Abstract
The fragrance of Clarkia breweri (Onagraceae), a California annual plant, includes three benzenoid esters: benzylacetate, benzylbenzoate, and methylsalicylate. Here we report that petal tissue was responsible for the benzylacetate and methylsalicylate emission, whereas the pistil was the main source of benzylbenzoate. The activities of two novel enzymes, acetyl-coenzyme A:benzylalcohol acetyltransferase (BEAT), which catalyzes the acetyl esterification of benzylalcohol, and S-adenosyl-l-methionine:salicylic acid carboxyl methyltransferase, which catalyzes the methyl esterification of salicylic acid, were also highest in petal tissue and absent in leaves. In addition, the activity of both enzymes in the various floral organs was developmentally and differentially regulated. S-Adenosyl-l-methionine:salicylic acid carboxyl methyltransferase activity in petals peaked in mature buds and declined during the next few days after anthesis, and it showed a strong, positive correlation with the emission of methylsalicylate. The levels of BEAT activity and benzylacetate emission in petals also increased in parallel as the buds matured and the flowers opened, but as emission began to decline on the 2nd d after anthesis, BEAT activity continued to increase and remained high until the end of the lifespan of the flower.
Flowers of Clarkia breweri ([Gray] Greene; Onagraceae), an annual plant native to California, emit a strong, sweet fragrance consisting of 8 to 12 different volatiles. These volatiles are derived from two biochemical pathways, one leading to monoterpenoids, the other to phenylpropanoids-benzenoids (Raguso and Pichersky, 1995). In the last group three are the esters benzylacetate, benzylbenzoate, and methylsalicylate (Raguso and Pichersky, 1995).
Volatile esters are common in floral scents, where they may contribute substantially to the total scent output. In C. breweri, for example, benzylacetate constitutes 20 to 40% (w/w) of the total scent output (depending on the particular C. breweri line), whereas the other two esters each constitute about 5% of the scent (Raguso and Pichersky, 1995). In addition to acting as attractants for pollinators, volatile esters such as methylsalicylate and methyljasmonate have been implicated in cell-to-cell signaling (Farmer and Ryan, 1990; Shulaev et al., 1997). To date, however, little is known about the enzymes that catalyze the condensation of the alcohol and acid moieties of such volatile esters in plants.
Here we report on the emission of the three ester constituents of the C. breweri scent: benzylacetate, benzylbenzoate, and methylsalicylate. We have also developed enzymatic assays to test for the activities of the biosynthetic enzymes that catalyze the formation of methylsalicylate and benzylacetate: SAMT and BEAT, respectively. The activity of benzoyl-CoA:benzylalcohol benzoyltransferase, the hypothetical enzyme that catalyzes the formation of benzylbenzoate (Gross, 1981; Croteau and Karp, 1991), was not tested because of the lack of a labeled substrate with a suitably high specific activity. The activities of SAMT and BEAT throughout the lifespan of the flower follow complex patterns. These patterns are compared with those of two previously described enzymes involved in scent biosynthesis in C. breweri flowers, LIS and IEMT (Pichersky et al., 1994; Dudareva et al., 1996; Wang et al., 1997). Overall, the data show that scent production in C. breweri is a complex process that involves spatial and temporal patterns of regulation that are not necessarily identical for all of the enzymes involved.
MATERIALS AND METHODS
Plant Material, Growth Conditions, Headspace Collection, and GC-MS Analysis
Details of the construction of true-breeding Clarkia breweri stocks, growing conditions, dynamic headspace collection on Tenax (Alltech Associates, Inc., Deerfield, IL) and activated charcoal sorbents, and chemical analyses via GC-MS are as described by Raguso and Pichersky (1995). All headspace collections were performed in a growth chamber (Conviron, Pembina, ND) under a 12-h light/12-h dark photoperiod. Temperature was set at 22°C during the light period and at 18°C during the dark period. In all experiments headspace collections from ambient air and from vegetative tissues were used as controls.
Time Course of Ester Production
Volatile production of esters in individual flowers of four separate plants was monitored over a 6-d period beginning on the day before anthesis and continuing until floral abscission. Headspace volatiles were collected as described by Raguso and Pichersky (1995). The collections were made at 12-h intervals, corresponding to the dark and light periods in the growth chamber.
Localization and Quantitation of Emission of Esters in Floral Parts
The specific floral parts responsible for scent emission were determined, and the emission levels were quantified by headspace collection, essentially as described by Raguso and Pichersky (1995). Headspace collections were made from attached, 2nd-d (hermaphroditic) intact flowers and from same-stage flowers in which floral organs had been systematically removed to leave only petals, only anthers, or only the pistil. To detect all volatiles emitted by a given flower part that could possibly emit different compounds at different times, a 24-h collection period was used.
SAMT and BEAT Enzyme Extraction and Assay
Enzyme Extraction
A crude protein extract was prepared as previously described (Wang et al., 1997). A ratio of extraction buffer:tissue fresh weight of 10:1 (v/w) was used. Protein concentrations in crude extracts were as follows: leaf, 10 mg/mL; sepal, 6.8 mg/mL; petal, 1.4 mg/mL; stigma, 4.1 mg/mL; style, 3.5 mg/mL; and stamen, 13.8 mg/mL. For each time point, flowers from three different plants were combined to prepare a crude extract, and at least two independent enzymatic assays were performed.
SAMT Enzyme Assays and Product Analysis
Assay samples were prepared by adding the following to a 1.5-mL microcentrifuge tube: 10 μL of crude extract, 10 μL of 5× assay buffer (250 mm Tris-HCl [pH 7.5] and 14 mm 2-mercaptoethanol), 1 μL of 50 mm salicylic acid, 1.0 μL (2 × 10−5 mCi) of 0.34 mm S-[methyl-14C]adenosyl-l-Met (NEN Research Products), and 28 μL of water to bring the assay volume to 50 μL. Assay samples were incubated at 30°C for 30 min in a heating block. The radioactively labeled methylated product was extracted by the addition of 100 μL of ethyl acetate, and 20 μL of the organic phase (on top and clear in color) was transferred to a scintillation vial with 2 mL of nonaqueous scintillation fluid (Bio-Safe NA, Research Products International, Mount Prospect, IL) and counted in a liquid-scintillation counter (model 2S6800, Beckman). The raw data (counts per minute) were converted to picokatals (picomoles of product produced per second) based on the specific activity of the substrate and using the appropriate correction factors for counting efficiency. Controls (for SAMT as well as for BEAT) included assays with boiled crude extracts and with buffers only, and background radioactivity produced in such assays was subtracted from all of the results. The specificity of SAMT was tested with several related substrates such as benzoic acid. No activity was detected with substrates other than salicylic acid.
The identity of the products was verified by several methods. First, 20 μL of reaction product was spotted onto a 10- × 20-cm silica-gel 60 F254-precoated TLC plate (EM Industries, Inc., Gibbstown, NJ), and 5 μL of a 5% (v/v) solution containing authentic standards was spotted on the same plate as the standard. Plates were run and analyzed as previously described (Wang et al., 1997). Products were also analyzed by GC-MS after organic extraction from scaled-up reactions of 1 mL of total volume, with both substrates nonradioactive and at a final concentration of 1 mm each. Because esters are hydrolyzed in concentrated acid solutions, the enzymatic assays were not stopped by the addition of concentrated acid, as is often done, but HCl hydrolysis (at a final concentration of 0.3 m) was also carried out to distinguish between methylation of the carboxyl OH group versus methylation of the 2-OH group on the benzyl ring. An enzymatic activity of the latter type was not found in the crude extracts of C. breweri flowers.
BEAT Enzyme Assays and Product Analysis
Assay samples were prepared by adding the following to a 1.5-mL microcentrifuge tube: 10 μL of crude extract, 10 μL of assay buffer (250 mm Tris-HCl [pH 7.5] and 14 mm 2-mercaptoethanol), 1 μL of 50 mm benzylalcohol, 0.4 μL (2 × 10−5 mCi) of 1 mm [14C]acetyl-CoA (Amersham), and 28.6 μL of water to bring the assay volume to 50 μL. Assay samples were incubated at 30°C for 15 min in a heating block. The radioactively labeled product was extracted by the addition of 100 μL of hexane. The product extracted into the organic phase was analyzed as described above for SAMT.
RESULTS AND DISCUSSION
Temporal Variation in Emission of Benzenoid Esters
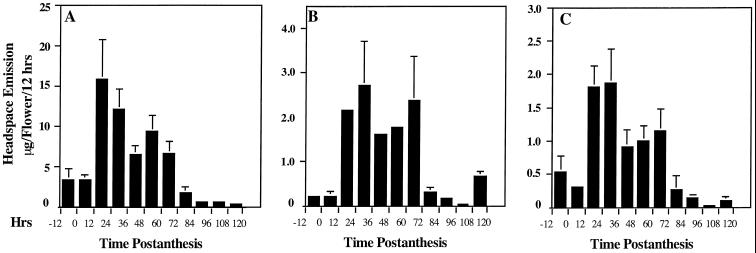
The strong, sweet floral scent of C. breweri is unique in its genus and is correlated with pollination by moths, a mode of reproduction that is novel among Clarkia spp. (McSwain et al., 1973). We have previously shown (Raguso and Pichersky, 1995) that three benzenoid esters, benzylacetate, benzylbenzoate, and methylsalicylate, are constituents of the scent of C. breweri flowers. To determine the amount of these compounds emitted at different stages of floral development, we performed time-course headspace collections at 12-h intervals, followed by GC-MS analysis. We began headspace collection with buds on the evening before they opened and ended it 5 d later.
Emission of all three esters began just before the flowers opened (10–20% from the maximal level) and remained relatively stable for the first 12 h after anthesis (Fig. 1, A–C). Emissions of benzylacetate (Fig. 1A), benzylbenzoate (Fig. 1B), and methylsalicylate (Fig. 1C) showed similar patterns over time. Emission of benzylacetate peaked on day 1, 12 h earlier than peak emission of benzylbenzoate. Emission of methylsalicylate also peaked on d 1, but was stable for the next 12 h. Emission of all three esters declined afterward, but remained high (50–75% of the peak level) and relatively stable during the next 36 to 48 h, with possibly a second minor peak on the 3rd d after anthesis. Emission of all three esters rapidly declined after d 3, although benzylbenzoate showed an additional minor peak on d 5. During the lifespan of the flower, marked variation in ester emission between the day and night periods was not observed. Quantitatively, emission of benzylacetate was the highest, peaking at 15.8 μg flower−1 12 h−1. Benzylbenzoate and methylsalicylate were emitted at much lower levels, peaking at 2.7 and 1.9 μg flower−1 12 h−1, respectively.
Figure 1.
Emission of benzenoid esters from C. breweri flowers as measured by headspace collection at 12-h intervals and GC-MS analysis. A, Emission of benzylacetate; B, emission of benzylbenzoate; and C, emission of methylsalicylate. Data are means ± se (n ≥ 3).
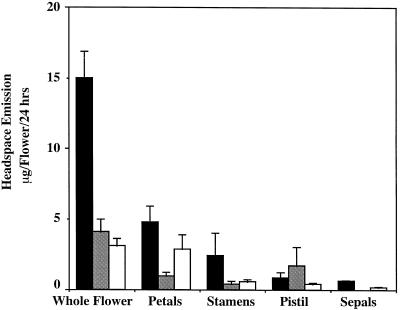
Localization and Quantification of Benzenoid Ester Emission from the Different Parts of the Flower
To determine the specific parts of the C. breweri flowers that emit these three volatile esters, we performed experiments in which living flowers were modified by selectively excising floral parts so that only one class of major floral organs (petal, stamen, and pistil) remained attached to the hypanthium. We then collected headspace volatiles from these modified flowers during a 24-h period. The data obtained were used to calculate the contribution of each floral part to the total emission of the flower (Fig. 2). These data revealed that the petals were the organs responsible for most of the emission of benzylacetate and methylsalicylate, although substantial emission of benzylacetate was also detected from stamens (one-half of the amount emitted by the petals). However, the pistil was the main source of benzylbenzoate emission, although some benzylbenzoate was also emitted by petals and stamens.
Figure 2.
Emission of benzenoid esters from C. breweri flowers and flower parts on d 1 of anthesis as measured by headspace collection at 24-h intervals and GC-MS analysis. Black bars, Benzylacetate; shaded bars, benzylbenzoate; and open bars, methylsalicylate. Data are means ± se (n = 5–8).
The emission of benzylalcohol and benzylacetate in the modified flowers was somewhat reduced. When values obtained for separate organs were totaled, emission of benzylacetate was 60% that of the intact flower, and emission of benzylbenzoate was 77%. However, emission of methylsalicylate by separate organs actually exceeded (123%) that of the intact flower. These results are similar to those previously observed with several phenylpropanoid scent components of C. breweri, in which the sum of (iso)eugenol emission decreased (by greater than 50%) and (iso)methyleugenol emission increased when flower parts were removed (Wang et al., 1997). Such results could mean that organs other than the emitting one are involved in controlling the flux of the pathway. However, the most likely explanation is that such decreases and increases were brought about by the injury sustained by the flowers in these experiments. It is noteworthy that emission of methylsalicylate, a compound known to be involved (together with salicylate) in the response of plant vegetative tissue to pathogen damage (Shulaev et al., 1997), increased in the injured flowers. Because salicylate is derived from the benzoic acid pathway (Yalpani et al., 1993), it is perhaps not surprising that increased synthesis of salicylate (as an intermediate in the synthesis of methylsalicylate, and possibly as an end product) resulted in the concomitant decrease in the biosynthesis of benzylacetate and benzylbenzoate.
BEAT and SAMT Activities in Flowers
BEAT and SAMT Activity in Flower Parts
In many plant species volatile benzyl esters contribute significantly to the total floral scent output (Knudsen et al., 1993). Specifically, benzylacetate, benzylbenzoate, and methylsalicylate are found in the scent of many moth-pollinated flowers (Kaiser, 1993; Knudsen and Tollsten, 1993). In addition, methylsalicylate is also reported to be important in plant defense and communication (Dicke et al., 1990; Shulaev et al., 1997). However, no enzymatic activities capable of forming these products have been reported, and the pathways leading to benzoate, benzylalcohol, and salicylic acid have only been partially elucidated. For example, it is known that the benzene ring is derived from trans-cinnamic acid (Yalpani et al., 1993; Lee et al., 1995) and that benzoic acid is converted to salicylic acid by benzoic acid 2-hydroxylase (Leon et al., 1993).
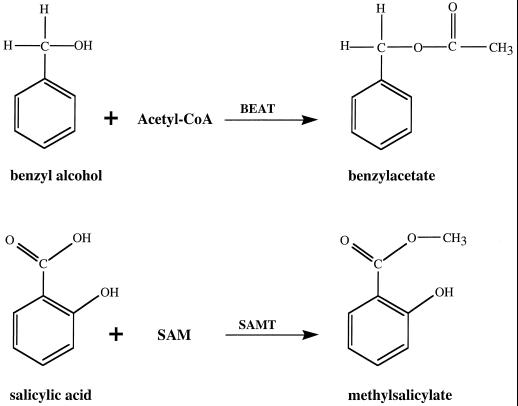
Although the immediate biochemical step leading to benzylacetate had not previously been determined, it appeared likely that benzylacetate could be synthesized by acetylation of benzylalcohol with acetyl-CoA as the donor of the acetyl group (Fig. 3). Therefore, we devised an enzymatic assay to test for BEAT activity. Crude extracts were prepared from different parts of flowers of different stages, incubated with benzylalcohol and [14C]acetyl-CoA, and the product was extracted and analyzed.
Figure 3.
The reactions catalyzed by BEAT and SAMT.
Methylsalicylate is likely to result from esterification of salicylic acid with SAM as the possible methyl donor (Fig. 3). Similar reactions of the SAM-dependent addition of a methyl group to the carboxylic groups of proteins in animals, yeasts, and plants are catalyzed by enzymes collectively termed carboxyl methyltransferases (Clarke, 1992). Therefore, we developed an enzymatic assay to test for SAMT activity using salicylic acid and [14C]SAM.
Levels of BEAT activity in petals on d 1 of anthesis were 7- to 10-fold higher than in any other floral organs on a fresh-weight basis (Table I). No BEAT activity was found in vegetative tissue. SAMT activity levels, when calculated on a fresh-weight basis, were also highest in petals, although other floral parts, in particular stigma and styles, had comparable levels of specific SAMT activity. Again, no activity was found in leaves (Table I).
Table I.
BEAT and SAMT activities in C. breweri on d 1 of anthesis
| Organ (Total wt per flower) | BEAT
|
SAMT
|
||
|---|---|---|---|---|
| Specific activity | Total activity | Specific activity | Total activity | |
| fkat/mg fresh wt | pkat/flower | fkat/mg fresh wt | pkat/flower | |
| Petal (64 mg) | 317.2 ± 22 | 20.3 ± 1.4 | 28.1 ± 1.3 | 1.8 ± 0.08 |
| Sepal (22.5 mg) | 33.3 ± 0.6 | 0.75 ± 0.01 | 1.3 ± 0.2 | 0.03 ± 0.005 |
| Stigma (6 mg) | 30.8 ± 6.2 | 0.18 ± 0.04 | 22.3 ± 1.2 | 0.13 ± 0.007 |
| Style (10 mg) | 32.3 ± 5.6 | 0.32 ± 0.06 | 13.0 ± 1.2 | 0.13 ± 0.01 |
| Stamen (24 mg) | 42.9 ± 3.4 | 1.03 ± 0.08 | 7.1 ± 1.1 | 0.17 ± 0.03 |
Values are averages of three independent measurements. Protein concentrations in the different organs are given in “Materials and Methods,” and can be used to calculate specific activities per milligram of protein. The total weight of each class of organs in the flowers is from Pichersky et al. (1994).
We also calculated the total BEAT and SAMT activity levels in each class of floral organs on d 1 of anthesis (Table I) using the mean values of 64, 22.5, 6, 10, and 24 mg for the total weight of the petals, sepals, stigma, styles, and stamens, respectively, of the C. breweri flower (Pichersky et al., 1994). Because petals possess the highest levels of BEAT- and SAMT-specific activities per milligram fresh weight among all floral organs, and because they constitute slightly more than one-half of the total mass of the flower, it is not surprising that 90% of the total BEAT activity and 80% of the total SAMT activity in the flower are found in the petals.
The total levels of both BEAT and SAMT activities in different floral tissues on d 1 of anthesis (Table I) correlated with the emission of benzylacetate and methylsalicylate by the same tissue (Fig. 2), with activity being highest in petals and absent in leaves. These results are very similar to those obtained for two other enzymes involved in floral scent production in C. breweri flowers, LIS and IEMT, in which a strong, positive correlation was observed between levels of enzyme activities and emissions of linalool and (iso)methyleugenol from floral tissues (Pichersky et al., 1994; Wang et al., 1997). Because no BEAT or SAMT activities were found in vegetative tissue, it is unlikely that this tissue makes a significant contribution to the final step of synthesis of benzylacetate and methylsalicylate, although earlier precursors may come from such parts of the plant.
The total BEAT activity per flower is approximately 10-fold greater than the total activity of LIS (which, like BEAT, also synthesizes a major scent component, linalool), and total SAMT activity per flower is similar to that of IEMT (which, like SAMT, also synthesizes the minor scent components methyleugenol and isomethyleugenol). The total BEAT and SAMT activities (as measured in vitro) in the flowers are in fact 10-fold greater than those needed for synthesizing the emitted amounts of benzylalcohol and methylsalicylate. This observation, however, does not necessarily mean that these two enzymes are not involved in controlling the pathway. Because they, as well as other enzymes, may depend on a common pathway for their substrates, a theoretical “excess” of enzyme may be necessary to produce the observed amount of volatiles in the competition for substrates. In addition, a portion of these esters may not be emitted.
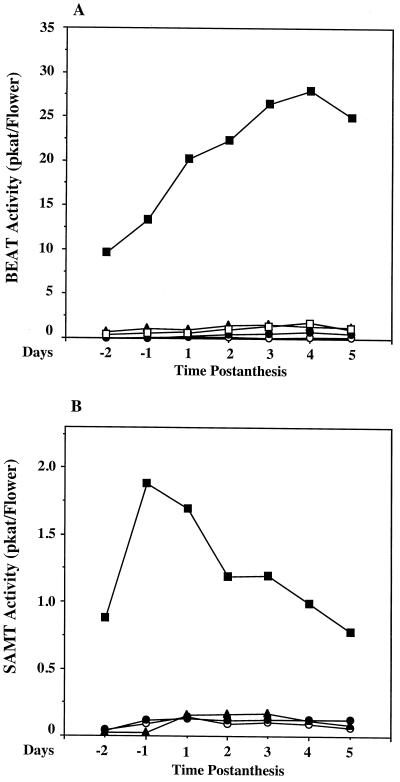
Temporal Variation in BEAT and SAMT Activities
The total BEAT and SAMT activity levels in each class of floral organs from 2 d before anthesis up to 5 d after anthesis are plotted in Figure 4. BEAT activity increased gradually in petals to achieve a maximum on the 4th d after anthesis, and then declined by 10% from the peak level on the 5th d. In other floral organs such as the stigma, style, stamen, and sepal, BEAT activity remained fairly constant during flower development (Fig. 4A).
Figure 4.
Levels of different BEAT and SAMT activities in different parts of the flower during the lifespan of the flowers. A, BEAT activity; and B, SAMT activity. For each data point, flowers from three different plants were combined for each assay, and two to three enzyme assays were conducted and the mean was obtained. se values for data points on d 1 of anthesis are given in Table I; se values for other time points are similar. ▪, Petals; ○, stigma; •, style; ▴, stamens; and □, sepals. pkat, Picomole of product per second.
The variation in total SAMT activity in petals during the development of the flower was different from that of BEAT. Petals of young flower buds (several days before flower opening) already possessed substantial enzymatic activity (approximately 40% of peak level) (Fig. 4B). Maximal SAMT activity was observed in mature flower buds 1 d before anthesis. In 1-d-old flowers the level of SAMT activity was approximately 80% of peak level. SAMT activity in petals declined from the peak level during the next few days and reached the initial level (equal to the level in young flower buds, 40% of peak level) on the 5th d after anthesis. As with BEAT, SAMT activity levels in the stigma, style, stamens, and sepals were relatively low throughout flower development.
The temporal variations in levels of SAMT activity and methylsalicylate emission are similar to those observed for LIS and linalool, in which the levels of enzyme activity in the petals peaked on the day of anthesis and fell afterward, in parallel with linalool emission (Pichersky et al., 1994). However, the temporal variation in levels of BEAT activity, which shows little or no decline at the end of the lifespan of the flower (although emission of benzylacetate does decline), is similar to that observed for IEMT, except that IEMT levels peaked on d 1 of anthesis and stayed stable afterward (Wang et al., 1997), whereas BEAT activity did not peak until the 4th d after anthesis (Fig. 4).
Together with our previous results (Pichersky et al., 1994; Dudareva et al., 1996; Wang et al., 1997) these data show the existence of at least two types of patterns for enzymes involved in scent production in C. breweri flowers. The activities of the first group of enzymes, such as LIS and SAMT, increase in young flowers and decline in old flowers, whereas the activities of the second group of enzymes, such as IEMT and BEAT, increase gradually during the lifespan of the flower and remain high in old flowers.
As discussed previously (Wang et al., 1997), the causes and consequences of high levels of activity of biosynthetic enzymes in old flowers, without concomitant emission of the volatile products, are not known. Although it is possible that the biosynthetic pathways in which these enzymes participate are blocked elsewhere, another possibility that remains to be investigated is that the products produced in the reactions catalyzed by these enzymes are required for additional processes in the flowers other than scent emission. A third possibility is that as the flower ages, substrates may be diverted to other compartments and are not accessible to the scent biosynthetic enzymes.
ACKNOWLEDGMENT
We thank John D'Auria for his help with the GC-MS analyses.
Abbreviations:
- BEAT
acetyl-CoA:benzylalcohol acetyltransferase
- IEMT
S-adenosyl-l-Met:(iso)eugenol O-methyltransferase
- LIS
linalool synthase
- SAM
S-adenosyl-l-Met
- SAMT
SAM:salicylic acid carboxyl methyltransferase
Footnotes
This research was funded by National Science Foundation grant no. IBN-9417582 to E.P. R.A.R. was supported in part by a National Institutes of Health/Genetics Training Grant fellowship.
LITERATURE CITED
- Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- Croteau R, Karp F (1991) Origin of natural odorants. In PM Muller, D Lamparsky, eds, Perfumes: Art, Science and Technology. Elsevier Applied Science, New York, pp 101–126
- Dicke M, Van Beek TA, Posthumus MA, Ben Dam N, Van Bokhoven H, de Groot A. Isolation and identification of volatile kairomone that affects acarine predator-prey interactions. J Chem Ecol. 1990;16:381–396. doi: 10.1007/BF01021772. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Cseke L, Blanc VM, Pichersky E. Evolution of floral scent in Clarkia: novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell. 1996;8:1137–1148. doi: 10.1105/tpc.8.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G (1981) Phenolic acids. In EE Conn, ed, The Biochemistry of Plants: A Comprehensive Treatise, Vol 7. Academic Press, New York, pp 301–316
- Kaiser R (1993) The Scent of Orchids. Elsevier Editiones Roche, Basel, Switzerland
- Knudsen JT, Tollsten L. Trends in floral scent chemistry in pollination syndromes: floral scent composition in moth-pollinated taxa. Bot J Linn Soc. 1993;113:263–284. [Google Scholar]
- Knudsen JT, Tollsten L, Bergstrom G. Floral scents: a check-list of volatile compounds isolated by head-space techniques. Phytochemistry. 1993;33:253–280. [Google Scholar]
- Lee H, Leon J, Raskin I. Biosynthesis and metabolism of salicylic acid. Proc Natl Acad Sci USA. 1995;92:4076–4079. doi: 10.1073/pnas.92.10.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon J, Yalpani N, Raskin I, Lawton MA. Induction of benzoic acid 2-hydroxylase in virus-inoculated tobacco. Plant Physiol. 1993;103:323–328. doi: 10.1104/pp.103.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacSwain J, Raven P, Thorp R. Comparative behavior of bees and Onagraceae. IV. Clarkia bees of the western United States. Univ Calif Publ Entomol. 1973;70:1–80. [Google Scholar]
- Pichersky E, Raguso RA, Lewinsohn E, Croteau R. Floral scent production in Clarkia (Onagraceae). I. Localization and developmental modulation of monoterpene emission and linalool synthase activity. Plant Physiol. 1994;106:1533–1540. doi: 10.1104/pp.106.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguso RA, Pichersky E. Floral volatiles from Clarkia breweri and C. concinna (Onagraceae): recent evolution of floral scent and moth pollination. Plant Syst Evol. 1995;194:55–67. [Google Scholar]
- Shulaev V, Silverman P, Raskin I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature. 1997;385:718–721. [Google Scholar]
- Wang J, Dudareva N, Bhakta S, Raguso RA, Pichersky E. Floral scent production in Clarkia breweri (Onagraceae). II. Localization and developmental modulation of the novel enzyme S-adenosyl-l-methionine:(iso)eugenol O-methyltransferase and phenylpropanoid emission. Plant Physiol. 1997;114:213–221. doi: 10.1104/pp.114.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N, Leon J, Lawton MA, Raskin I. Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol. 1993;103:315–321. doi: 10.1104/pp.103.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]