lancet 3459–13. [DOI] [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y.et al. (1998Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients Blood 921549–1555. [PubMed] [Google Scholar]
- Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C., and, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H.et al. (2005Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers J Exp Med 202379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Christin A, Khalil M, Weiss H, Gee AP, Brenner MK.et al. (2008Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy J Virol 82546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ.et al. (2009Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation Blood 1144283–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdemann U, Christin AS, Vera JF, Ramos CA, Fujita Y, Liu H.et al. (2009Nucleofection of DCs to generate Multivirus-specific T cells for prevention or treatment of viral infections in the immunocompromised host Mol Ther 171616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudorfer J, Schmidt B, Huster KM, Anderl F, Schiemann M, Holzapfel G.et al. (2007Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens J Immunol Methods 320119–131. [DOI] [PubMed] [Google Scholar]
- Rauser G, Einsele H, Sinzger C, Wernet D, Kuntz G, Assenmacher M.et al. (2004Rapid generation of combined CMV-specific CD4+ and CD8+ T-cell lines for adoptive transfer into recipients of allogeneic stem cell transplants Blood 1033565–3572. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Tonn T, Busch DH, Grigoleit GU, Einsele H, Odendahl M.et al. (2011Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation Transfusion 51591–599. [DOI] [PubMed] [Google Scholar]
- Mackinnon S, Thomson K, Verfuerth S, Peggs K., and, Lowdell M. Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood Cells Mol Dis. 2008;40:63–67. doi: 10.1016/j.bcmd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Moosmann A, Bigalke I, Tischer J, Schirrmann L, Kasten J, Tippmer S.et al. (2010Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells Blood 1152960–2970. [DOI] [PubMed] [Google Scholar]
- Feuchtinger T, Matthes-Martin S, Richard C, Lion T, Fuhrer M, Hamprecht K.et al. (2006Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation Br J Haematol 13464–76. [DOI] [PubMed] [Google Scholar]
- Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H.et al. (2010Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother 33305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdemann U, Vera JF, Rooney CM., and, Leen AM. Generation of multivirus-specific T cells to prevent/treat viral infections after allogeneic hematopoietic stem cell transplant. J Vis Exp. 2011. p. 2736. [DOI] [PMC free article] [PubMed]
- Kern F, Faulhaber N, Frommel CC, Khatamzas E, Prösch S, Schönemann C.et al. (2000Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides Eur J Immunol 301676–1682. [DOI] [PubMed] [Google Scholar]
- Haque T, Taylor C, Wilkie GM, Murad P, Amlot PL, Beath S.et al. (2001Complete regression of posttransplant lymphoproliferative disease using partially HLA-matched Epstein Barr virus-specific cytotoxic T cells Transplantation 721399–1402. [DOI] [PubMed] [Google Scholar]
- Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P.et al. (2007Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial Blood 1101123–1131. [DOI] [PubMed] [Google Scholar]
- Haque T, McAulay KA, Kelly D., and, Crawford DH. Allogeneic T-cell therapy for Epstein-Barr virus-positive posttransplant lymphoproliferative disease: long-term follow-up. Transplantation. 2010;90:93–94. doi: 10.1097/TP.0b013e3181d7c424. [DOI] [PubMed] [Google Scholar]
- Barker JN, Doubrovina E, Sauter C, Jaroscak JJ, Perales MA, Doubrovin M.et al. (2010Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes Blood 1165045–5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J.et al. (2012Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation Blood 1192644–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]