Abstract
Angiopoietin-like proteins (ANGPTLs) play major roles in the trafficking and metabolism of lipids. Inactivation of ANGPTL3, a gene located in an intron of DOCK7, results in very low levels of LDL-cholesterol (C), HDL-C and triglyceride (TAG). We identified another ANGPTL family member, ANGPTL8, which is located in the corresponding intron of DOCK6. A variant in this family member (rs2278426, R59W) was associated with lower plasma LDL-C and HDL-C levels in three populations. ANGPTL8 is expressed in liver and adipose tissue, and circulates in plasma of humans. Expression of ANGPTL8 was reduced by fasting and increased by refeeding in both mice and humans. To examine the functional relationship between the two ANGPTL family members, we expressed ANGPTL3 at physiological levels alone or together with ANGPTL8 in livers of mice. Plasma TAG level did not change in mice expressing ANGPTL3 alone, whereas coexpression with ANGPTL8 resulted in hypertriglyceridemia, despite a reduction in circulating ANGPTL3. ANGPTL8 coimmunoprecipitated with the N-terminal domain of ANGPTL3 in plasma of these mice. In cultured hepatocytes, ANGPTL8 expression increased the appearance of N-terminal ANGPTL3 in the medium, suggesting ANGPTL8 may activate ANGPTL3. Consistent with this scenario, expression of ANGPTL8 in Angptl3−/− mice failed to promote hypertriglyceridemia. Thus, ANGPTL8, a paralog of ANGPTL3 that arose through duplication of an ancestral DOCK gene, regulates postprandial TAG and fatty acid metabolism by controlling activation of its progenitor, and perhaps other ANGPTLs. Inhibition of ANGPTL8 provides a new therapeutic strategy for reducing plasma lipoprotein levels.
The angiopoietin-like (ANGPTL) genes encode a family of secreted proteins with pleiotropic effects on vascular cells (1), lipid metabolism (2), and stem cell biology (3). The family members share a common architecture, comprising an extended N-terminal domain and a C-terminal fibrinogen-like domain. Genetic studies have revealed that two closely related family members, ANGPTL3 and ANGPTL4, play pivotal roles in the trafficking and metabolism of lipids and lipoproteins (4–7). Mutations that disrupt ANGPTL3 are associated with greatly reduced plasma levels of triacylglycerol (TAG) and cholesterol in mice (5) and in humans (4). Mice lacking ANGPTL4 also have markedly reduced levels of plasma TAG and cholesterol (8, 9), and sequence variations in ANGPTL4 are associated with lower plasma TAG levels in humans (7).
TAG synthesized in the gut and liver are incorporated into chylomicrons and very low density lipoproteins (VLDL), respectively, and delivered to peripheral tissues where they interact with lipoprotein lipase (LPL). LPL hydrolyzes the TAGs, releasing fatty acids to the adjacent tissues. Other intravascular lipases, including hepatic lipase and endothelial lipase, further remodel lipoprotein particles. Several lines of evidence suggest that ANGPTL3 and ANGPTL4 contribute to the partitioning of TAGs among tissues (2). During fasting, ANGPTL4 levels increase in adipose tissue (10) where it inhibits LPL, thus preventing fatty acid uptake from circulating lipoproteins (2, 11). After a meal, expression of ANGPTL4 is markedly reduced, relieving the inhibition of intravascular lipolysis and promoting uptake of dietary lipids into adipose tissue.
The role of ANGPTL3 is less well understood. ANGPTL3 is expressed almost exclusively in the liver (5, 6), and is only modestly regulated by food intake (12). Angptl3−/− mice have increased LPL activity (8, 13, 14), and recombinant ANGPTL3 inhibits LPL in vitro (6, 13, 15, 16), leading several investigators to propose that ANGPTL3 raises plasma TAG levels by inhibiting LPL activity (13, 14). However, the concentrations of ANGPTL3 used in these studies were supraphysiological. Moreover in some (14, 17), although not all (8) studies, the increase in LPL activity in Angptl3−/− mice has been modest. ANGPTL3 also inhibits the activity of endothelial lipase (18, 19), which catalyzes the hydrolysis of phospholipids in circulating lipoproteins. Angptl3−/− mice have a ∼50% reduction in HDL levels and a ∼50% increase in heparin-releasable phospholipase activity (19). Thus, ANGPTL3 may raise HDL levels by inhibiting endothelial lipase.
ANGPTL3 is activated by cleavage at a proprotein convertase consensus site (221RAPR224) to release the N-terminal domain (20). Cleavage is essential for ANGPTL3-mediated inhibition of lipases; disruption of the consensus site markedly reduces the effect of the recombinant protein on plasma TAG levels (20).
Here we show that ANGPTL3 is activated by ANGPTL8, a paralog of ANGPTL3 that is highly regulated by fasting and refeeding in mice and humans. We provide biochemical evidence that ANGPTL8 binds to ANGPTL3 and promotes the appearance of the cleaved form, and genetic evidence in mice that the two proteins are mechanistically interdependent. We also show that genetic variation in ANGPTL8 is associated with reductions in HDL-C and LDL-C in three populations, thus confirming a role for ANGPTL8 in lipoprotein metabolism in humans.
Results
Degenerate ANGPTL Family Member Evolutionarily Related to ANGPTL3.
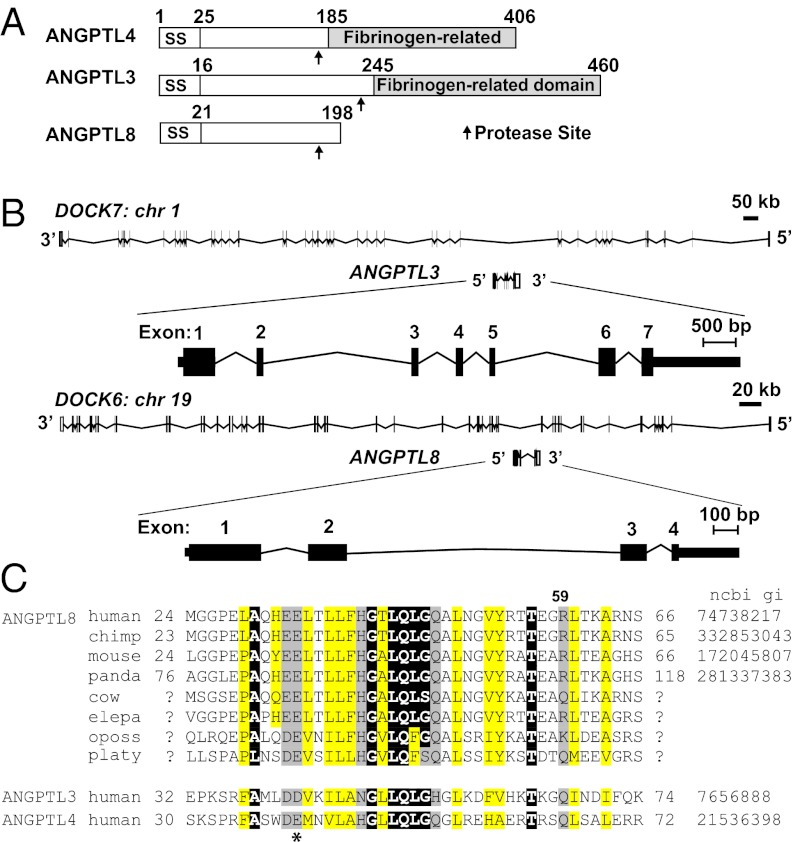
A database search for proteins related to the ANGPTL family identified ANGPTL8, formerly known as TD26, hepatocellular carcinoma-associated gene, C19orf80, refeeding-induced fat and liver (21), and Lipasin (22). ANGPTL8 shares ∼20% identity with the N-terminal domains of ANGPTL3 and ANGPTL4. The protein terminates at residue 198 and therefore lacks a C-terminal fibrinogen-related domain (Fig. 1A). Sequencing of polyA RNA from both human and mouse liver revealed only a single species of ANGPTL8 transcript. ANGPTL8 and ANGPTL3 are located in corresponding introns of DOCK6 and DOCK7, respectively (Fig. 1B), a configuration that was already established in monotremes. Thus, ANGPTL8 presumably arose through duplication of an ancestral DOCK gene that occurred before the mammalian radiation.
Fig. 1.
Domain structure and sequence similarity of ANGPTL3, ANGPTL4, and ANGPTL8. (A) Domain organization of ANGPTL4, ANGPTL3, and ANGPTL8. (B) Chromosomal location and exon-intron structure of ANGPTL3 and ANGPTL8. (C) Multiple sequence alignment of a conserved N-terminal motif in three ANGPTLs. A conserved acidic residue at amino acid 40 in ANGPTL4 is indicated by an asterisk. Positions with semi-invariant, uncharged, and hydrophobic residues are highlighted in black, yellow, and gray, respectively. National Center for Biotechnology Information gene identifier (NCBI gi) numbers are shown on the right (where available). Consensus was computed on the PROMALS3D alignment of all sequences.
The spacing of semi-invariant and uncharged residues in the N-terminal domain of ANGPTL3 and ANGPTL4 is conserved in ANGPTL8. The glutamate at residue 40 of ANGPTL4, which is required for lipase inhibition (23), is conserved in ANGPTL8 (Fig. 1C). These data suggested ANGPTL8 may have similar functions to the other two family members.
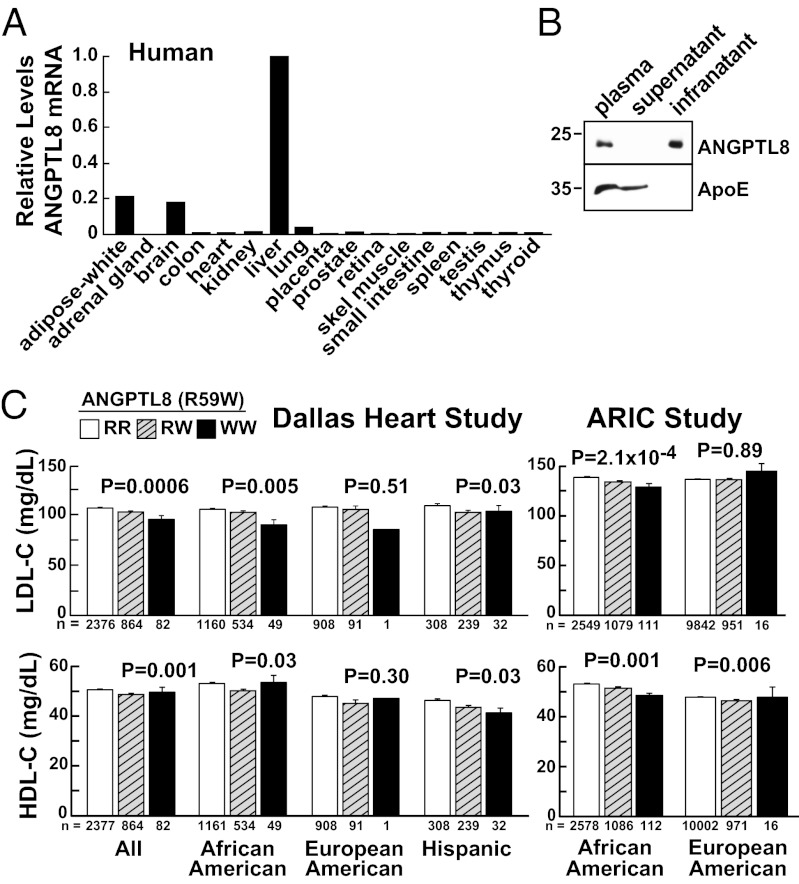
The distribution of ANGPTL8 mRNA in human tissues was also consistent with a role in lipid metabolism (see Fig. 2A). Expression was highest in liver and adipose tissue. Immunoblot analysis using polyclonal antibodies raised against full-length recombinant ANGPTL8 revealed a protein of the expected size (22 kDa) in plasma. The protein was recovered in the infranatant after ultracentrifugation of plasma at density >1.21 g/mL (Fig. 2B). Because some lipoprotein-associated proteins dissociate from lipoproteins at the high salt concentrations used for ultracentrifugation, we also size-fractionated human plasma by fast-performance liquid chromatography. ANGPTL8 eluted between HDL and LDL, well above the size of a monomer, in fractions that contain small amounts of cholesterol (Fig. S1). Thus, ANGPTL8 is a secreted protein that circulates as part of a high molecular weight complex. The protein is not tightly bound to lipoproteins in the circulation, but may be weakly associated with a specific subset of lipoproteins with diameters intermediate between LDL and HDL.
Fig. 2.
Tissue distribution of ANGPTL8 expression in humans and genetic association of sequence variation in ANGPTL8 with plasma lipoprotein levels. (A) ANGPTL8 mRNA levels in human tissues were determined by real-time PCR, and normalized to expression levels of 36B4. Levels were expressed as a ratio relative to liver expression, which was set to 1. (B) Distribution of ANGPTL8 in human plasma. Plasma lipoproteins were isolated by ultracentrifugation and equal proportions of each fraction were subjected to immunoblot analysis with antibodies to ANGPTL8 and ApoE. (C) Mean (± SE) LDL-C and HDL-C values in the DHS and ARIC participants.
Nonsynonymous Sequence Variation in ANGPTL8 Is Associated with Reduced Plasma Levels of LDL-Cholesterol and HDL-Cholesterol.
To determine if ANGPTL8 plays a role in lipoprotein metabolism, we screened public SNP repositories for nonsynonymous variants in ANGPTL8. A nucleotide transition (c.194C > T, rs2278426) was identified that substitutes tryptophan for arginine at residue 59 (Fig. 1C). In the Dallas Heart Study (DHS), a multiethnic, population-based study of Dallas County (24), the 59W variant was more common among Hispanics [minor allele frequency (MAF) = 26%] and African Americans (MAF = 18%) than among European-Americans (MAF = 5%) (Table S1). The 59W variant was significantly associated with lower plasma levels of LDL-cholesterol (C) and HDL-C in African Americans and Hispanics. Among African Americans, the largest group in the DHS, plasma levels of LDL-C were 15% lower in WW homozygotes than in RR homozygotes. Heterozygotes had intermediate LDL-C levels. The association was not apparent in European-Americans, presumably because the 59W variant is much less common in this population. The R59W variant was not associated with plasma levels of TAG (P = 0.23) (Fig. S2A), or with body mass index (BMI), fasting glucose or homeostatic model assessment-insulin resistance in any ethnic group (Table S1).
We replicated the association between the 59W variant and plasma HDL-C and LDL-C levels in African American participants in the Atherosclerosis Risk in Communities Study (ARIC), a large, prospective, biracial study of heart disease (25) and in the Dallas Biobank, a convenience sample of 4,500 unrelated African Americans from Dallas County (Fig. S2B). Among European-Americans in ARIC, the 59W variant was associated with significantly lower plasma levels of HDL-C (0.006) (Fig. 2C), but not LDL-C (Table S2). The variant was not associated with plasma TAG levels in ARIC or in the Dallas Biobank (Fig. S2, and Tables S2 and S3). In a genome-wide association study that predominantly included individuals of European ancestry (26), imputed R59W genotypes were associated with both HDL-C (P = 3.87 × 10−7) and LDL-C (P = 0.006) at the nominal significance threshold.
Thus, the phenotype resulting from the R59W substitution in ANGPTL8 (i.e., lower plasma levels of LDL-C and HDL-C without changes in TAG) is similar, but not identical to that of conferred by defective ANGPTL3 (6).
ANGPTL8 Expression in Livers of Mice Causes Hypertriglyceridemia That Is Exacerbated by Coexpression of ANGPTL3.
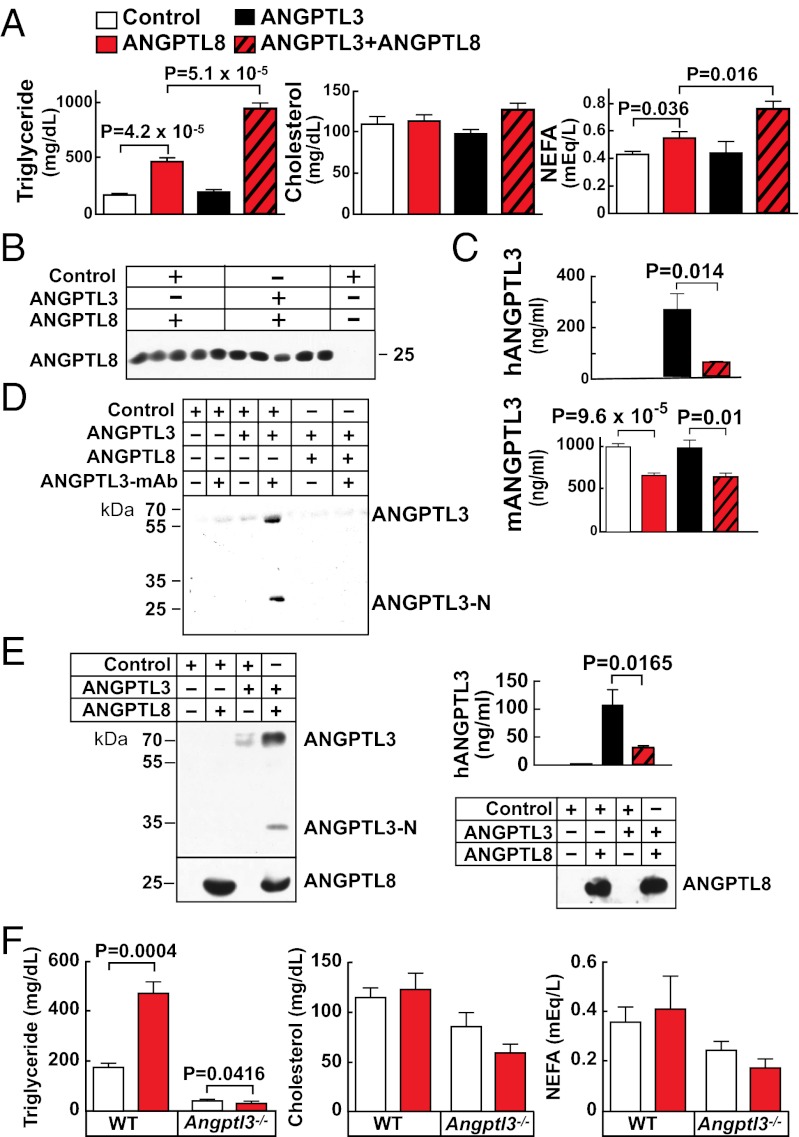
As a first step toward elucidating the specific role of ANGPTL8, we used recombinant adenoviruses to express human ANGPTL8 in livers of mice. ANGPTL8 protein was readily detectable in liver and plasma of mice infected with the ANGPTL8 adenovirus, but not in mice given empty virus (Fig. S3A). Expression of ANGPTL8 significantly increased plasma levels of TAG and nonesterified fatty acids (NEFA) (Fig. 3A and Fig. S3 B and C). Similar results were obtained when the 59W allele was expressed at comparable levels (Fig. S3D), suggesting that the substitution has modest effects on ANGPTL8 function. In contrast to ANGPTL8, adenovirus-mediated expression of ANGPTL3 at levels comparable to those present in human plasma (∼300 ng/mL) (27) did not elicit an increase in plasma TAG or NEFA (Fig. 3A). To test for functional interaction between ANGPTL8 and its progenitor, ANGPTL3, we coexpressed the two proteins. Plasma levels of TAG and NEFA were both dramatically increased in the doubly-infected mice (Fig. 3A) without changes in liver enzymes (Fig. S4A). Expression of ANGPTL8 did not alter the hepatic mRNA levels of the exogenous or endogenous ANGPTL3 (Fig. S4B). Expression of the transgenes did not alter TAG hydrolase activity in pre- or postheparin plasma (Fig. S4C). Expression of ANGPTL8 also failed to increase VLDL secretion as determined using Triton-WR1335 (Fig. S4D).
Fig. 3.
Overexpression of human ANGPTL8 and ANGPTL3 in livers of wild-type and Angptl3−/− mice. (A) Plasma levels of TAG, cholesterol, and NEFA were measured in 10-wk-old C57Bl6/J male mice (n = 4–5 per group) 3 d after being injected with adenovirus. (B) Immunoblot analysis of plasma ANGPTL8. (C) Human and mouse ANGPTL3 were measured by ELISA, as described in Materials and Methods. (D) In an independent experiment, ANGPTL3 was immunoprecipitated from pooled plasma samples (n = 3 per group) of mice infected with the indicated viruses using a mouse anti-human ANGPTL3 mAb. Immunoprecipates were subjected to immunoblotting with an anti-ANGPTL3 polyclonal antibody. (E) Coimmunoprecipitation of ANGPTL3 with ANGPTL8-FLAG from plasma of mice expressing human ANGPTL8-FLAG and ANGPTL3 (n = 6 per group). The immunoprecipitated proteins were blotted with polyclonal antibodies to human ANGPTL8 and the N terminus of human ANGPTL3 (Left). Plasma levels of ANGPTL3 and ANGPTL8 (input) were assessed by ELISA and by immunoblotting, respectively (Right). (F) Wild-type and Angptl3−/− mice (n = 3–5 per group) were infected with recombinant ANGPTL8 adenoviruses. After 3 d, the mice were fasted for 4 h, killed, and plasma levels of TAG, cholesterol and NEFA were measured. The experiments were all performed three times with similar results and representative data are shown.
Coexpression may potentiate the activities of ANGPTL3 and ANGPTL8 by increasing the circulating levels of one or both proteins. To test this hypothesis, we assayed the levels of the two proteins in plasma from adenovirus-treated mice. Expression of ANGPTL3 had little effect on circulating levels of ANGPTL8 (Fig. 3B). Plasma levels of both human and mouse ANGPTL3 were measured using an ELISA and both were reduced with coexpression of ANGPTL8 (Fig. 3C). The reduction in human ANGPTL3 was confirmed by immunoprecipitating the protein from mouse plasma (Fig. 3D). The plasma levels of both full-length ANGPTL3 and the N-terminal fragment were reduced in the mice coexpressing the two viruses.
To test if ANGPTL8 physically interacts with ANGPTL3, mice were infected with adenoviruses encoding ANGPTL3 and a fusion protein of ANGPTL8 containing a FLAG epitope tag at the C terminus. ANGPTL8 was immunoprecipitated from the plasma using an anti-FLAG antibody, and immunoblot analysis of the precipitated proteins using an anti–N-terminal ANGPTL3 antibody revealed that both the full-length and N-terminal fragment of ANGPTL3 coimmunoprecipitated with ANGPTL8 (Fig. 3E). Thus, ANGPTL8 interacts with ANGPTL3 in vivo.
ANGPTL8 Requires ANGPTL3 to Promote Hypertriglyceridemia.
If ANGPTL8 functionally interacts with ANGPTL3, we would predict that ANGPTL8 would not increase plasma TAG levels when expressed in Angptl3−/− mice. In three independent experiments, expression of ANGPTL8 increased plasma TAG levels more than twofold in wild-type mice, but did not increase TAG or NEFA levels in Angptl3−/− mice (Fig. 3F). In all three experiments, the levels of TAG and VLDL-TAG actually fell in Angptl3−/− mice receiving the ANGPTL8-expressing virus despite robust expression of ANGPTL8 in these animals (Fig. 3F and Fig. S5 A and B). Liver enzyme levels were not increased in these mice (Fig. S5C).
ANGPTL8 Promotes Cleavage of ANGPTL3 in Cultured Hepatocytes.
The finding that coexpression of ANGPTL8 and ANGPTL3 increased plasma TAG and decreased circulating ANGPTL3 levels was paradoxical, because inactivation of ANGPTL3 in mice causes a marked reduction in plasma TAG levels (5). Ono et al. reported that ANGPTL3 must undergo cleavage to increase plasma TAG levels (20). Therefore, a possible explanation for the anomalous effects of ANGPTL8 on ANGPTL3 levels and activity is that ANGPTL8 may promote cleavage of ANGPTL3, a process that would simultaneously increase the activity of the peptide and decrease the amount of full-length ANGPTL3.
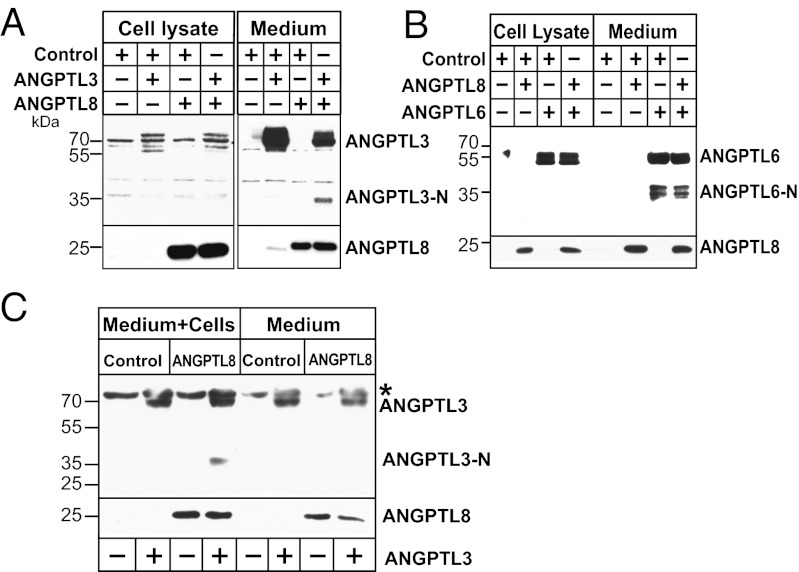
To test if ANGPTL8 promotes cleavage of ANGPTL3 in cultured cells, we coexpressed the two proteins in HepG2 cells (Fig. 4A). In medium from cells expressing ANGPTL3 alone, only full-length ANGPTL3 was detected. Coexpression of ANGPTL3 with ANGPTL8 resulted in the appearance of a ∼33-kDa band corresponding to the N-terminal domain of ANGPTL3. In contrast, coexpression of ANGPTL8 with ANGPTL6 did not increase the appearance of the N-terminal domain of ANGPTL6. Thus, the effect of ANGPTL8 on ANGPTL3 is not a nonspecific effect in these cells.
Fig. 4.
ANGPTL8 promotes cleavage of ANGPTL3 in cultured hepatocytes. ANGPTL8-FLAG was expressed alone or together with either ANGPTL3 (A) or ANGPTL6 (B) in HepG2 cells as described in Materials and Methods. Immunoblot analysis was performed to detect ANGPTL8, ANGPTL3, and ANGPTL6. (C) Cells are required for cleavage of ANGPTL3. Recombinant mouse ANGPTL3 protein (1 μg/mL) was added to the conditioned medium of HuH7 cells infected with ANGPTL8 or control adenoviruses. ANGPTL3 was also added to the same medium in the absence of cells. Cells were grown for 16 h at 37 °C, 5% CO2. Medium was subjected to immunoblot analysis with anti-ANGPTL8 and anti-mANGPTL3 antibodies as described in Materials and Methods. The asterisk represents a nonspecific band.
To determine if cells are required for the generation of the N-terminal fragment, we added recombinant mouse ANGPTL3 to medium of cells infected with adenoviruses expressing either ANGPTL8 or the vector alone (control) (Fig. 4C). Cleaved ANGPTL3 was present in the medium of the ANGPTL8-expressing cells, whereas no increase in N-terminal ANGPTL3 was detected when the protein was incubated with the same medium in the absence of cells.
These data are consistent with a model in which ANGPTL8 activates ANGPTL3 by binding to the N-terminal domain of the full-length ANGPTL3 protein and promoting its cleavage, perhaps by changing the conformation of ANGPTL3 to make the cleavage site more accessible or by recruiting a cell-associated proteases. After cleavage, ANGPTL8 remains bound to the N-terminal domain of ANGPTL3, and may form part of an active complex that orchestrates trafficking of lipids among tissues. The interaction between ANGPTL8 and ANGPTL3 appears to also promote the egress of ANGPTL3 from the circulation.
ANGPTL8 Expression Is Regulated by Food Intake in an SREBP-1c–Independent Manner.
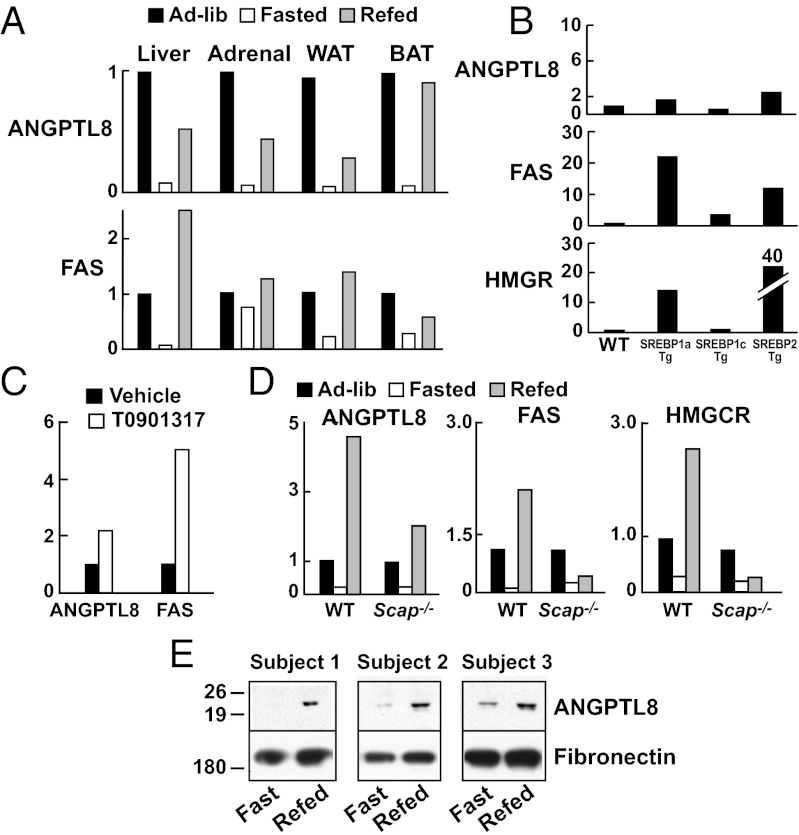
To elucidate the physiological context in which ANGPTL8 and ANGPTL3 interact, we examined the tissue distribution and regulation of ANGPTL8 mRNA in mice. As this manuscript was being prepared, ANGPTL8 mRNA was reported to be most abundantly expressed in the livers of mice, with lower levels in brown and white adipose tissue (21). We found a similar distribution of ANGPTL8 mRNA in mice, but in addition found substantial expression of the transcript in the adrenals (Fig. S6). In all four tissues, ANGPTL8 mRNA levels were markedly reduced by fasting and restored by refeeding (Fig. 5A). Whereas Ren et al. (21) identified ANGPTL8 as an insulin target gene in adipocytes, ANGPTL8 expression in liver was not increased in mice overexpressing sterol regulatory binding protein (SREBP)-1c, which coordinates the lipogenic response to insulin in the liver (Fig. 5B) (28). A modest increase in ANGPTL8 expression was seen in mice expressing SREBP-1a and SREBP-2. Moreover, a potent agonist of LXR, which is required for the insulin-induced increase in SREBP-1c (29), only modestly increased ANGPTL8 mRNA levels (Fig. 5C). The fasting-refeeding response of ANGPTL8 was preserved, although attenuated, in mice lacking Scap, a protein required for SREBP activation (30, 31) (Fig. 5D).
Fig. 5.
Regulation of ANGPTL8 mRNA in mouse tissues. (A) C57BL/6J mice (n = 5 per group) were fasted for 12 h, or fasted for 12 h and then refed with a high-carbohydrate diet for 12 h. Messenger RNA samples from liver were pooled and analyzed by Real-time PCR. Values are expressed as ratios compared with levels in the ad libitum fed group. (B) Expression of ANGPTL8 in livers from SREBP-1a, SREBP-1c, SREBP-2 transgenic (Tg) mice. Fatty acid synthase (FAS) and HMG-CoA reductase (HMGCR) mRNA levels were used as positive controls. (C) ANGPTL8 and FAS in livers of mice treated with the LXR agonist T0901317 (0.015%) for 4 d. (D) Liver-specific Scap−/− mice (4 mice per group) and littermate controls were fed ad libitum, fasted for 24 h, or fasted for 24 h and then refed for 12 h with a high-carbohydrate diet. (E) Immunoblot analysis of ANGPTL8 in plasma from humans after 12-h fasting and 6 h after ingestion of a meal. Fibronectin was used as a loading control.
Finally, we examined the regulation of ANGPTL8 levels in humans in response to food intake. Circulating levels were very low after a 12-h fast and increased significantly within 3 h of feeding (Fig. 5E). Thus, the fasting-refeeding response in mice was also seen in humans.
Discussion
The major finding of this study is that a recently identified member of the ANGPTL family, ANGPTL8, plays an important role in lipoprotein metabolism through a functional interaction with ANGPTL3. Our results provide evidence for a close evolutionary relationship, direct physical interaction, and functional interdependence between the two ANGPTLs. ANGPTL8 and ANGPTL3 are located in corresponding introns of two related genes and share significant sequence similarity. The two proteins coimmunoprecipitated from the plasma of mice when coexpressed via recombinant adenoviruses, and neither protein was fully active without the other. ANGPTL3 did not alter plasma levels of TAG when expressed at physiological concentrations in wild-type mice, whereas coexpression of ANGPTL8 with ANGPTL3 markedly increased plasma TAG levels. Conversely, expression of ANGPTL8 increased plasma levels of TAG in wild-type mice but caused a small decrease in plasma TAG in Angptl3−/− animals. In cultured cells, ANGPTL8 stimulated appearance of the N-terminal fragment of ANGPTL3. Taken together, our data indicate that ANGPTL8 is a degenerate paralog of ANGPTL3 that regulates TAG metabolism in concert with ANGPTL3.
The present study used adenoviral transgenesis to probe the role of ANGPTL8, but we provide two lines of evidence that our data are not an artifact of transgene overexpression. First, the ANGPTL8 adenovirus, which caused substantial hypertriglyceridemia in wild-type mice, did not increase TAG levels in Angptl3−/− mice. Thus, ANGPTL8 requires ANGPTL3 to raise TAG levels. Second, levels of recombinant ANGPTL3 in mice injected with the ANGPTL3 adenovirus were comparable to those observed in humans. At these levels, the recombinant ANGPTL protein had no detectable effect on plasma TAG in wild-type mice unless exogenous ANGPTL8 was coexpressed. These data provide direct support for the conclusion that ANGPTL8 normally acts to increase plasma TAG levels and that it requires ANGPTL3 to do so. Consistent with this notion, Angptl8−/− mice generated as part of a library of mice with mutations in membrane and secreted proteins had markedly decreased serum TAG levels compared with their sex-matched wild-type littermates (32).
The finding that ANGPTL3 expression did not increase plasma TAG levels in wild-type mice indicates that ANGPTL3 is usually present in excess and that ANGPTL8 is rate-limiting for ANGPTL3 action. Our data are most consistent with a model in which ANGPTL8 activates ANGPTL3 by promoting its cleavage, but we cannot exclude alternative explanations. It remains possible that ANGPTL3 activates ANGPTL8, or that neither protein activates the other but instead the two proteins simply form a functional complex. Expression of ANGPTL3 in cultured HepG2 cells increases the level of ANGPTL8 in the medium (Fig. 4A). It is also possible that ANGPTL8 stabilizes the N-terminal domain of the protein. In preliminary studies we did not find evidence that ANGPTL8 stabilizes ANGPTL3 in cultured cells, but in vivo studies with physiologically relevant concentrations of both proteins will be required to further elucidate the relationship between ANGPTL3 and ANGPTL8.
The underlying mechanism and physiological significance of the marked reduction in circulating ANGPTL3 in mice expressing ANGPTL8 is unclear. ANGPTL8 may promote intracellular degradation of ANGPTL3 and thus reduce its secretion. Alternatively, ANGPTL8 may recruit ANGPTL3 to the surfaces of cells, sequestering the protein from the circulation. Irrespective of the mechanism by which ANGPTL8 expression reduces ANGPTL3 levels, the net effect of ANGPTL8 expression is a potentiation of the action of ANGPTL3.
The location of ANGPTL3 and ANGPTL8 in the introns of larger genes is common to several ANGPTL family members. In humans, ANGPTL1 and ANGPTL2 are located in introns of RALGPS2 and RALGPS1, respectively, and ANGPTL7 is located in an intron of MTOR. A similar arrangement is apparent in the zebrafish genome. Thus, the dispersal of ANGPTLs and their host genes was already well underway before the divergence of bony fish and amphibians.
Whereas the locations of certain ANGPTLs within larger genes is highly conserved, the functional significance of this arrangement, if any, is not known. DOCK6 and -7, and RALGPS1 and -2 are all guanine nucleotide exchange factors, but their biological roles appear to be unrelated to those of the ANGPTL genes they host. The phenotypes of mice lacking Dock7, generalized hypopigmentation and white-spotting (33), are not seen in Angptl3−/− mice. Mutations in DOCK6 cause Adams–Oliver syndrome, a rare disorder affecting skin and limb development, without noted changes in plasma lipid levels (34). Mice lacking Angptl8 were hypotriglyceridemic but no other notable phenotype was observed in a broad phenotypic screen (32). Thus, ANGPTL3 and ANGPTL8 appear to function independently of DOCK7 and DOCK6. These data suggest that the location of ANGPTLs within the introns of larger genes reflects the shared origin, rather than function of these genes.
The finding that ANGPTL8 fails to increase TAG in Angptl3−/− mice indicates that ANGPTL8 does not act independently of ANGPTL3 to increase TAG levels. A recent report from Zhang (22) found that increasing concentrations of ANGPTL8 (referred to as lipasin in that report) had modest incremental effects on LPL activity in vitro, so that even very high concentrations (100 nM) of recombinant ANGPTL8 inhibited LPL activity by less than 50%. Whereas Zhang proposed that ANGPTL8 elevated circulating TAG levels by inhibiting LPL, our data are not consistent with this hypothesis; overexpression of ANGPTL8 in the liver of mice caused hypertriglyceridemia without any significant decrease in pre- or postheparin plasma lipolytic activity (Fig. S4C). Moreover, ANGPTL8 did not increase TAG levels in the Angptl3−/− mice. These data suggest that ANGPTL8 does not function as a direct inhibitor of LPL but instead acts in concert with ANGPTL3 and perhaps other ANGPTL family members, to modulate TAG metabolism.
Alterations in the activities of ANGPTL8 and ANGPTL3 have similar, but not identical outcomes in mice and humans. Inactivation of either gene in mice results in hypotriglyceridemia (5, 32), and both genes elevate plasma TAG levels when expressed at high levels. Humans with complete ANGPTL3 deficiency have very low plasma levels of LDL-C, HDL-C, and TAG (4). Thus, the reduction in LDL-C and HDL-C levels associated with the R59W variant in ANGPTL8 resembles features of ANGPTL3 deficiency (4), and is most consistent with the substitution conferring a loss of function. However, the R59W substitution was not associated with low plasma TAG levels (Fig. S2), which is a cardinal feature of ANGPTL3 deficiency in humans and mice (4, 5). Moreover, expression of ANGPTL8 lowered TAG levels in Angptl3−/− mice. These differences indicate that ANGPTL8 has actions that are independent of ANGPTL3.
The finding that ANGPTL8 is expressed in a broader range of tissues than ANGPTL3 is also consistent with ANGPTL3-independent functions. One possibility is that ANGPTL8 interacts with ANGPTL4. Further studies will be required to determine whether ANGPTL8 affects the function of other ANGPTL family members.
Expression of ANGPTL3 and ANGPTL8 in mice altered circulating fatty acid levels in parallel with TAGs. By controlling the activation of ANGPTL3, and possibly regulating other family members, ANGPTL8 may coordinate trafficking of fatty acids following a meal, redirecting them from the liver to peripheral tissues. The resulting effects on plasma lipoprotein levels make ANGPTL8 a potential target for lipid-lowering therapy, and perhaps the treatment of other disorders of fuel homeostasis.
Materials and Methods
Reagents.
Rabbit polyclonal antibodies were raised against full-length human ANGPTL8 and the N-terminal domain of human ANGPTL3 (amino acids 13–200) and ANGPTL6 expressed as histidine-tagged proteins in bacteria. Monoclonal anti-FLAG M2 antibodies were purchased from Sigma, rabbit polyclonal anti-calnexin antibodies from Enzo Life Science, and goat polyclonal anti-ApoE antibodies from Calbiochem. A rabbit polyclonal antibody to N-terminal mouse ANGPTL3 was purchased from Santa Cruz. Anti-mouse, anti-rabbit, and anti-goat IgG HRP-linked secondary antibodies (1:10,000) were from Amersham Biosciences. Mouse ANGPTL3 was expressed as a fusion protein with a polyhistidine (n = 6) at the C terminus in Chinese Hamster Ovarian cells. The protein was purified using nickel affinity chromatography and was estimated to be >90% pure by Coomassie staining.
Study Populations.
Association studies were performed in the DHS (24), the ARIC study (25), and the Dallas Biobank, a convenience sample of Dallas County residents (SI Materials and Methods). The study protocols were approved by the Institutional Review Board of the University of Texas Southwestern Medical Center, and all subjects provided written informed consent.
Genotyping.
The R59W variant was assayed in the DHS using oligonucleotide hybridization as previously described (35) and by real-time PCR in the DHS and the Dallas Biobank, and by using the Sequenom MassArray system in the ARIC cohort.
Mice.
The Angptl3−/− mice were developed by ablating Angptl3 in embryonic stem cells using the Velocigene method (36) (Velocigene ID: VG1425). A bacterial artificial chromosome containing the Angptl3 gene was used to replace the coding region with the β-galactosidase (β-gal) reporter gene followed by a hygromysin cassette. F1 heterozygous mice were bred together to generate F2 wild-type and knockout mice, which were used for the experiments. All research protocols involving mice were reviewed and approved by the Institutional Animal Care and Use Committee (University of Texas Southwestern Medical Center). C57BL/6J male mice between 10–12 wk of age (Jackson Laboratory) were housed in a room maintained in a controlled environment (12-h light/12-h dark daily cycle, 23 ± 1 °C, 60–70% humidity), and fed ad libitum with standard chow diet (Harlan Teklad). In fasting-refeeding experiments, the high-carbohydrate diet was from MP Biomedicals.
Real-Time PCR Assay of mRNA Levels.
The expression of mRNAs in mouse tissues was determined by quantitative real-time PCR (6) using 36B4 as an internal control as described in SI Materials and Methods. The distribution of ANGPTL8 in human tissues was assayed using cDNAs prepared from 17 human tissues (Human Total RNA Master Panel II; Clontech Laboratories). Total RNA was isolated from mouse tissues using STAT-60 reagent (TEL-TEST).
ELISA Assays.
Human and mouse ANGPTL3 levels were assayed using commercial ELISA kits as described in SI Materials and Methods.
Adenoviral Infection of Mice.
Recombinant adenoviruses expressing human ANGPTL8-FLAG, and wild-type ANGPTL3 were generated using a commercial system (AdEasy Vector System; Qbiogene) as described in the SI Materials and Methods. A total of 1.25 × 1011 viral particles were injected into the tail vein of each mouse and the mice were killed 3 d later after a 4-h fast. In each experiment, mice were injected with the same amount of virus (vector alone virus plus recombinant viruses in mice receiving only ANGPTL3 or only ANGPTL8).
Measurement of Lipids and Lipolytic Activity.
Blood was collected in EDTA tubes and plasma was separated by centrifugation. Plasma levels of liver enzymes, lipids, and lipoproteins were measured as described in SI Materials and Methods. Lipolytic activity of mouse plasma obtained before and 15 min after injection of heparin (30 U per mouse) was determined as described in SI Materials and Methods.
Statistical Analysis.
The relationship between rs2278426 genotype and clinical variables was assessed using linear regression models as described in SI Materials and Methods.
Cell Culture.
Cultured hepatocytes (HepG2 and HuH7) were grown in six-well dishes and infected with adenoviruses as described in SI Materials and Methods.
Immunoblot and Immunoprecipitation Analysis.
Immunoblot analysis of ANGPTL3, ANGPTL6, and ANGPTL8 was performed as described in SI Materials and Methods. ANGPTL8-FLAG and ANGPTL3 were immunoprecipitated as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Liangcai Nie, Stephanie Blankenship, Zifen Wang, and Christina Zhao for technical assistance; and George D. Yancopoulos for contributions to the angiopoietin-like protein-3 knockout mouse. This work was supported by National Institutes of Health Grants P01 HL20948 and RL1 HL092550.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217552109/-/DCSupplemental.
References
- 1.Camenisch G, et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J Biol Chem. 2002;277(19):17281–17290. doi: 10.1074/jbc.M109768200. [DOI] [PubMed] [Google Scholar]
- 2.Mattijssen F, Kersten S. Regulation of triglyceride metabolism by Angiopoietin-like proteins. Biochim Biophys Acta. 2012;1821(5):782–789. doi: 10.1016/j.bbalip.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Zhang CC, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12(2):240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musunuru K, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010;363(23):2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koishi R, et al. Angptl3 regulates lipid metabolism in mice. Nat Genet. 2002;30(2):151–157. doi: 10.1038/ng814. [DOI] [PubMed] [Google Scholar]
- 6.Romeo S, et al. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J Clin Invest. 2009;119(1):70–79. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romeo S, et al. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet. 2007;39(4):513–516. doi: 10.1038/ng1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Köster A, et al. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: Regulation of triglyceride metabolism. Endocrinology. 2005;146(11):4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 9.Desai U, et al. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci USA. 2007;104(28):11766–11771. doi: 10.1073/pnas.0705041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kersten S, et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem. 2000;275(37):28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 11.Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci USA. 2006;103(46):17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge H, et al. Differential regulation and properties of angiopoietin-like proteins 3 and 4. J Lipid Res. 2005;46(7):1484–1490. doi: 10.1194/jlr.M500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Shimizugawa T, et al. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J Biol Chem. 2002;277(37):33742–33748. doi: 10.1074/jbc.M203215200. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto K, Koishi R, Shimizugawa T, Ando Y. Angptl3-null mice show low plasma lipid concentrations by enhanced lipoprotein lipase activity. Exp Anim. 2006;55(1):27–34. doi: 10.1538/expanim.55.27. [DOI] [PubMed] [Google Scholar]
- 15.Shan L, et al. The angiopoietin-like proteins ANGPTL3 and ANGPTL4 inhibit lipoprotein lipase activity through distinct mechanisms. J Biol Chem. 2009;284(3):1419–1424. doi: 10.1074/jbc.M808477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EC, et al. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL) J Biol Chem. 2009;284(20):13735–13745. doi: 10.1074/jbc.M807899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ando Y, et al. A decreased expression of angiopoietin-like 3 is protective against atherosclerosis in apoE-deficient mice. J Lipid Res. 2003;44(6):1216–1223. doi: 10.1194/jlr.M300031-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Jin W, et al. Hepatic proprotein convertases modulate HDL metabolism. Cell Metab. 2007;6(2):129–136. doi: 10.1016/j.cmet.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimamura M, et al. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler Thromb Vasc Biol. 2007;27(2):366–372. doi: 10.1161/01.ATV.0000252827.51626.89. [DOI] [PubMed] [Google Scholar]
- 20.Ono M, et al. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J Biol Chem. 2003;278(43):41804–41809. doi: 10.1074/jbc.M302861200. [DOI] [PubMed] [Google Scholar]
- 21.Ren G, Kim JY, Smas CM. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am J Physiol Endocrinol Metab. 2012;303(3):E334–E351. doi: 10.1152/ajpendo.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem Biophys Res Commun. 2012;424(4):786–792. doi: 10.1016/j.bbrc.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 23.Yin W, et al. Genetic variation in ANGPTL4 provides insights into protein processing and function. J Biol Chem. 2009;284(19):13213–13222. doi: 10.1074/jbc.M900553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Victor RG, et al. Dallas Heart Study Investigators The Dallas Heart Study: A population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93(12):1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 25.Anonymous . ARIC Manual of Operations: No 2, Cohort Component Procedures. Chapel Hill: ARIC Coordinating Center, School of Public Health, Univ of North Carolina; 1987. [Google Scholar]
- 26.Teslovich TM, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robciuc MR, Tahvanainen E, Jauhiainen M, Ehnholm C. Quantitation of serum angiopoietin-like proteins 3 and 4 in a Finnish population sample. J Lipid Res. 2010;51(4):824–831. doi: 10.1194/jlr.M002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horton JD, Goldstein JL, Brown MS. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Repa JJ, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14(22):2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horton JD, Goldstein JL, Brown MS. SREBPs: Transcriptional mediators of lipid homeostasis. Cold Spring Harb Symp Quant Biol. 2002;67:491–498. doi: 10.1101/sqb.2002.67.491. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda M, et al. SREBP cleavage-activating protein (SCAP) is required for increased lipid synthesis in liver induced by cholesterol deprivation and insulin elevation. Genes Dev. 2001;15(10):1206–1216. doi: 10.1101/gad.891301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang T, et al. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol. 2010;28(7):749–755. doi: 10.1038/nbt.1644. [DOI] [PubMed] [Google Scholar]
- 33.Blasius AL, et al. Mice with mutations of Dock7 have generalized hypopigmentation and white-spotting but show normal neurological function. Proc Natl Acad Sci USA. 2009;106(8):2706–2711. doi: 10.1073/pnas.0813208106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaheen R, et al. Recessive mutations in DOCK6, encoding the guanidine nucleotide exchange factor DOCK6, lead to abnormal actin cytoskeleton organization and Adams-Oliver syndrome. Am J Hum Genet. 2011;89(2):328–333. doi: 10.1016/j.ajhg.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romeo S, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenzuela DM, et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol. 2003;21:652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.