Abstract
Pathogens are sensed by Toll-like receptors (TLRs) and a growing number of non-TLR receptors. Integrins constitute a family of signaling receptors exploited by viruses and bacteria to access cells. By gain- and loss-of-function approaches we found that αvβ3-integrin is a sensor of and plays a crucial role in the innate defense against herpes simplex virus (HSV). αvβ3-integrin signaled through two pathways. One concurred with TLR2, affected activation/induction of interferons type 1 (IFNs-1), NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), and a polarized set of cytokines and receptors. The virion glycoproteins gH/gL sufficed to induce IFN1 and NF-κB via this pathway. The other pathway was TLR2-independent, involved sarcoma (SRC)-spleen tyrosine kinase (SYK)-Caspase recruitment domain-containing protein 9 (CARD9)-TRIF (TIR-domain-containing adapter-inducing interferon-β), and affected interferon regulatory factor 3 and 7 (IRF3-IRF7). The importance of αvβ3-integrin-mediated defense is reflected in the observation that HSV evolved the immediate-early infected cellular protein 0 (ICP0) protein to counteract it. We propose that αvβ3-integrin is considered a class of non-TLR pattern recognition receptors, a role likely exerted toward viruses and bacteria that interact with integrins and mount an innate response.
The ability of a virus to establish an infection is the outcome of the encounter of the virus with a cell that carries receptor(s) for that virus, of the innate response of the cell aimed to limit the infection within the initially infected cell and in adjacent cells through the secretion of type-1 IFNs and inflammatory cytokines, and lastly of the virus’s ability to combat and evade the host response. The innate response, which is also critical in eliciting the adaptive response, follows the recognition of pathogen-associated molecular patterns (PAMPs) by evolutionarily ancient pattern recognition receptors (PRRs), which constitute the first line of defense against invaders. In humans, Toll-like receptor (TLR) signaling converges in the transcription factors NF-κB, interferon regulatory factor 3 and 7 (IRF3 and IRF7), and in the production of cytokines, especially type-1 IFNs, and chemokines (1, 2). PRRs other than TLRs (non-TLRs) emerged recently as important contributors to innate immunity (3). They comprise a heterogeneous collection of membrane-bound, cytoplasmic, or soluble proteins, exemplified by the C-type lectin (CLRs), nucleotide oligomerization domain receptors (NOD)-like receptors (NLRs), retinoic acid-inducible gene 1 (RIGI)-like (RLRs), and absent in melanoma 2 (AIM2) receptors, in addition to scavenger receptors and others (for reviews, see refs. 1 and 4–7). Typically, non-TLR PRRs signal through autonomous pathways and may synergize with TLRs (8).
Herpes simplex virus 1 (HSV-1) infection is widespread among humans (9). In the human body, the virus preferentially targets epithelial and neuronal cells; it persists lifelong in neurons in a latent-reactivable state. Hitherto, the known innate defenses against HSV consist of TLR2, located at or around cholesterol-rich membrane microdomains, the endosomal TLR3 and TLR9, and the cytosolic RNA and DNA sensors (9–13). Opposing the host defenses are an array of viral proteins exemplified by the virion–host–shutoff Rnase, the immediate-early infected cell protein 0 (ICP0) and ICP27 (9, 11–13).
HSV-1 enters cells through a complex process that involves at least four essential glycoproteins (gD, gH/gL, and gB) and a number of cellular receptors, among which are the gD receptors nectin1 and herpesvirus entry mediator (for reviews, see refs. 14–16). HSV entry may occur by different pathways—that is, uptake into acidic or neutral endosomes or direct fusion at the plasma membrane. The choice of the entry pathway is entirely dictated by the cell (17). Recently, the epithelial/endothelial αvβ3-integrin emerged as the cellular factor that routes HSV to the acidic endosomal pathway. Specifically, αvβ3-integrin relocalizes the nectin1 receptor, and consequently HSV, to cholesterol-rich microdomains and thus enables virus uptake into dynamin2-dependent acidic endosomes (18, 19).
Here, we asked whether, by relocalizing HSV to the cholesterol-rich microdomains where TLR2 resides, αvβ3-integrin participates in the innate response to the virus. By gain- and loss-of-function assays, we show that type-1 IFNs, NF-κB, and a specific set of inflammatory cytokines are induced by αvβ3-integrin. αvβ3-integrin physically interacts with the virion glycoproteins gH/gL, and with TLR2, and thus cross-links the virion and the PRR. The importance of the αvβ3-integrin defense mechanism is reflected in the observation that it was counteracted by the viral protein ICP0; indeed, a HSV mutant deleted in ICP0 replicated to a higher extent in cells in which β3-integrin was silenced.
Results
β3-Integrin–Silenced 293T Cells Support HSV Replication.
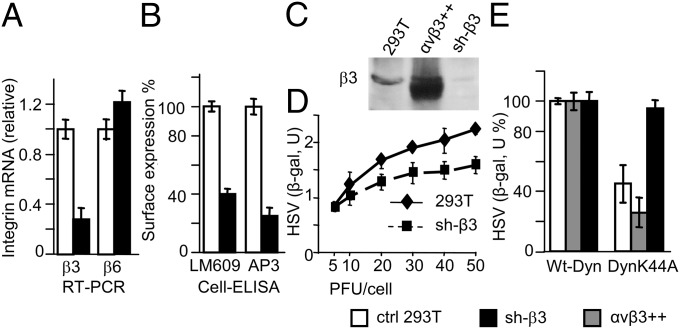
To ask the question whether αvβ3-integrin plays a role in the innate response to HSV-1, we performed loss-of-function experiments and silenced β3-integrin in 293T cells. Silencing was carried out by means of lentiviruses encoding two sh-RNA (reagents provided by and described in ref, 20). One of the stably silenced 293T cell lines was designated as sh-β3 and used in the experiments described below. We verified that β3-integrin mRNA and surface expression were reduced by q-RT-PCR and cell-ELISA (enzyme-linked immunosorbent assay). In either assay, reduction was about 70% (Fig. 1 A and B) relative to control mock-silenced cells, generated by a control nontargeting sh-RNA lentivirus (20). Immunoblotting (IB) of β3-integrin confirmed the decrease (Fig. 1C). The β3-integrin silencing was specific, as β6-integrin (Fig. 1A) was unaffected.
Fig. 1.
β3-Integrin–silenced 293T cells support HSV-1 infection. Silencing of β3-integrin in 293T cells by lentivirus encoding shRNA (20) to generate sh-β3 cells was quantified by q-RT-PCR (A), cell-ELISA (B), and IB (C) relative to control mock-silenced cells (ctrl 293T). (A) Relative changes in gene expression between control mock-silenced and sh-β3 cell samples were determined by q-RT-PCR using the 2−ΔΔCt method. The results were normalized relative to those of control mock-silenced cells. (B) Cell-ELISA was performed by MAb LM609 to αvβ3-integrin (Chemicon) or MAb AP3 to β3-integrin (gift from B. Scott, State University of New York, Upstate Medical University, Syracuse, NY) (19). (C) IB of lysates of control mock-silenced, 293T cells overexpressing αvβ3-integrin (αvβ3++), or sh-β3 cells performed by polyclonal antibody (PAb) to β3-integrin (Chemicon). (D) sh-β3 and wt-293T (293T) cells were exposed to 5–50 PFU/cell of R8102, a wt-recombinant HSV expressing β-gal driven by the α27 immediate-early promoter (21). β-gal expression was measured at 6 h after infection and expressed as arbitrary units (U). (E) Pathway of R8102 entry in control mock-silenced cells, 293T cells overexpressing αvβ3-integrin (αvβ3++), or sh-β3 cells, as determined by sensitivity to DN Dyn-K44A. The indicated cells were transfected with plasmids encoding wt-dynamin2 (wt-Dyn) or Dyn-K44A, infected with R8102 (5 PFU/cell). Extent of infection was assessed as β-gal expression. In panels A, B, D, and E, data represent the average of triplicates or quadruplicates ± SD.
To rule out that any decrease in innate responses was due to reduced infection, we verified that sh-β3 293T cells remained susceptible to HSV. Wt- and sh-β3 293T cells were infected with R8102, a recombinant carrying β-gal under the immediate-early α27 promoter (21). Reduction of infection results in decreased β-gal expression. Fig. 1D shows that sh-β3 293T cells support HSV infection with similar efficiency as wt-cells at 5–10 PFU/cell. Replication in wt- and control mock-silenced cells was indistinguishable (Fig. S1A). Sh-β3 and wt-293T cells produced similar amounts of immediate-early, early, and late viral proteins (Fig. S1B). β3-Integrin silencing resulted in the functional suppression of the β3-integrin– and dynamin2–dependent pathway of HSV entry (19). To this end, we transfected sh-β3, mock-silenced, or αvβ3-integrin-overexpressing cells with a dominant negative (DN) dynamin2 (Dyn-K44A). R8102 infection was unaffected by Dyn-K44A in sh-β3 cells (Fig. 1E), and it was inhibited in αvβ3-integrin-overexpressing or control cells (19).
NF-κB Response Is Inhibited in β3-Integrin–Silenced 293T Cells, Particularly in the Presence of TLR2.
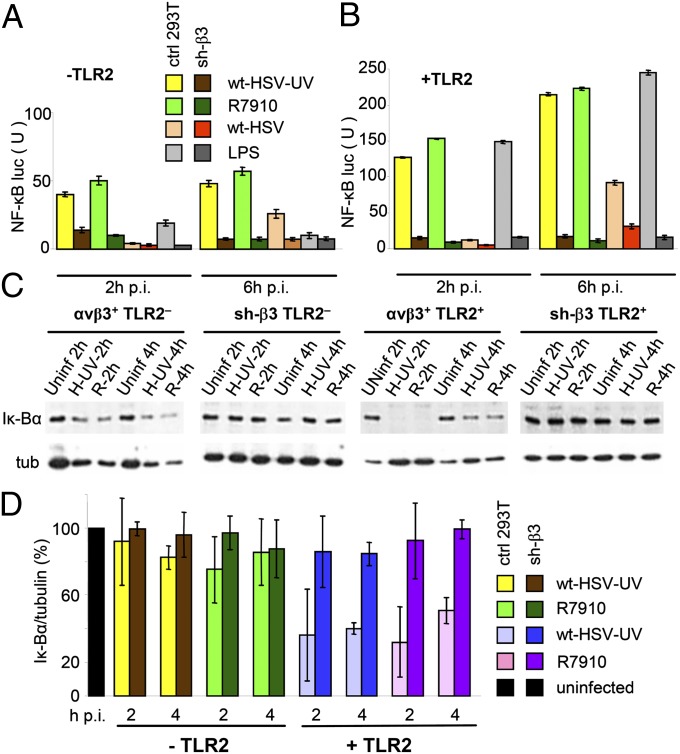
The NF-κB response induced by incoming HSV, including UV-inactivated or entry-defective virions, is detectable at 2 h postinfection (p.i.), and more sustained at 6–8 h p.i (9, 22–24). It is best seen with the ΔICP0 recombinant R7910 (25), as ICP0 counteracts part of the host defense (26), and in its absence, the host response is augmented. NF-κB activation was dramatically inhibited in β3-integrin–silenced cells (Fig. 2 A and B). Specifically, 293T cells express no endogenous TLR2. Control mock-silenced or sh-β3 cells were transfected or not with TLR2 and plasmids encoding NF-κB-driven luciferase (NF-κB-luc) and Renilla-luciferase and then infected with wt-HSV, UV-inactivated wt-HSV, or R7910. In β3-integrin–silenced cells, both the TLR2-independent and -dependent NF-κB activation was dramatically reduced (Fig. 2 A and B). Interestingly, the inhibition was seen also when NF-κB was activated by a commercial source of lipopolysaccharide (LPS) from Escherichia coli 0111:B4, able to activate TLR2 (Fig. 2 A and B). The results imply that αvβ3-integrin–mediated innate response was activated also by bacterial components.
Fig. 2.
HSV-induced NF-κB activation is inhibited in sh-β3 293T cells. (A and B) Control (ctrl) or sh-β3 293T cells were transfected with TLR2 (panel B) or empty plasmid (panel A) plus NF-κB-luc and Renilla luciferase. At 12 h after transfection cells were cultured in pre-exhausted medium for 48–72 h. Cells were then infected with 20 PFU/cell of wt-HSV, UV-inactivated wt-HSV (wt-HSV-UV), ΔICP0 recombinant R7910, or exposed to LPS (Sigma-Aldrich, # L2630) (100 ng/mL) for 2, 4, or 6 h. NF-κB-luc was measured as described (51) and expressed as arbitrary units (U) of luciferase:renilla ratio. The histogram shows the NF-κB-luc activity following infection or LPS treatment, and the decrease in sh-β3 cells relative to control cultures, at 2 and 6 h after infection. Each value represents the average of triplicate samples from two independent experiments. (C and D) Quantification of endogenous NF-κB activity through detection of Iκ-Bα. Control (ctrl) or sh-β3 293T cells, transfected with TLR2 (+TLR2) or an empty plasmid (–TLR2), were infected with UV-inactivated wt-HSV (wt-HSV-UV) (H-UV) or R9710 (R) (20 PFU/cell) for 2, 4, and 6 h. Lysates were subjected to SDS PAGE; Iκ-Bα and tubulin were detected by IB. (D) Quantification of bands, expressed as densitometric units (DUs), was performed by Image-J software. Shown are the 2 and 4 h Ik-Bα/tubulin ratios, expressed as percentage, relative to the uninfected cell value taken at each time point (black column). Vertical bars represent SD. In A and B, the differences between control and sh-β3 293T cells were statistically significant by two-way ANOVA test, except for the panel A samples 2 h wt-HSV and 6 h LPS, TLR2-minus.
To validate the decrease in NF-κB activation, we measured the endogenous NF-κB. Of note, NF-κB activation is exerted through phosphorylation and degradation of the Iκ-Bα inhibitor. Lysates of control or sh-β3 cells, positive or negative for TLR2, infected with R7910 or UV-HSV-1, were subjected to SDS/PAGE; Iκ-Bα was detected by IB. The results of Fig. 2C, quantified in Fig. 2D, show that in TLR2+ mock-silenced cells, Iκ-Bα was decreased by about 65% in infected cells, relative to uninfected cells. In sh-β3 cells, Iκ-Bα did not undergo the decrease seen in the mock-silenced cells, as exemplified by the 2 and 4 h samples. These loss-of-function experiments provide the first line of evidence that αvβ3-integrin plays a prominent role in NF-κB response induced by HSV or bacterial components in 293T cells, particularly in the presence of TLR2.
Expression of IFNβ, IFNα, IL2, and IL10 (Group 1) Cytokines Is Strongly Inhibited in TLR2+ β3-Integrin–Silenced 293T Cells.
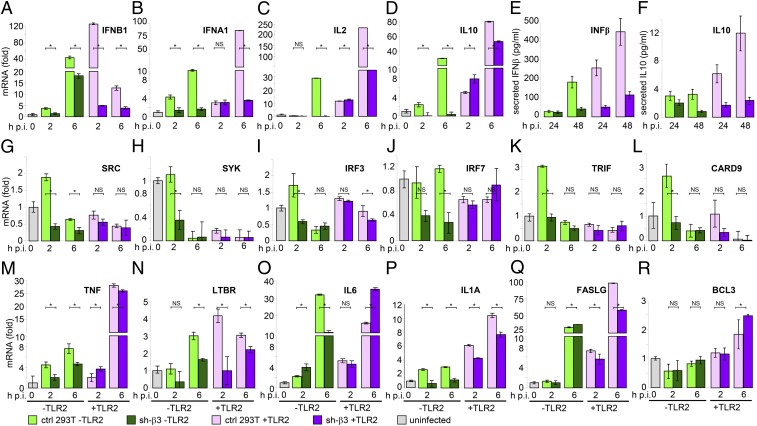
To obtain a snapshot of the signaling pathways affected by αvβ3-integrin, we first profiled the NF-κB-related genes in sh-β3 293T cells, positive or negative for TLR2, by microarray and validated the most highly affected genes and genes of interest by q-RT-PCR. Variations ≥twofold were considered as significant. Based on the effect of β3-integrin silencing, we clustered the genes into three groups. The expression of group 1 cytokine genes—IFNB1, IFNA1, IL2, and IL10—was dramatically decreased in sh-β3 293T cells, independent of whether they were negative or positive for TLR2 (Fig. 3 A–D). These genes were among the most highly induced by HSV, particularly in the presence of TLR2 (note the fold increase in Fig. 3). A variant to this group was IL2RA, whose expression was increased (not decreased) by β3-integrin silencing, only in a TLR2-independent manner (Fig. S2A). Thus, β3-integrin silencing exerts an opposite effect on IL2 and its receptor, suggesting a fine-tuning capability. IFNβ and IL10 decreases were validated at the protein level (Fig. 3 E and F). These results show that HSV-induced production of IFNα, IFNβ, IL2, and IL10 is dictated by αvβ3-integrin. Inasmuch as this signaling is enhanced by TLR2, it may well have the transcription factor NF-κB as its master regulator. The down-regulation of group 1 cytokine genes seen in β3-integrin–silenced and TLR2− cells may reflect the TLR2-independent ability of αvβ3-integrin to activate NF-κB, documented here through antibody cross-linking (Fig. S3) and elsewhere (27).
Fig. 3.
IFN-α and -β, IL2, and IL10 expression are selectively decreased in β3-integrin–silenced 293T cells. (A–D, G–R) Control mock-silenced (ctrl) or sh-β3 293T cells, transfected with irrelevant plasmid (–TLR2) or TLR2 (+TLR2), were cultured in pre-exhausted medium from 12 to 48–72 h after transfection and then infected with UV-inactivated wt-HSV-1(F) (50 PFU/cell) and harvested at 2 h p.i. or infected with R7910 (20 PFU/cell) and harvested at 6 h p.i. RNA was extracted and retrotranscribed by standard techniques for q-RT-PCR. To compare infected versus uninfected cell (uninfected) samples, relative changes in gene expression were determined using the 2−ΔΔCt method. Data were expressed as relative mRNA levels relative to the uninfected cell value, taken as 1 (column, uninfected). Each value represents the average of triplicate samples ± SD. The significance of the difference between each pair of nonsilenced versus silenced samples was determined by two-way ANOVA and Bonferroni posttest: An asterisk denotes statistically significant and NS denotes nonstatistically significant differences. (E and F) Secretion of IFN-β and IL10 by control or sh-β3 293T cells, infected with R7910. The media were harvested 24 or 48 h after infection. Each value represents average of triplicates ± SD.
Expression of SRC, SYK, IRF3, IRF7, TRIF, and CARD9 (Group 2) Genes Is Moderately Affected by β3-Integrin Silencing.
The expression of group 2 genes was down-regulated in sh-β3 cells, only in the absence of TLR2 (Fig. 3 G–L). These genes were not significantly modified in the presence of TLR2 and modestly up-regulated by HSV. SRC is an effector in integrin signaling. IRF3 and IRF7 control the IFN enhancesome. TRIF is an adaptor in the MYD88-independent signaling pathways. Collectively, these genes identify a TLR2-independent signaling pathway, distinct from the one defined by group 1 genes.
Expression of TNF, LTRB, IL1A, IL6, BCL3, FASL, and PTAFR (Group 3) Genes Is Unaffected by β3-Integrin Silencing.
The expression of group 3 genes, which included TNF, LTBR, IL1A, IL6, BCL3, FASLG (Fig. 3 M–R), and PTAFR (Fig. S2B), was not modified in sh-β3 cells, irrespective of TLR2. We also included in this group LTBR because, like the TNF and FASL ligands, it belongs to the death domain receptor family. Beyond its intrinsic significance, group 3 genes show that β3-integrin silencing selectively affects the cell transcriptome. Altogether, the profile of affected genes indicates that αvβ3-integrin–mediated induction of cytokines is highly polarized.
Gain-of-Function Experiments: The NF-κB and Type-1 IFN Response Is Increased in K562 Cells Expressing αvβ3-Integrin.
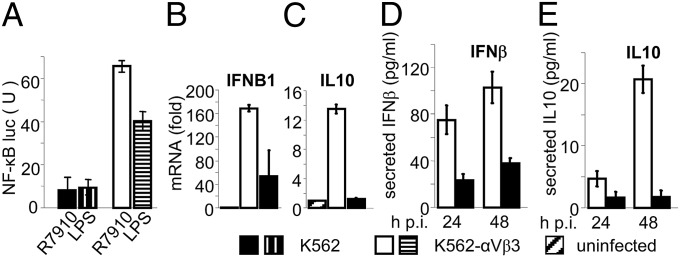
We verified the above conclusion in a gain-of-function experiment, independent of silencing approach. The myelomonocytic wt-K562 cells fail to express αv- and β3-integrins (28, 29). Stable transformants expressing αvβ3-integrin were described (28); the αv- and β3-integrin expression profile in wt- and αvβ3-integrin+ K562 cells was previously assessed by RT-PCR, immunofluorescence, and fluorescence activated cell sorting (29). Here, wt-K562 cells and the αvβ3-integrin+ K562 were transfected with NF-κB-luc, Renilla-luciferase, and TLR2 and infected with R7910. The virus-induced NF-κB-luc was several-fold higher in αvβ3-integrin–positive than in wt-K562 (Fig. 4A). The increase was seen also with LPS (Fig. 4A), again indicating that the αvβ3-integrin–mediated effect is not restricted to HSV. Next, we compared the extent of IFNB and IL10 induction between αvβ3-integrin–positive and –negative K562 cells. Their expression and secretion were higher in the β3-integrin–positive than in the β3-integrin–negative K562 cells (Fig. 4 B–E). Thus, even in K562 cell system, αvβ3-integrin was critical for the induction of IFNB1 and IL10.
Fig. 4.
Gain-of-function assay. HSV-induced NF-κB activation and IFNβ1 and IL10 expression and production are increased in K562 cells expressing αvβ3-integrin, relative to wt-integrin–negative-K562. (A) NF-κB activity in K562 cells expressing or not expressing αvβ3-integrin. The integrin-negative K562 cells (K562) and their counterparts expressing αvβ3-integrin (K562-αvβ3) were transfected with NF-κB-luc and Renilla luciferase plus TLR2 and infected 96 h later with R7910 (20 PFU/cell) or exposed to LPS, both for 6 h. NF-κB activity was expressed as luciferase:renilla ratio, as in panels A and B of Fig. 2. (B and C) Expression of IFNB and IL10 in K562-αvβ3 and K562 cells infected with R7910 (50 PFU/cell) for 6 h. Results are the average of two independent experiments. All details as in legend to Fig. 3. (D and E) Production of IFN-β1 and IL10 in the media of K562-αvβ3 and K562 cells infected with R7910 (50 PFU/cell) for 24 or 48 h. Each value represents the average of triplicate samples ± SD.
αvβ3-integrin Physically Interacts with the Virion Glycoproteins gH/gL and with TLR2.
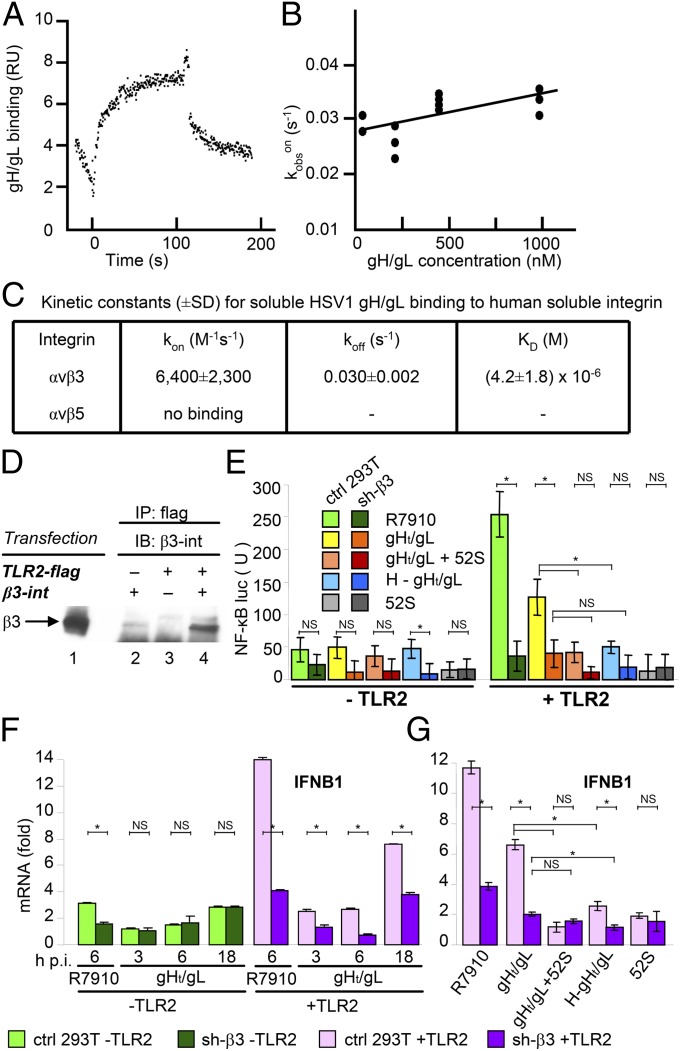
Mechanistically, the involvement of αvβ3-integrin in the innate response to HSV may be exerted through its physical interaction with virions and/or with TLR2. With respect to the virion components, we focused on the envelope heterodimeric glycoproteins gH/gL because, in other herpesviruses, they interact with integrins (30–32). Moreover, gH/gL from HSV and from other herpesviruses interacts with TLR2 (33). The αvβ3-integrin–gH/gL physical interaction was assessed by Biacore (Fig. 5 A–C). The thermodynamic KD was KD = (4.2 ± 1.8) × 10−6 M (Fig. 5 B and C). Thus, αvβ3-integrin binds gH/gL at relatively low affinity. We note that αvβ3-integrin does not play an essential role in HSV entry, and cells that lack β3-integrin are readily infected (29). To document the interaction of αvβ3-integrin with TLR2, we cotransfected 293T cells with TLR2-flag and with αvβ3-integrin and carried out coimmunoprecipitation experiments. TLR2-flag coimmunoprecipitated β3-integrin (Fig. 5D), in agreement with a previous report (20).
Fig. 5.
The virion gH/gL glycoproteins physically interact with αvβ3-integrin and induce NF-κB and IFNB activation in wt-293T cells but not in sh-β3 293T cells. (A) SPR analysis of binding of soluble gH/gL (29) to immobilized integrin αvβ3. (B) Plot of kobson versus gH/gL concentration. Kobs values were calculated by Biacore software. Correlation coefficient was R = 0.72. The plot of kobson versus gH/gL concentration was linear, indicating a single-step binding reaction. The intersection with the y axis corresponds to the dissociation rate constant [koff = (0.027 ± 0.001)s−1] and the slope corresponds to the association rate constant [kon = (6,400 ± 2,300)M−1⋅s−1], giving a KD = (4.2 ± 1.8) × 10−6 M. (C) Summary of kinetic constants. (D) Coimmunoprecipitation of β3-integrin by TLR2-flag. Cells were transfected with αvβ3-integrin (1, 2), TLR2-flag (3), or cotransfected with αvβ3-integrin + TLR2-flag (4). At 24 h posttransfection TLR2 was immunoprecipitated by anti-flag Mab (Sigma-Aldrich). The proteins retained by Protein A/G Sepharose were separated by SDS/PAGE and analyzed by WB with anti–β3-integrin PAb. Lane 1 shows the migration position of β3-integrin. (E) Control (ctrl) or sh-β3 293T cells were transfected with TLR2 (Right) or an empty plasmid (Left) plus NF-κB-luc and Renilla luciferase plasmids. Cells were infected with R7910 or exposed to gHt/gL (1.5 µM) for 6 h. In the “gH/gL + 52S” sample, gHt/gL was preincubated with neutralizing MAb 52S (34) for 1 h at 37 °C. In the “H–gHt/gL” sample, gH/gL was heat-inactivated for 1 h at 95 °C. In the “52S” sample, cells were exposed to MAb alone. NF-κB-luc was measured as detailed in Fig. 2. (F and G) Control mock-silenced (ctrl) or sh-β3 293T cells, transfected with irrelevant plasmid (–TLR2) or TLR2 (+TLR2), were infected with R7910 (6 h) or exposed to gHt/gL (1.5 µM) for 3, 6, and 18 h (F) or for 18 h (G), as detailed in E. RNA extraction and IFNB1 mRNA expression were as detailed in Fig. 3. Each value represents the average of triplicate samples ± SD. The significance of the difference between each pair of nonsilenced versus silenced samples was determined by the two-way ANOVA and Bonferroni posttest: An asterisk denotes statistically significant and NS denotes nonstatistically significant differences.
Recently, we reported that soluble gHt/gL was sufficient to induce NF-κB activation (24). Here, we ascertained that this activation is αvβ3-integrin–dependent, as it was strongly decreased in sh-β3 293T cells. The gHt/gL-mediated activation of NF-κB was specific, as it was inhibited by gHt/gL preincubation with its neutralizing MAb 52S (34) or heat-denaturation (Fig. 5E).
We further demonstrate that gHt/gL was sufficient for IFNB induction and that this induction is αvβ3-integrin–dependent, in that it was strongly decreased in sh-β3 293T cells (Fig. 5 F and G).
Defective Replication of the ΔICP0 R7910 Mutant Is Partially Rescued in β3-Integrin–Silenced 293T Cells.
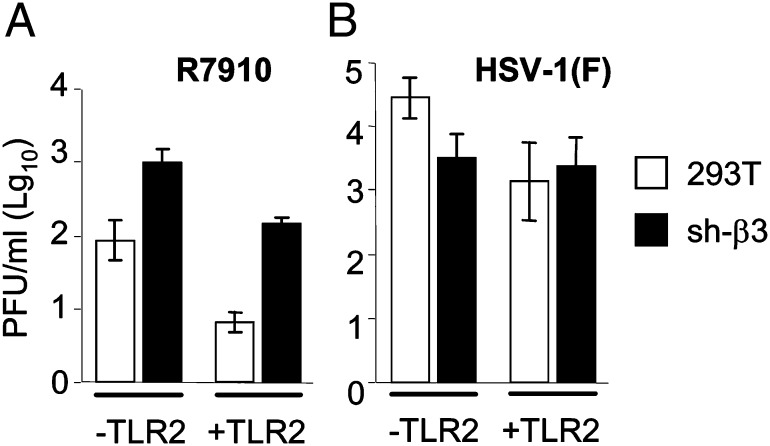
It is well established that some of the innate responses limit viral replication within the initially infected cell and ΔICP0 mutants are highly impaired in replication (9).We measured the yield of wt and ΔICP0 R7910 in β3-integrin–silenced and mock-silenced 293T cells. Surprisingly, the growth defect of R7910, apparent in αvβ3-integrin+ TLR2+ cells, was partly rescued in the β3-integrin–silenced cells, particularly when TLR2 was absent (Fig. 6 A and B)—that is, under conditions in which the NF-κB response was severely decreased. Of note, the replication of wt-HSV was not increased in β3-integrin-silenced cells relative to the nonsilenced cells and was either unmodified or slightly decreased.
Fig. 6.
(A and B) Yield of wt HSV-1(F) and R7910 in wt or sh-β3 293T cells, negative or positive for TLR2. Replicate cultures were infected (1 PFU/cell) and harvested 4 h (0 time) or 24 h after virus adsorption. The virus present in cell debris plus medium was titrated in Vero [HSV-1(F)] or U2OS (R7910) cells. Columns show the 24 h yield (PFU/mL) after subtraction of 0-time values. Results are average of three experiments ± SD.
Discussion
The innate response to a viral infection plays a fundamental role in establishment of the infection and in eliciting adaptive immunity. By loss- and gain-of-function assays we show that αvβ3-integrin is a major sensor and activator of specific components of the innate responses to HSV. Specifically, αvβ3-integrin is critical for the production of type 1-IFNs and of a specific set of cytokines, is a major determinant in NF-κB activation, and reduces viral growth in a single replication cycle. The cumulative picture emerging from current and previous reports (18, 19) is that αvβ3-integrin relocates the HSV receptor nectin1, and thus HSV, to cholesterol-rich membrane microdomains, from where the virus is endocytosed, and simultaneously initiates the innate response. We propose that αvβ3-integrin be considered a non-TLR PRR for HSV and possibly for a number of other viral and bacterial pathogens. Our finding has implications for the design of anti-HSV vaccines.
We classified genes related to the innate response to HSV into three groups based on how they respond to β3-integrin silencing. The specificity of the αvβ3-integrin–mediated response was evident from the observation that β3-integrin silencing resulted in a highly polarized effect—that is, in strong decrease of group 1 cytokines (IFNβ, IFNα, IL2, and IL10), a moderate effect on group 2 genes (SRC, SYK, IRF3, IRF7, TRIF, and CARD9), and no effect on group 3 genes (TNF, LTBR, IL1A, IL6, BCL3, FASL, and PTAFR), even though some of them are known to be dependent on NF-κB (8). Collectively, group 1 and group 2 genes delineate two distinct αvβ3-integrin–mediated signaling pathways.
The dependence of the activation of type 1-IFNs on αvβ3-integrin and TLR2 was unexpected. To date, the sensors recognized as important for HSV-induced type-1 IFN production have been RLRs, TLR9 in plasmacytoid cells, and TLR3 (10–12). Furthermore, until recently TLR2 was thought to be involved mainly in antibacterial defense and inflammatory cytokine induction, not in the activation of type-1 IFN (35). A role for TLR2 in type-1 IFN induction by murine cytomegalovirus and vaccinia virus was recently documented in macrophages (36).
A snapshot of the signaling pathway of group 1 cytokine genes highlighted that it overlapped in part but was independent of the pathway regulated by TLR2. In fact, β3-integrin was required for the activation of group 1 cytokines in both TLR2+ and TLR2− cells; hence, TLR2 enhanced but was not the determinant factor in up-regulation of the cytokine response.
The signaling pathway defined by group 2 genes (SRC, SYK, IRF3, IRF7, TRIF, and CARD9) differed from that of group 1 cytokines in that it was activated exclusively by β3-integrin and not by TLR2. It included TRIF, a MYD88-independent adaptor in TLR3 signaling, which leads to activation of IRF3 and IRF7, major transcription factors of type-1 IFN. It bears a striking resemblance to a recently described pathway initiated by immunoreceptor tyrosine-based activation motifs, leukocyte integrin, or the non-TLRs CLRs; the latter is fundamental in the innate defense against fungi (4, 7, 37).
αvβ3-integrin sensed HSV by binding the virion glycoproteins gH/gL (Fig. 5), which, in turn, bind TLR2 (24). αvβ3-integrin physically interacted with TLR2 and thus bridged the virion and TLR2. Remarkably, soluble gH/gL was sufficient for the αvβ3-integrin/TLR2-dependent induction of IFN-1 and NF-κB activation, testifying a signaling capability of this glycoprotein. Because gH/gL are essential for HSV entry, it follows that entry of the virus into the cell inevitably induces an innate immune response. By deploying PRRs whose PAMPs are the very same virion glycoproteins essential for virus entry, the cell ensures that the virus cannot escape its first line of defense.
How does HSV escape the αvβ3-integrin–mediated defense? It is well established that HSV uses the virion–host–shutoff RNase encoded by the UL41 gene to selectively degrade some of the NF-κB–dependent and –independent RNAs induced after infection (38). ICP0 and ICP27, acting immediately after the viral protein synthesis onset, constitute a secondary line of defense (9, 26, 39, 40). The importance of the αvβ3-integrin defense mechanism is underscored by the observation that it was targeted by ICP0. Silencing of β3-integrin enabled the ΔICP0 mutant to better replicate, particularly when even the TLR2 defense arm was absent. The counteraction of the integrin defense by ICP0 was unexpected but not surprising. The multifunctional protein ICP0 operates in both the nucleus and cytoplasm of the infected cell. In the nucleus, by degrading promyelocytic leukemia nuclear bodies and dispersing nuclear domain 10 bodies, ICP0 blocks exogenous IFN from interfering with viral replication (41). In the cytoplasm, ICP0 interacts with several cellular proteins (9, 11, 12, 42), inhibits IRF3 dimerization, and promotes degradation of the MYD88 adaptor (11, 26).
Several considerations justify the conclusion that αvβ3-integrin is an important non-TLR PRR for HSV and possibly for a number of other viral and bacterial pathogens. Integrins are evolutionarily ancient and highly conserved. They recognize specific molecular patterns in many viral and bacterial pathogens (43, 44). They exhibit signaling activities that lead to a polarized cytokine production. Monocyte integrins—e.g., αM (CD11b)—are already considered as non-TLRs PRRs and regulators of the innate response (4, 45). Importantly, a number of viruses, including the herpesviruses cytomegalovirus, Epstein–Barr virus and Kaposi’s sarcoma herpesvirus, adenoviruses, reoviruses, HIV, and hepatitis C virus, make use of integrins, in most cases as portals of entry into cell, and induce a strong innate response (30–32, 43, 46–49). With respect to bacteria, β3-integrin delivers bacterial lipopeptide to TLR2, inducing inflammatory cytokines; β-integrins physically interact with bacterial pathogens (20, 44).
Materials and Methods
Detailed methods are available in SI Materials and Methods.
K562 Cells.
The wt-K562 (αvβ3-integrin−) and their αvβ3-integrin+ stable transformants were described (28, 29).
β3-Integrin Silencing, Western Blot (IB), and Cell-ELISA of Silencing.
Lentiviruses expressing two different sh-RNAs to β3-integrin (ITGB3) were produced by transfection of pFuGW-ITGB3-1 or pFuGW-ITGB3-2 and additional plasmids into 293T cells, according to ref, 20. The control mock-silenced cells were generated by plasmid pFuGW-control. αvβ3-integrin cell surface expression was measured by cell-ELISA (29).
Infection.
R8102 carries Lac-Z under the α27 immediate-early promoter (21). R7910 carries the deletion of the α0 gene (ΔICP0) (25). R8102 infection was revealed at 6 h (21). For virus yield determinations, titrations were performed in Vero [HSV-1(F)] or U2OS (R7910) cells.
Reverse Transcription and q-RT-PCR.
Total RNA was purified with Total RNA Isolation kit (Macherey-Nagel) and reverse transcribed with high-capacity cDNA reverse transcription kit (Applied Biosystems). Real-time PCR primers were the inventoried TaqMan gene expression assays (Applied Biosystems) (see list in Table S1).
Cytokine Quantification.
IFN-β and human IL10 were detected by VeriKine kit (Pestka Biomedical Laboratories, PBL IFN Source) or Elisa kit (Thermo Scientific, Pierce) from infected cell media.
BIAcore.
Kinetic measurements of the interaction between soluble gH/gL and soluble αvβ3-integrin purified from 293-B3 AVAP cells (a gift of Stephen Nishimura, University of California, San Francisco, CA) were as described (50). MAb LS-C44264 to AP (LifeSpan BioScience) was used to capture the integrins on the surface of the sensor chip.
Supplementary Material
Acknowledgments
We thank A. Zychlinsky, J. L. de Diego, E. Kurt-Jones, S. Nishimura, and B. Scott for cells or reagents. G.C.F. was supported by Investigator Grants from AIRC (Associazione Italiana per la Ricerca sul Cancro) Milan, by Fondazione del Monte di Bologna e Ravenna, by Roberto and Cornelia Pallotti Funds from the Department of Experimental Pathology, by Programmi di ricerca di Rilevante Interesse Nazionale (PRIN) projects from Italian Ministry for University and Research, and by University of Bologna Ricerca Fondamentale Orientata (RFO). L.M.H.-F. and L.S.C. were supported by National Institutes of Health Public Health Service Grant DE016669 (to L.M.H.-F.) from the National Institute of Dental and Craniofacial Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212597109/-/DCSupplemental.
References
- 1.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7(2):131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Sansonetti PJ. The innate signaling of dangers and the dangers of innate signaling. Nat Immunol. 2006;7(12):1237–1242. doi: 10.1038/ni1420. [DOI] [PubMed] [Google Scholar]
- 4.Brown GD. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6(1):33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 5.Inohara N, Nuñez G. NODs: Intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3(5):371–382. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- 6.Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011;34(5):680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abram CL, Lowell CA. Convergence of immunoreceptor and integrin signaling. Immunol Rev. 2007;218:29–44. doi: 10.1111/j.1600-065X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 8.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7(3):179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 9.Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, et al., editors. Fileds Virology. 5th Ed. Vol 2. New York: Lippincott Williams & Wilkins; 2007. pp. 2501–2601. [Google Scholar]
- 10.Reinert LS, et al. TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J Clin Invest. 2012;122(4):1368–1376. doi: 10.1172/JCI60893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paludan SR, Bowie AG, Horan KA, Fitzgerald KA. Recognition of herpesviruses by the innate immune system. Nat Rev Immunol. 2011;11(2):143–154. doi: 10.1038/nri2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paladino P, Mossman KL. Mechanisms employed by herpes simplex virus 1 to inhibit the interferon response. J Interferon Cytokine Res. 2009;29(9):599–607. doi: 10.1089/jir.2009.0074. [DOI] [PubMed] [Google Scholar]
- 13.Finberg RW, Knipe DM, Kurt-Jones EA. Herpes simplex virus and toll-like receptors. Viral Immunol. 2005;18(3):457–465. doi: 10.1089/vim.2005.18.457. [DOI] [PubMed] [Google Scholar]
- 14.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. Fusing structure and function: A structural view of the herpesvirus entry machinery. Nat Rev Microbiol. 2011;9(5):369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campadelli-Fiume G, et al. The multipartite system that mediates entry of herpes simplex virus into the cell. Rev Med Virol. 2007;17(5):313–326. doi: 10.1002/rmv.546. [DOI] [PubMed] [Google Scholar]
- 16.Campadelli-Fiume G, Menotti L, Avitabile E, Gianni T. Viral and cellular contributions to herpes simplex virus entry into the cell. Curr Opin Virol. 2012;2(1):28–36. doi: 10.1016/j.coviro.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Nicola AV, McEvoy AM, Straus SE. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol. 2003;77(9):5324–5332. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gianni T, Campadelli-Fiume G. αVβ3-integrin relocalizes nectin1 and routes herpes simplex virus to lipid rafts. J Virol. 2012;86(5):2850–2855. doi: 10.1128/JVI.06689-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianni T, Gatta V, Campadelli-Fiume G. alphaVbeta3-integrin routes herpes simplex virus to an entry pathway dependent on cholesterol-rich lipid rafts and dynamin2. Proc Natl Acad Sci USA. 2010;107(51):22260–22265. doi: 10.1073/pnas.1014923108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerold G, et al. A Toll-like receptor 2-integrin beta3 complex senses bacterial lipopeptides via vitronectin. Nat Immunol. 2008;9(7):761–768. doi: 10.1038/ni.1618. [DOI] [PubMed] [Google Scholar]
- 21.Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72(12):9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLeod IJ, Minson T. Binding of herpes simplex virus type-1 virions leads to the induction of intracellular signalling in the absence of virus entry. PLoS ONE. 2010;5(3):e9560. doi: 10.1371/journal.pone.0009560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taddeo B, Luo TR, Zhang W, Roizman B. Activation of NF-kappaB in cells productively infected with HSV-1 depends on activated protein kinase R and plays no apparent role in blocking apoptosis. Proc Natl Acad Sci USA. 2003;100(21):12408–12413. doi: 10.1073/pnas.2034952100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leoni V, Gianni T, Salvioli S, Campadelli-Fiume G. Herpes simplex virus glycoproteins gH/gL and gB bind Toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-κB. J Virol. 2012;86(12):6555–6562. doi: 10.1128/JVI.00295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaguchi Y, Bruni R, Roizman B. Interaction of herpes simplex virus 1 alpha regulatory protein ICP0 with elongation factor 1delta: ICP0 affects translational machinery. J Virol. 1997;71(2):1019–1024. doi: 10.1128/jvi.71.2.1019-1024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Lint AL, et al. Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-kappaB signaling. J Virol. 2010;84(20):10802–10811. doi: 10.1128/JVI.00063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5(10):816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 28.Blystone SD, Graham IL, Lindberg FP, Brown EJ. Integrin alpha v beta 3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor alpha 5 beta 1. J Cell Biol. 1994;127(4):1129–1137. doi: 10.1083/jcb.127.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gianni T, et al. Herpes simplex virus glycoproteins H/L bind to cells independently of alphaVbeta3 integrin and inhibit virus entry, and their constitutive expression restricts infection. J Virol. 2010;84(8):4013–4025. doi: 10.1128/JVI.02502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feire AL, Koss H, Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci USA. 2004;101(43):15470–15475. doi: 10.1073/pnas.0406821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandran B. Early events in Kaposi’s sarcoma-associated herpesvirus infection of target cells. J Virol. 2010;84(5):2188–2199. doi: 10.1128/JVI.01334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proc Natl Acad Sci USA. 2009;106(48):20464–20469. doi: 10.1073/pnas.0907508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boehme KW, Guerrero M, Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177(10):7094–7102. doi: 10.4049/jimmunol.177.10.7094. [DOI] [PubMed] [Google Scholar]
- 34.Showalter SD, Zweig M, Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathinam VA, Fitzgerald KA. Innate immune sensing of DNA viruses. Virology. 2011;411(2):153–162. doi: 10.1016/j.virol.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10(11):1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: Signaling cross talk. Cold Spring Harb Perspect Biol. 2011;3(3):pii: a002352. doi: 10.1101/cshperspect.a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taddeo B, Zhang W, Roizman B. The U(L)41 protein of herpes simplex virus 1 degrades RNA by endonucleolytic cleavage in absence of other cellular or viral proteins. Proc Natl Acad Sci USA. 2006;103(8):2827–2832. doi: 10.1073/pnas.0510712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taddeo B, Zhang W, Lakeman F, Roizman B. Cells lacking NF-kappaB or in which NF-kappaB is not activated vary with respect to ability to sustain herpes simplex virus 1 replication and are not susceptible to apoptosis induced by a replication-incompetent mutant virus. J Virol. 2004;78(21):11615–11621. doi: 10.1128/JVI.78.21.11615-11621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melchjorsen J, Sirén J, Julkunen I, Paludan SR, Matikainen S. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J Gen Virol. 2006;87(Pt 5):1099–1108. doi: 10.1099/vir.0.81541-0. [DOI] [PubMed] [Google Scholar]
- 41.Chee AV, Lopez P, Pandolfi PP, Roizman B. Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects. J Virol. 2003;77(12):7101–7105. doi: 10.1128/JVI.77.12.7101-7105.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paladino P, Collins SE, Mossman KL. Cellular localization of the herpes simplex virus ICP0 protein dictates its ability to block IRF3-mediated innate immune responses. PLoS ONE. 2010;5(4):e10428. doi: 10.1371/journal.pone.0010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart PL, Nemerow GR. Cell integrins: Commonly used receptors for diverse viral pathogens. Trends Microbiol. 2007;15(11):500–507. doi: 10.1016/j.tim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Ulanova M, Gravelle S, Barnes R. The role of epithelial integrin receptors in recognition of pulmonary pathogens. J Innate Immun. 2009;1(1):4–17. doi: 10.1159/000141865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han C, et al. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11(8):734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- 46.Danthi P, et al. From touchdown to transcription: The reovirus cell entry pathway. Curr Top Microbiol Immunol. 2010;343:91–119. doi: 10.1007/82_2010_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cicala C, Arthos J, Fauci AS. HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV. J Transl Med. 2011;9(Suppl 1):S2. doi: 10.1186/1479-5876-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lütschg V, Boucke K, Hemmi S, Greber UF. Chemotactic antiviral cytokines promote infectious apical entry of human adenovirus into polarized epithelial cells. Nat Commun. 2011;2:391. doi: 10.1038/ncomms1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nemerow GR. A new link between virus cell entry and inflammation: Adenovirus interaction with integrins induces specific proinflammatory responses. Mol Ther. 2009;17(9):1490–1491. doi: 10.1038/mt.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chesnokova LS, Hutt-Fletcher LM. Fusion of Epstein-Barr virus with epithelial cells can be triggered by αvβ5 in addition to αvβ6 and αvβ8, and integrin binding triggers a conformational change in glycoproteins gHgL. J Virol. 2011;85(24):13214–13223. doi: 10.1128/JVI.05580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurt-Jones EA, et al. The role of toll-like receptors in herpes simplex infection in neonates. J Infect Dis. 2005;191(5):746–748. doi: 10.1086/427339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.