Abstract
Histone H3 lysine-9 methyltransferase G9a/EHMT2/KMT1C is a key corepressor of gene expression. However, activation of a limited number of genes by G9a (independent of its catalytic activity) has also been observed, although the precise molecular mechanisms are unknown. By using RNAi in combination with gene expression microarray analysis, we found that G9a functions as a positive and a negative transcriptional coregulator for discrete subsets of genes that are regulated by the hormone-activated Glucocorticoid Receptor (GR). G9a was recruited to GR-binding sites (but not to the gene body) of its target genes and interacted with GR, suggesting recruitment of G9a by GR. In contrast to its corepressor function, positive regulation of gene expression by G9a involved G9a-mediated enhanced recruitment of coactivators CARM1 and p300 to GR target genes. Further supporting a role for G9a as a molecular scaffold for its coactivator function, the G9a-specific methyltransferase inhibitor UNC0646 did not affect G9a coactivator function but selectively decreased G9a corepressor function for endogenous target genes. Overall, G9a functioned as a coactivator for hormone-activated genes and as a corepressor in support of hormone-induced gene repression, suggesting that the positive or negative actions of G9a are determined by the gene-specific regulatory environment and chromatin architecture. These findings indicate distinct mechanisms of G9a coactivator vs. corepressor functions in transcriptional regulation and provide insight into the molecular mechanisms of G9a coactivator function. Our results also suggest a physiological role of G9a in fine tuning the set of genes that respond to glucocorticoids.
Keywords: methylation, transcription, enhancer, H3K9
The histone code of posttranslational modifications has a pivotal role in organizing nuclear architecture, which, in turn, is involved in regulating transcription. Methylation of histone H3 at lysine 9 (i.e., H3K9) is involved in gene repression and heterochromatin formation. G9a/EHMT2/KMT1C is the major enzyme in euchromatin of mammalian cells, which mono- and dimethylates H3K9 (1, 2). Disruption of the G9a gene in mice results in early embryonic lethality (3). G9a interacts with a variety of repressive transcription factors such as CDP/Cut (4), Gfi1 (5), PRDM1 (6), NRSF/REST (7), and UHRF1 (8), and has canonically been characterized as a transcriptional corepressor. The catalytic function located in the G9a C-terminal SET domain plays key roles in regulation of gene expression, proviral silencing (9, 10), establishment and maintenance of DNA methylation (11–13), and genome stability (14).
G9a is overexpressed in various human cancers including leukemia (15), prostate carcinoma (14, 15), hepatocellular carcinoma (16), and lung cancer (17), and depletion of G9a inhibits cancer cell growth (14, 18); potent and selective inhibitors have been developed to prevent gene repression by G9a-mediated H3K9 methylation (19–22), and this may represent a potential approach for cancer treatment. However, we have shown that G9a can also positively regulate gene expression by acting as a transcriptional coactivator for nuclear hormone receptors (NRs) (23, 24). In luciferase reporter gene assays, the methyltransferase activity of G9a is not required for its coactivator function with NRs (23). Indeed, in such assays, the N-terminal portion of G9a is necessary and sufficient to reproduce full coactivator function (24), suggesting distinct mechanisms for G9a coactivator and corepressor functions. Although G9a coactivator function is poorly documented and not well understood, it may be of equal physiological relevance, as positive gene regulation by G9a has been shown to play an important role in adult erythroid cell differentiation (25) and T helper cell differentiation and function (26). However, to date, a limited number of endogenous target genes that are positively regulated by G9a have been described, and the molecular mechanisms by which G9a contributes positively to transcription complex assembly and activation on target genes remain unknown. Moreover, it is not well understood how the switch between G9a repressor and activator functions is regulated.
To address these questions, we studied G9a function as a transcriptional coregulator for the Glucocorticoid Receptor (GR), which belongs to the steroid hormone receptor subclass of NRs. GR is activated by its cognate natural steroid ligand cortisol as well as many synthetic compounds, causing it to bind specific DNA sequences that are associated with and serve as regulatory enhancer or silencer elements for specific genes (27). Hormone-activated GR can also associate with specific genes through protein–protein interactions with other DNA-binding transcription factors. Most mammalian tissues and cell types contain GR, and GR ligands typically cause activation or repression of hundreds of target genes in any given cell type (28–30). Transcriptional regulation by GR and other NRs involves recruitment of many coregulator proteins (coactivators and corepressors), which remodel chromatin conformation and regulate the recruitment and activation of transcription complexes containing RNA polymerase II (27, 31). Positive and negative regulation by G9a have been documented for a few endogenous target genes of steroid hormone receptors, but the overall extent of G9a influence on steroid hormone-regulated gene expression has not been defined for any cell type.
Here we identify the global repertoire of endogenous GR target genes in A549 lung adenocarcinoma cells and define the subset of GR target genes that require positive or negative actions by G9a for their hormonal regulation. Genes thus identified were used as models for dissecting the molecular mechanisms and protein partners involved in the positive vs. negative gene-regulating functions of G9a. Surprisingly, we found that G9a depletion did not affect basal levels of gene expression; instead, G9a was required as a true coactivator or corepressor for specific subsets of GR target genes and acts by direct association with GR target genes. We investigated the mechanism by which G9a is recruited to those genes and the mechanism of G9a coactivator function by studying protein–protein interactions and testing how the depletion of G9a affected the assembly of an active transcription complex on GR target genes; and we tested whether G9a methyltransferase activity is required for its coactivator and corepressor functions. Our results support a model whereby G9a functions as a coactivator by acting as a scaffold for the recruitment/stabilization of additional coactivators to GR-binding sites (GBSs). These findings begin to elucidate the molecular mechanisms of G9a coactivator function and the switch mechanism that controls the dual coactivator and corepressor actions of G9a.
Results
G9a Positively and Negatively Regulates Transcription of Specific Subsets of GR Target Genes.
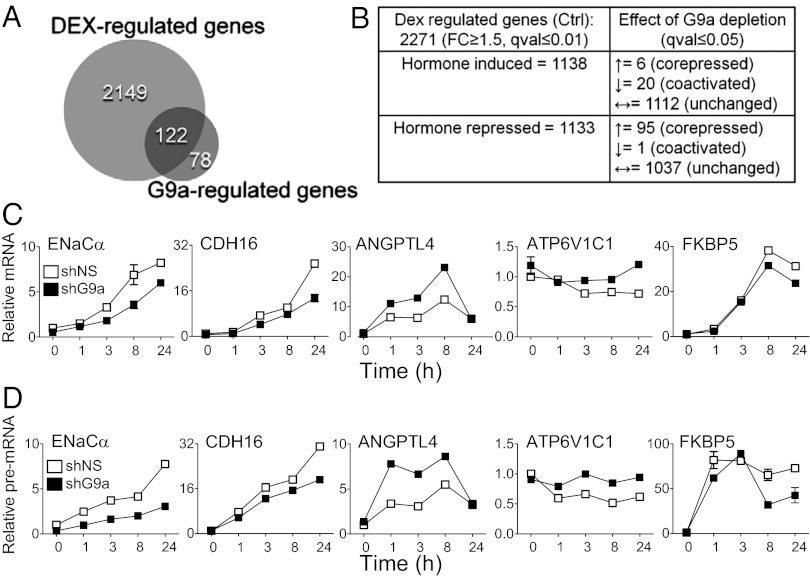
To characterize in an unbiased genome-wide manner the repertoire of endogenous genes positively and negatively regulated by G9a, we performed gene expression microarray profiling of noninfected A549 cells as well as cells depleted of G9a with lentivirus-transduced shRNA (shG9a) and cells expressing a control nonspecific shRNA (shNS). G9a protein and mRNA were efficiently depleted by shG9a, whereas the level of G9a-like protein (GLP or EHMT1), a heterodimer partner for G9a in gene repression (32), was unaffected (Fig. S1 A and B). Glucocorticoid-regulated genes and the GBSs near GR target genes have been well defined in A549 cells (28, 29, 33). Noninfected cells and cells infected with lentivirus expressing shNS and shG9a were treated (or untreated) with the synthetic GR agonist dexamethasone (dex), and the impact of G9a depletion on the hormonal regulation of GR target genes was investigated (Fig. S1C). To impose stringent reproducibility criteria, RNA samples from four independent experiments conducted on separate days were analyzed. From analysis of the microarray results, we identified 2,271 genes for which the mRNA level was changed significantly and by at least 1.5 fold after 24 h of dex treatment (Fig. 1 A and B and Dataset S1). Almost equal numbers of genes were induced or repressed by hormone (Fig. 1B). Because the cell phenotype is determined more by the levels of mRNAs after dex treatment than the fold change in the mRNA levels, we assessed the effect of G9a depletion by comparing gene expression profiles in the dex-treated control cells (uninfected cells and cells infected with the virus encoding shNS) and the dex-treated shG9a cells (Fig. S1D). Overall, the expression of 200 genes was significantly different (no fold cutoff was imposed) in the dex-treated shG9a cells vs. the dex-treated control cells (small Venn diagram, Fig. 1A), and 122 of the total 200 G9a-regulated genes (61%) also belonged to the dex-regulated set of genes (Fig. 1A). Surprisingly, we found that G9a depletion did not significantly affect basal levels of gene expression; instead, G9a served as a required coactivator or corepressor for a specific subset of GR target genes. Thus, serving as a coregulator for GR represents a major function for G9a in dex-treated A549 cells. Notably, among all 200 genes regulated by G9a, 28% were positively regulated by G9a (Dataset S2). Thus, positive regulation of gene expression represents a significant portion of G9a-mediated regulation.
Fig. 1.
G9a negatively and positively regulates specific subsets of GR target genes. (A) Large Venn diagram represents the total number of dex-regulated genes from the microarray analysis: genes with significantly different expression (q ≤ 0.01 and ≥1.5-fold increase or decrease) after treatment with 100 nM dex for 24 h compared with untreated cells; cells were uninfected or infected with lentivirus encoding nonspecific shRNA (i.e., shNS). Small Venn diagram represents the number of G9a-regulated genes with significantly different expression (q ≤ 0.05) in dex-treated cells expressing shG9a vs. dex-treated control cells (uninfected cells and cells infected with the virus encoding shNS). Overlap area indicates the number of genes belonging to both sets. Complete lists of genes for each set depicted are found in Datasets S1 and S2. (B) Table summarizing results of bioinformatics analysis of Illumina microarray results for the dex-regulated gene set. (C and D) RT-qPCR quantification of mRNA (C) or pre-mRNA (D) levels for the indicated genes. Cells expressing shNS (open symbols) or shG9a (filled symbols) were treated with 100 nM dex for the indicated times (in hours). Results shown are mean ± SD for three PCR reactions performed on the same cDNA sample and are representative of three independent experiments.
Of the 26 hormone-induced genes that were significantly regulated by G9a, 20 were induced less upon G9a depletion, indicating a positive role (i.e., putative coactivator function) for G9a (Fig. 1B and Datasets S1 and S2). For example, the microarray data shows that the dex-mediated induction of ENaCα and CDH16 gene expression is compromised upon G9a depletion, illustrating a positive supporting role by G9a in dex-induced gene expression (Fig. S1E). In contrast, dex-induced expression of only six genes was enhanced upon G9a depletion (Fig. 1 B and Datasets S1 and S2). Indeed, the hormonal induction of ANGPTL4 gene expression is further enhanced upon G9a depletion (Fig. S1E), indicating that G9a can also restrict or oppose hormone-induced expression. Considering the 96 hormone-repressed genes that were significantly regulated by G9a, 95 were less well repressed upon G9a depletion, indicating that G9a functioned almost exclusively as a corepressor in support of dex-regulated repression by GR (Fig. 1B). For example, dex-mediated repression of ATP6V1C1 gene expression was eliminated upon G9a depletion (Fig. S1E). Thus, our data show that, in general (with a few exceptions), G9a supported the activity of hormone-activated GR, helping (rather than opposing) its activation or repression of genes. Given that the dex-regulated level of GR-target gene expression was influenced by G9a depletion in only 122 of 2,271 cases, G9a is a very selective coregulator for GR (Fig. 1 A and B, Fig. S1E, and Dataset S1). Although G9a protein was not completely eliminated by shG9a (Fig. S1A), it seems unlikely that the small residual amount of G9a protein was responsible for the low percentage of GR target genes that were affected by G9a depletion.
G9a Acts at Transcriptional Level.
The effect of G9a depletion on expression of selected dex-regulated genes was validated by quantitative RT-PCR (RT-qPCR) analysis of cells treated with dex for varying times (Fig. 1C). The effect of G9a depletion on mRNA levels agreed well with data from the microarray analysis (Fig. S1E). As steady-state mRNA levels are determined by a multistep process (including RNA synthesis, splicing, polyadenylation, export, and turnover) (34), we quantified the levels of nascent transcript as a more accurate assessment of the effect of G9a depletion on the instantaneous transcriptional activity on the gene promoter at any given time. To assess pre-mRNA levels, we performed RT-qPCR with PCR primers that span an early exon/intron boundary for each gene of interest. The effects of G9a depletion on pre-mRNA levels (Fig. 1D) were very similar to the effects observed for mRNA steady-state levels (Fig. 1C). Thus, both positive and negative G9a-mediated regulation of GR target gene expression occurs at the gene transcriptional level.
G9a Is Recruited to GR-Binding Sites in a Hormone-Dependent Manner.
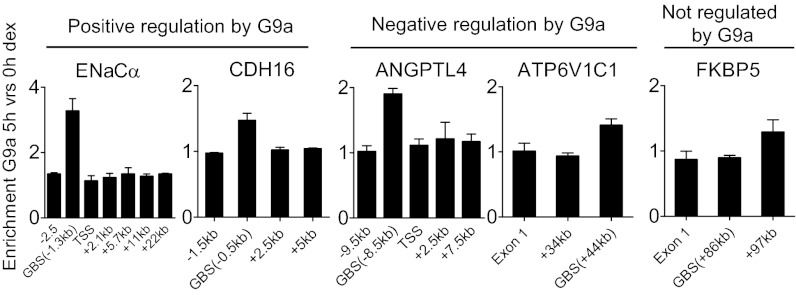
Although G9a regulates GR target genes at the transcriptional level (Fig. 1D), it is possible that G9a acts through a direct mechanism (i.e., G9a associates with the GR target genes) or an indirect mechanism (i.e., G9a regulates a second gene whose product then regulates a GR target gene). As a direct mechanism would require that G9a physically associate with the genes it regulates, we performed ChIP analyses with antibody against G9a. G9a was specifically observed at the GBS located 1.3 kb upstream of the transcription start site (TSS) of the ENaCα gene (positively regulated by G9a) at 1 and 5 h after onset of dex treatment but not in the absence of hormone (Fig. S2A). In contrast, G9a enrichment was not observed at other sites on the ENaCα gene (Fig. S2 A and B and Fig. 2, Left). The same was found to be true for another gene positively regulated by G9a, CDH16 (Fig. 2). Importantly, hormone-induced G9a occupancy at the GBSs of the ENaCα and CHD16 genes was abolished upon depletion of G9a, indicating the specificity of the G9a ChIP signal obtained by using this antibody (Fig. S2B). G9a occupancy was also specifically enriched in a hormone-dependent manner at the GBS of the ANGPTL4 and ATP6V1C1 genes, which were negatively regulated by G9a (Fig. 2). In contrast, G9a occupancy at the GBS of the FKBP5 gene, which did not require G9a for dex-induced expression, was not enriched in response to hormone. Thus, dex treatment caused gene-specific and site-specific recruitment of G9a to GBS of GR target genes where G9a exerted positive and negative regulation.
Fig. 2.
G9a is recruited to GR target genes that require G9a for dex-regulated expression. ChIP was performed on A549 cells untreated or treated with 100 nM dex for 0 or 5 h. Immunoprecipitated DNA was analyzed by qPCR by using the indicated primers (sequences given in Dataset S3), and results are normalized to input chromatin and shown as the ratio of 5 h vs. 0 h of dex treatment. Results shown are mean ± SD (n = 3) of PCR reactions from a single experiment, and are representative of at least three independent experiments.
G9a Binds to GR in Vitro and in Vivo.
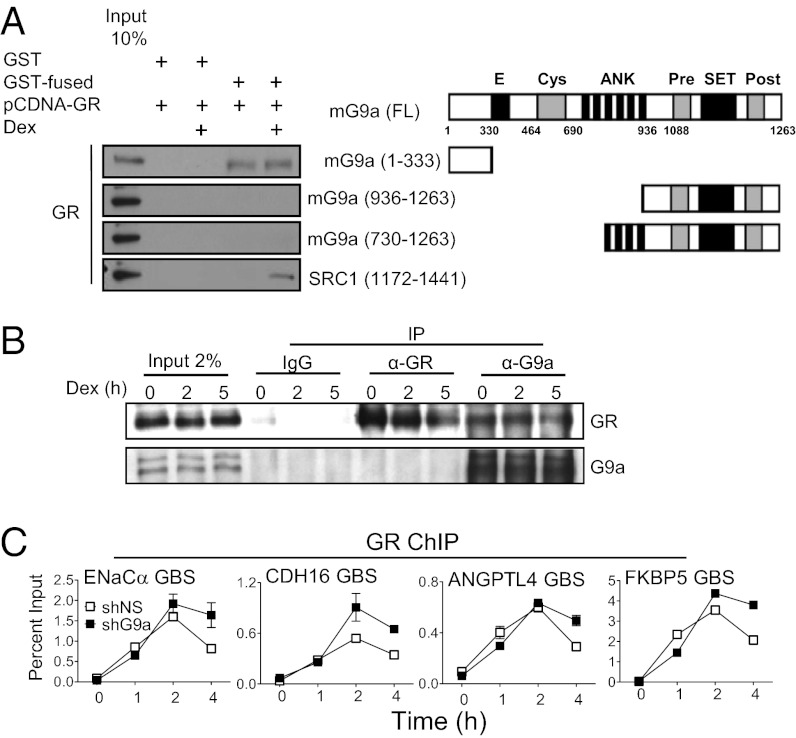
To explore the mechanism of G9a recruitment to the GBS of its target genes, we tested for a physical interaction between G9a and GR. In GST pull-down experiments, full-length human GR translated in vitro failed to interact with the C-terminal SET domain or central ankyrin repeat domain of mouse G9a (mG9a), but bound specifically to an N-terminal mG9a fragment consisting of amino acids 1 to 333 (Fig. 3A). In contrast to the hormone-dependent nature of the interaction between GR and SRC-1 (used as a positive control), the interaction between GR and G9a N-terminal domain was not hormone-dependent (Fig. 3A). Further GST pull-down experiments demonstrated that the GST-tagged mG9a N-terminal fragment bound to the C-terminal ligand binding domain of rat GR, but not to the rat GR DNA-binding domain (Fig. S3A). Importantly, the interaction between G9a and GR was also confirmed by endogenous coimmunoprecipitation experiments (immunoprecipitation with antibodies against G9a) in A549 cells and was also hormone-independent (Fig. 3B); coimmunoprecipitated G9a was not detected when antibodies against GR were used for immunoprecipitation, possibly because of the weak input Western blot signal generally obtained for G9a. The interaction of G9a with GR provides a potential mechanism for hormone-dependent recruitment of G9a to the GBS of GR target genes.
Fig. 3.
G9a interacts with GR in vitro and in vivo. (A) Right: Diagram of full-length mG9a (FL) and mG9a fragments used in this experiment showing amino acid sequence numbers and specific domains: Glu-rich (marked with “E”), Cys-rich ring finger-like (Cys), ankyrin repeat (ANK), methyltransferase (SET), and Cys-rich Pre-SET (Pre) and post-SET (Post). Left: Full-length human GR was synthesized in vitro, incubated with 1 µM dex or vehicle for 4 h, and incubated with GST or GST fused to mG9a fragments or to an SRC1a C-terminal fragment bound to glutathione-agarose beads as indicated. Bound human GR protein was detected by immunoblot with anti-GR antibody. A 10% input sample was loaded for comparison. (B) Coimmunoprecipitation of endogenous GR with G9a from lysates of A549 cells treated with 100 nM dex for the indicated times (in hours). Immunoprecipitation was performed with anti-G9a, anti-GR, or control IgG antibodies. Western blots were performed with anti-GR (Upper) and anti-G9a (Lower) antibodies. A 2% input sample was loaded for comparison. (C) Effect of G9a depletion on GR binding to target genes. ChIP was performed on A549 cells expressing shNS (open symbols) or shG9a (filled symbols) and treated with 100 nM dex for the indicated times. Immunoprecipitated DNA was analyzed by qPCR by using primers that amplify the GBS region of the indicated GR target genes. Results shown are mean ± SD of PCR reactions (n = 3) performed with DNA samples from a single experiment, and are representative of three independent experiments.
G9a Is Important for Recruitment of p300 and CARM1 to GR Target Genes.
The specific steps in transcription complex assembly facilitated by G9a in its role as a transcriptional coactivator have not previously been investigated to our knowledge. Our microarray (Datasets S1 and S2) and immunoblot analyses (Fig. S3B) ruled out the possibility that G9a depletion reduced GR mRNA or protein levels. Furthermore, the recruitment of GR to GBSs in response to hormone was not significantly affected by G9a depletion (Fig. 3C). Although the experiment shown features a slight decrease in GR recruitment upon G9a depletion at 4 h, this decrease was not consistently observed across multiple independent experiments.
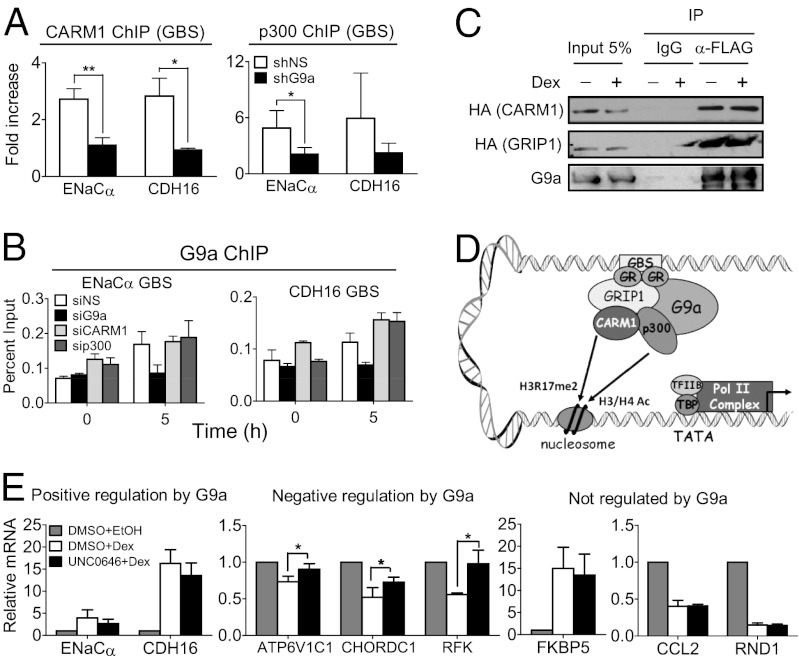
Formaldehyde-assisted isolation of regulatory elements (FAIRE) experiments coupled with qPCR showed that the hormone-inducible chromatin remodeling of the GBS is not affected by G9a depletion (Fig. S4). Thus, G9a must contribute to the assembly of an active transcription complex on GR target gene promoters by facilitating events occurring subsequent to the binding of GR, dex-induced remodeling of the GBS and TSS regions, and recruitment of G9a to the GBS. Our previous transient reporter gene studies indicated that G9a acts as a coactivator in synergy with the coregulators GRIP1, CARM1, and p300 (23, 24). We therefore tested whether G9a facilitates recruitment of CARM1 and p300 to the DNA-bound GR complex. Indeed, G9a depletion specifically resulted in deficient p300 and CARM1 recruitment to GBSs associated with GR target genes (ENaCα and CHD16) that require G9a coactivator function (Fig. 4A). Furthermore, siRNA-mediated depletion of CARM1 or p300 reduced the hormonal induction of the G9a-coactivated genes ENaCα and CDH16 (Fig. S5A), indicating that CARM1 and p300 are critical for the hormonal regulation of these genes. Depletion of G9a did not affect p300 or CARM1 protein levels, nor did depletion of CARM1 or p300 reduce G9a protein level (Fig. S5 B and C). We next investigated whether CARM1 and/or p300 were reciprocally required for G9a recruitment, which would suggest cooperative recruitment of these three coregulators. Depletion of p300 or CARM1 did not affect G9a recruitment to its target genes, whereas, in the control experiment, depletion of G9a abolished G9a recruitment as expected (Fig. 4B). These results show that G9a is required for recruitment of CARM1 and p300, but CARM1 and p300 are not required for G9a recruitment, indicating that G9a recruitment to the GBS is a prerequisite for CARM1 and p300 recruitment.
Fig. 4.
G9a facilitates recruitment of p300 and CARM1 for activation of G9a-dependent GR target genes. (A) A549 cells expressing shNS (open bars) or shG9a (filled bars) were treated with dex for 0 or 5 h. ChIP was performed with antibodies against CARM1 (Left) or p300 (Right), and immunoprecipitated DNA was analyzed by qPCR by using primers specific for the GBS of the indicated genes. Results are normalized to input chromatin and the mean ± SD of the ratio between 5 h and 0 h dex treatment for three independent experiments are shown. (B) A549 cells transfected with the indicated siRNA were treated with dex for 0 or 5 h. ChIP was performed with antibody against G9a, and immunoprecipitated DNA was analyzed by qPCR by using primers specific for the GBS of the indicated gene. Results shown are mean ± SD of triplicate PCR reactions performed on the same DNA sample, are from a single experiment, and are representative of two independent experiments. (C) COS-7 cells were transfected with pSG5-2XFLAG-hG9a (full-length) and pSG5-HA-mCARM1 (full-length) or pSG5-HA-mGRIP1 (full-length) and treated with dex for 0 or 4 h. G9a was immunoprecipitated from cell extracts with an anti-FLAG antibody or nonimmune IgG (for background estimation), and bound protein was analyzed by immunoblot with anti-G9a antibody (Lower) to control for levels of immunoprecipitated G9a or with anti-HA antibody to detect CARM1 or GRIP1. A 5% input sample was loaded for comparison. (D) Model for positive G9a-mediated regulation of GR target gene expression. First, hormone-activated GR binds to GBS. Next, G9a is recruited to GBS at least in part through interaction with GR. G9a then facilitates recruitment or stabilizes the association at the GBS of p300 and CARM1 coactivators, which acetylate histones H3 and H4 (H3/H4 Ac) and dimethylate histone H3 at R17 (H3R17me2), respectively, leading to the recruitment of basal transcription factors (TFIIB and TBP) and RNA polymerase II (Pol II) complex. (E) RT-qPCR quantification of mRNA levels for the indicated genes normalized to β-actin mRNA. Cells were treated with 100 nM dex (white and black bars) or the equivalent volume of EtOH (vehicle; gray bars) for 8 h. At 1 h before hormone or EtOH treatment, cells were treated with 2 µM UNC0646 (black bars) or equivalent volume of DMSO (vehicle; white and gray bars). Results shown are mean ± SD for at least three independent experiments (*P ≤ 0.05, paired t test).
To explore the mechanism of G9a recruitment of p300 and CARM1, coimmunoprecipitation experiments were performed. GRIP1 interacts with CARM1 and p300 and has previously been implicated through transient reporter gene assays in the recruitment of CARM1 and p300 to NR target genes (35). Immunoprecipitation of FLAG-tagged full-length G9a caused coimmunoprecipitation of HA-tagged CARM1 and GRIP1 (Fig. 4C), consistent with a mechanism of G9a coactivator function that involves G9a-assisted recruitment of p300 and CARM1 to GR target genes (Fig. 4D).
G9a Catalytic Activity Is Not Involved in Its Coactivator Function.
We have previously shown in luciferase reporter gene assays that the G9a SET domain is not required for its coactivator function in this artificial system. However, it is not clear whether, in addition to recruiting CARM1 and p300, G9a catalytic activity is also involved in its coactivator function for the dex regulation of endogenous genes in the context of chromatin. Treatment of A549 cells with the G9a methyltransferase inhibitor UNC0646 (36) for 48 h caused reduced cellular levels of H3K9 dimethylation (Fig. S6), as expected. Treatment with UNC0646 beginning 1 h before hormone delivery partially but significantly relieved the hormonal repression of all three G9a corepressed genes tested; indeed, approximately half or more of the inhibitory effect of dex is lost upon cotreatment with the G9a enzyme inhibitor (Fig. 4E, Center). However, the inhibitor did not significantly affect the hormonal induction of genes coactivated by G9a (Fig. 4E, Left) or the hormonal activation or repression of genes that are not regulated by G9a (Fig. 4E, Right). Thus, whereas the G9a corepressor function for endogenous genes involves its methyltransferase activity, G9a coactivator function is methyltransferase activity-independent. Our results provide robust evidence that G9a functions as a coactivator mainly by acting as a molecular bridge (or assembly platform) between the NR and transcriptional coactivators.
Discussion
Role of G9a in Regulating the Expression of Target Genes of GR.
Although G9a has canonically been characterized as a transcriptional corepressor for a variety of transcription factors, positive regulation of gene expression by G9a has recently been described (23–26). However, the molecular mechanisms of G9a-mediated positive regulation of gene expression are poorly understood. In particular, it has not been determined whether G9a enhances expression of genes by direct positive actions or through an indirect cascade of gene regulation that involves G9a repression of genes encoding other transcriptional repressors. In this study, we defined the global extent of positive and negative gene regulation by G9a in a specific cell line in the context of glucocorticoid-regulated gene expression. We found that G9a regulates positively or negatively the transcriptional efficiency of specific subsets of GR target genes. G9a had a positive action on the transcription of a relevant portion (17%) of the 122 genes that are regulated by dex and G9a, emphasizing the physiological relevance of positive gene regulation by G9a. Furthermore, in most cases, G9a facilitated, rather than opposed, the positive or negative regulation of genes by dex. Specifically, G9a functioned as a coactivator for 20 of 26 genes that were induced by dex and affected by G9a depletion; and G9a served as a corepressor for 95 of 96 genes that were repressed by dex and affected by G9a depletion (Fig. 1B). These results suggest that the switch that controls G9a function as a positive or negative regulator of gene expression likely involves the gene-specific chromatin architecture and/or context of protein–protein interactions with GR or other coregulators at the GBS. Indeed, negative regulation of gene expression by G9a is often linked to its histone methyltransferase function, but histones that are acetylated at H3K9 (which is expected at genes where histone acetyltransferases have been recruited) are not substrates for G9a methyltransferase catalytic activity. In addition, the switch between G9a coactivator and corepressor functions could be regulated by posttranslational modifications of G9a or protein–protein interactions. Thus, it seems likely that G9a, through its coactivator and corepressor functions, can fine-tune the hormone response (i.e., the exact set of genes that is regulated by hormone) and thus is likely to have an impact on the physiological outcome of the hormone action. Another key finding of our global gene regulation analysis is that most (122 of 200) of the genes affected by G9a depletion were dex-regulated genes. These results suggest that the primary role of G9a in A549 cells is to modulate or facilitate acute gene regulation by regulated transcription factors (such as GR), rather than control basal expression of genes.
Molecular Mechanism of G9a Coregulator Function on GR Target Genes.
Previous studies showed that dimethylation of H3K9 was present in the coding region of transcriptionally active genes (37), suggesting that positive regulation of gene expression by G9a might involve enzymatic actions by G9a within the coding region to facilitate transcription elongation. However, for GR target genes regulated positively and negatively by G9a, we observed hormone-dependent recruitment of G9a to distal GR-binding regions associated with GR target genes, but not to the gene TSS and body (Fig. 2 and Fig. S2). Furthermore, negative and positive gene regulation by G9a occurs at the level of transcription, as pre-mRNA levels measured for each gene of interest were affected by G9a depletion (Fig. 1D).
Our analyses indicate that G9a recruitment to GBSs is most likely mediated through direct interaction between G9a and GR. Supporting these findings, the lower fold enrichment of G9a occupancy at the ATP6V1C1 GBS (Fig. 2) is consistent with previous reports of weaker occupancy of GR at hormone-repressed genes compared with hormone-induced genes (38). The N-terminal region of G9a interacted physically with GR in vitro and in vivo (Fig. 3). Furthermore, the same region of G9a also bound to estrogen receptor (ER)-α, was necessary and sufficient to recapitulate full G9a coactivator function in luciferase reporter gene assays with ER-α, and was sufficient for hormone-dependent recruitment of G9a to endogenous target genes of ER-α (24). Given that there is no known enzymatic activity associated with this N-terminal domain of G9a, these data suggest that G9a coactivator function is most likely mediated through G9a acting as a bridge for protein–protein interactions between GR and coactivators or basal transcription machinery.
We found that G9a action occurred subsequent to the dex-induced recruitment of GR and G9a to the GBS (Fig. 3C) and chromatin remodeling at the GBS (Fig. S4), but before the recruitment of CARM1 and p300 (Fig. 4B). We also showed that G9a coactivator function, in contrast to its corepressor function, is mediated through G9a acting as a molecular scaffold to facilitate recruitment of coactivators CARM1 and p300 to endogenous GR target genes that are positively regulated by hormone and by G9a (Fig. 4). These data provide a unique molecular mechanism to explain the synergy among G9a, CARM1, and p300 previously observed in transient reporter gene assays (23). However, we cannot rule out the possibility that G9a may stabilize the association of CARM1 and p300 with the GBS rather than facilitate their recruitment. A detailed time course of GR, G9a, p300, and CARM1 binding to GBS after the onset of dex treatment would further help to dissect the order of different steps leading to activation.
Importantly, our data show that G9a coactivator function for endogenous genes is independent of its catalytic activity (Fig. 4E). In agreement with these findings, artificial luciferase reporter gene assays previously showed that the N-terminal domain consisting of the first 333 aa (and lacking the catalytic SET domain) was necessary and sufficient to recapitulate full coactivator function (24). Thus, if G9a inhibitors become more commonly used in drug cancer therapy and induced pluripotent stem cell technology, one must take into account that these compounds will inhibit G9a-mediated regulation of corepressed gene targets but are unlikely to affect G9a-mediated regulation of coactivated gene targets. In conclusion, these data begin to elucidate the molecular mechanisms of G9a-mediated positive regulation of gene expression and how the switch between G9a coactivator and corepressor functions is regulated.
Materials and Methods
Cell culture, plasmids, immunoblot, and lentivirus production and delivery of anti-G9a shRNA are described in SI Materials and Methods.
Real-Time RT-qPCR Analysis.
Total RNA isolation, cDNA synthesis, and quantitative PCR analysis were performed as described in SI Materials and Methods by using PCR primers described in Dataset S3. All mRNA and pre-mRNA levels were normalized to the level of β-actin mRNA.
Gene Expression Analysis by Illumina Microarray.
Global gene expression analysis was performed with Illumina Human-Ref8v3 microarrays by using total RNA samples from four biological replicates from four independent experiments performed on different days. Each experiment included noninfected A549 cells or A549 cells infected with lentivirus encoding shNS or shG9a, with or without treatment with 100 nM dex for 24 h (24 samples total). Experimental details and methods of data analysis are described in SI Materials and Methods and Dataset S4.
ChIP.
ChIP assays were performed according to previously described protocols (39) with minor modifications, as detailed in SI Materials and Methods.
Protein Interaction Assays.
The procedure for GST pull-down assays was described previously (40) with minor modifications, as detailed in SI Materials and Methods.
FAIRE.
The procedure for FAIRE was performed as described previously (41).
siRNA Transfection.
siRNA transfection protocol and siRNA sequences used are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the following colleagues at the University of Southern California (USC): Dr. Chen-Yin Ou for CARM1 siRNA, Dr. Judd C. Rice and Dr. Chendhore S. Veerappan for advice on the G9a inhibitor UNC0646, and Kelly Chang for technical assistance. We thank Ben Schiller (University of California, San Francisco) for information on GR-binding sites in A549 cells, Dr. Alex So (California Institute of Technology) for guidance with lentiviral shRNA delivery system, and Dr. Jian Jin (University of North Carolina) for the gift of the UNC0646 compound. Gene expression microarray data were generated by the Genomics Core Facility and analyzed with support from the Biostatistics Core Facility of the USC Norris Comprehensive Cancer Center. This work was supported by National Institutes of Health (NIH) Grant R01 DK055274 (to M.R.S.), USC Norris Comprehensive Cancer Center (Core) Grant P30 CA 014089-36 (to K.D.S), a California Institute of Regenerative Medicine postdoctoral training grant (to D.B.), and NIH Training Grant T32 GM067587 (to D.-Y.W.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE35962).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211803109/-/DCSupplemental.
References
- 1.Tachibana M, Sugimoto K, Fukushima T, Shinkai Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J Biol Chem. 2001;276(27):25309–25317. doi: 10.1074/jbc.M101914200. [DOI] [PubMed] [Google Scholar]
- 2.Shinkai Y, Tachibana M. H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev. 2011;25(8):781–788. doi: 10.1101/gad.2027411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tachibana M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16(14):1779–1791. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishio H, Walsh MJ. CCAAT displacement protein/cut homolog recruits G9a histone lysine methyltransferase to repress transcription. Proc Natl Acad Sci USA. 2004;101(31):11257–11262. doi: 10.1073/pnas.0401343101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan Z, Zarebski A, Montoya-Durango D, Grimes HL, Horwitz M. Gfi1 coordinates epigenetic repression of p21Cip/WAF1 by recruitment of histone lysine methyltransferase G9a and histone deacetylase 1. Mol Cell Biol. 2005;25(23):10338–10351. doi: 10.1128/MCB.25.23.10338-10351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyory I, Wu J, Fejér G, Seto E, Wright KL. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat Immunol. 2004;5(3):299–308. doi: 10.1038/ni1046. [DOI] [PubMed] [Google Scholar]
- 7.Roopra A, Qazi R, Schoenike B, Daley TJ, Morrison JF. Localized domains of G9a-mediated histone methylation are required for silencing of neuronal genes. Mol Cell. 2004;14(6):727–738. doi: 10.1016/j.molcel.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Kim JK, Estève PO, Jacobsen SE, Pradhan S. UHRF1 binds G9a and participates in p21 transcriptional regulation in mammalian cells. Nucleic Acids Res. 2009;37(2):493–505. doi: 10.1093/nar/gkn961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong KB, et al. DNA methylation in ES cells requires the lysine methyltransferase G9a but not its catalytic activity. EMBO J. 2008;27(20):2691–2701. doi: 10.1038/emboj.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung DC, et al. Lysine methyltransferase G9a is required for de novo DNA methylation and the establishment, but not the maintenance, of proviral silencing. Proc Natl Acad Sci USA. 2011;108(14):5718–5723. doi: 10.1073/pnas.1014660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epsztejn-Litman S, et al. De novo DNA methylation promoted by G9a prevents reprogramming of embryonically silenced genes. Nat Struct Mol Biol. 2008;15(11):1176–1183. doi: 10.1038/nsmb.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estève PO, et al. Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. Genes Dev. 2006;20(22):3089–3103. doi: 10.1101/gad.1463706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikegami K, et al. Genome-wide and locus-specific DNA hypomethylation in G9a deficient mouse embryonic stem cells. Genes Cells. 2007;12(1):1–11. doi: 10.1111/j.1365-2443.2006.01029.x. [DOI] [PubMed] [Google Scholar]
- 14.Kondo Y, et al. Downregulation of histone H3 lysine 9 methyltransferase G9a induces centrosome disruption and chromosome instability in cancer cells. PLoS ONE. 2008;3(4):e2037. doi: 10.1371/journal.pone.0002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, et al. G9a and Glp methylate lysine 373 in the tumor suppressor p53. J Biol Chem. 2010;285(13):9636–9641. doi: 10.1074/jbc.M109.062588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo Y, et al. Alterations of DNA methylation and histone modifications contribute to gene silencing in hepatocellular carcinomas. Hepatol Res. 2007;37(11):974–983. doi: 10.1111/j.1872-034X.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe H, et al. Deregulation of histone lysine methyltransferases contributes to oncogenic transformation of human bronchoepithelial cells. Cancer Cell Int. 2008;8:15. doi: 10.1186/1475-2867-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGarvey KM, et al. Silenced tumor suppressor genes reactivated by DNA demethylation do not return to a fully euchromatic chromatin state. Cancer Res. 2006;66(7):3541–3549. doi: 10.1158/0008-5472.CAN-05-2481. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, et al. Protein lysine methyltransferase G9a inhibitors: Design, synthesis, and structure activity relationships of 2,4-diamino-7-aminoalkoxy-quinazolines. J Med Chem. 2010;53(15):5844–5857. doi: 10.1021/jm100478y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, et al. Discovery of a 2,4-diamino-7-aminoalkoxyquinazoline as a potent and selective inhibitor of histone lysine methyltransferase G9a. J Med Chem. 2009;52(24):7950–7953. doi: 10.1021/jm901543m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubicek S, et al. Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol Cell. 2007;25(3):473–481. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Chang Y, et al. Structural basis for G9a-like protein lysine methyltransferase inhibition by BIX-01294. Nat Struct Mol Biol. 2009;16(3):312–317. doi: 10.1038/nsmb.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DY, Northrop JP, Kuo MH, Stallcup MR. Histone H3 lysine 9 methyltransferase G9a is a transcriptional coactivator for nuclear receptors. J Biol Chem. 2006;281(13):8476–8485. doi: 10.1074/jbc.M511093200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purcell DJ, Jeong KW, Bittencourt D, Gerke DS, Stallcup MR. A distinct mechanism for coactivator versus corepressor function by histone methyltransferase G9a in transcriptional regulation. J Biol Chem. 2011;286(49):41963–41971. doi: 10.1074/jbc.M111.298463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaturvedi CP, et al. Dual role for the methyltransferase G9a in the maintenance of beta-globin gene transcription in adult erythroid cells. Proc Natl Acad Sci USA. 2009;106(43):18303–18308. doi: 10.1073/pnas.0906769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehnertz B, et al. Activating and inhibitory functions for the histone lysine methyltransferase G9a in T helper cell differentiation and function. J Exp Med. 2010;207(5):915–922. doi: 10.1084/jem.20100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E. The human glucocorticoid receptor: Molecular basis of biologic function. Steroids. 2010;75(1):1–12. doi: 10.1016/j.steroids.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JC, et al. Chromatin immunoprecipitation (ChIP) scanning identifies primary glucocorticoid receptor target genes. Proc Natl Acad Sci USA. 2004;101(44):15603–15608. doi: 10.1073/pnas.0407008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogatsky I, et al. Target-specific utilization of transcriptional regulatory surfaces by the glucocorticoid receptor. Proc Natl Acad Sci USA. 2003;100(24):13845–13850. doi: 10.1073/pnas.2336092100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galon J, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16(1):61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- 31.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14(2):121–141. [PubMed] [Google Scholar]
- 32.Tachibana M, et al. Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3-K9. Genes Dev. 2005;19(7):815–826. doi: 10.1101/gad.1284005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy TE, et al. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res. 2009;19(12):2163–2171. doi: 10.1101/gr.097022.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bittencourt D, et al. Cotranscriptional splicing potentiates the mRNA production from a subset of estradiol-stimulated genes. Mol Cell Biol. 2008;28(18):5811–5824. doi: 10.1128/MCB.02231-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen D, Huang SM, Stallcup MR. Synergistic, p160 coactivator-dependent enhancement of estrogen receptor function by CARM1 and p300. J Biol Chem. 2000;275(52):40810–40816. doi: 10.1074/jbc.M005459200. [DOI] [PubMed] [Google Scholar]
- 36.Liu F, et al. Optimization of cellular activity of G9a inhibitors 7-aminoalkoxy-quinazolines. J Med Chem. 2011;54(17):6139–6150. doi: 10.1021/jm200903z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine 9 methylation and HP1gamma are associated with transcription elongation through mammalian chromatin. Mol Cell. 2005;19(3):381–391. doi: 10.1016/j.molcel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Engel KB, Yamamoto KR. The glucocorticoid receptor and the coregulator Brm selectively modulate each other’s occupancy and activity in a gene-specific manner. Mol Cell Biol. 2011;31(16):3267–3276. doi: 10.1128/MCB.05351-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee YH, Campbell HD, Stallcup MR. Developmentally essential protein flightless I is a nuclear receptor coactivator with actin binding activity. Mol Cell Biol. 2004;24(5):2103–2117. doi: 10.1128/MCB.24.5.2103-2117.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeong KW, Lee YH, Stallcup MR. Recruitment of the SWI/SNF chromatin remodeling complex to steroid hormone-regulated promoters by nuclear receptor coactivator flightless-I. J Biol Chem. 2009;284(43):29298–29309. doi: 10.1074/jbc.M109.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon JM, Giresi PG, Davis IJ, Lieb JD. Using formaldehyde-assisted isolation of regulatory elements (FAIRE) to isolate active regulatory DNA. Nat Protoc. 2012;7(2):256–267. doi: 10.1038/nprot.2011.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.