Abstract
Naturally arising IgM antibodies, which recognize neo-determinants on apoptotic cell (AC) membranes, are present from birth and can be further induced by AC challenge. Such naturally arising IgM antibodies can suppress proinflammatory responses to purified agonists for Toll-like receptors (TLRs), as well as block the induction of IgG immune complex-induced in vitro and in vivo pathogenic responses. To investigate the responsible mechanisms, we studied the regulatory effects of IgM anti-AC antibody on responses in bone marrow-derived dendritic cells mediated by a range of different TLRs and found that addition of IgM anti-AC inhibited the activation of the primary MAPKs: ERK1/2, JNK, and particularly p38. This was dependent on the recruitment of either C1q or mannose-binding lectin, which are both early complement factors that tag ACs for innate immune recognition. Strikingly, MAPK inhibition of responses to TLR agonists, and to lupus IgG autoantibody-chromatin immune complexes, was found to correlate with, and had an absolute requirement for, the induction and nuclear localization of MAPK phosphatase-1, a factor known to mediate glucocorticoid suppression of immune responses. Further experiments showed that natural IgM antibodies in serum exhibited the same inhibitory properties. These studies elucidate a novel homeostatic pathway by which natural antibodies, which are products of the adaptive immune system, can directly blunt inflammatory responses by recruitment and coordination of a primitive regulatory pathway of the innate immune system.
Keywords: apoptotic cell, autoimmunity, Dusp-1, inflammation, MBL
Inflammation represents a protective host response to foreign challenge or tissue injury that is ultimately beneficial. However, to limit and resolve inflammatory responses and maintain homeostasis, layered inhibitory mechanisms have arisen that are often intertwined with the recognition and clearance of damaged host cells. Indeed, recent findings suggest that innate phagocytic cell functions can be modulated by a class of naturally arising IgM antibodies (NAbs) (reviewed in ref. 1), which are present from birth in humans and mice (2). In the mouse, the major source of IgM–NAbs is the B-1 subset of mature B lymphocytes that express a repertoire believed initially selected by self-antigens and which includes recurring B-cell clones that enhance host defenses (3). For example, murine strains express a B-1 clonotype, termed T15, defined by canonical Ig variable region rearrangements (4), that binds phosphorylcholine (PC)-containing determinants (5) and targets certain microbial pathogens (reviewed in ref. 6). Notably, PC determinants are also expressed on oxidatively modified low-density lipoprotein and in atherosclerotic plaques, and these altered self-antigens are likewise recognized by T15 clonotype antibodies (7).
We previously postulated that the regulatory properties of certain anti-apoptotic cell (AC) NAbs are linked to the recognition of newly exposed oxidation-associated PC head groups on ACs, which on healthy cells are sequestered within the cell membrane and inaccessible to antibodies (8). Intravenous immunization of ACs can induce much higher levels of IgM antibodies to PC and other oxidation-associated neo-determinants, such as malondialdehyde (MDA) (9), which suggests that baseline levels of such IgM antibodies reflect physiologic immune responses to natural AC exposure. Indeed, over 100 billion cells die every day by apoptosis, and must be rapidly eliminated to prevent release of autoantigens and danger-associated signals that can mediate inflammatory responses.
AC clearance is believed to be primarily carried out by macrophages through a specialized process, termed “efferocytosis,” which is enhanced by the addition of natural IgMs (10, 11) including those with anti-PC specificity (9, 12). Immature dendritic cells (DCs) also have the capacity for efferocytosis and can reinforce immunologic tolerance by presenting self-antigens acquired through this process (13). However, when fully activated, DCs serve as immune sentinels that trigger robust inflammatory responses (13). Thus, it is critical to maintain these antigen-presenting cells in an inactivated state. AC membranes can also block inflammatory responses by cell–cell contact mechanisms that do not have an absolute requirement for phagocytic uptake (14).
In recent studies we have shown that natural IgM can suppress inflammatory responses mediated by Toll-like receptors (TLRs) (9, 12), which are important sensors of pathogen-associated molecular patterns that activate innate and adaptive immunity (15). Such anti-AC NAbs also have a direct inhibitory effect on inflammatory responses induced in vitro by lupus-associated IgG–nucleic acid immune complexes (16). In addition, infusions of AC-reactive IgM markedly reduced clinical disease activity and synovial leukocytic infiltrates in the collagen-induced arthritis model (9) and in responses to pathogenic anticollagen IgG autoantibodies (9). Therefore, anti-AC NAbs facilitate efficient clearance of ACs and the inhibition of activation of phagocytes.
Here, we examine the mechanisms by which IgM–NAbs to ACs can broadly suppress inflammatory responses in DCs and document the efficient inhibition of the MAPK system. The magnitude and duration of MAPK signaling is dependent on the balance between upstream activators and deactivation by phosphatases. We found that the blockade of TLR-mediated MAPK signaling mediated by anti-AC IgM had an absolute requirement for the early up-regulated expression of MAPK phosphatase-1 [MKP-1, also known as dual specificity phosphatase-1 (DUSP-1)], a prototypic counter-regulatory factor for the primary MAPKs (reviewed in ref. 17). These studies elucidate a MKP-1–dependent pathway by which NAbs inhibit innate inflammatory responses.
Results
NAb to ACs Inhibits LPS-Induced MAPK Activation and Up-Regulates MKP-1.
To begin to define the cellular mechanism of NAb-mediated inhibition of LPS stimulation, we evaluated the effects of anti-AC IgM on downstream MAPK signaling events in bone marrow-derived (BM) DCs. As previously documented, there are always sufficient amounts of apoptotic DCs in these cultures, making it unnecessary to add exogenous ACs (9). We initially confirmed that LPS stimulation of DCs induced phosphorylation of all primary MAPKs—p38, JNK, and ERK1/2, as well as ELK-1, a downstream nuclear transcription factor (Fig. 1A). Addition of the T15 anti-AC IgM in the presence of C1q, however, inhibited LPS-induced activation of these MAPKs and ELK-1. This activation pattern was unaffected by the addition of C1q alone or a control IgM that does not bind ACs. Furthermore, the inhibitory effect of the anti-AC IgM appeared to be specific for the MAPK pathway and, for example, had no effect on IFN-induced Janus-activated kinase activation (Fig. S1). As this anti-AC IgM can recruit several-fold higher levels of C1q onto ACs (9), these findings suggested that, in a setting of TLR-induced stimulation, anti-AC IgM antibody and C1q cooperatively down-modulate the inflammatory MAPK-signaling pathway.
Fig. 1.
Anti-AC IgM with LPS stimulation inhibits MAP kinase activation and induces MKP-1. (A) Immunoblot analysis of BM-DCs incubated for 30 min in serum-free media, under the indicated conditions, with IgM at 20 μg/mL and C1q at 80 μg/mL. Whole-DC extracts were used for all replicate immunoblots. Data are representative of five or more studies. (B) Quantitative transcript analysis from BM-DCs incubated in complete media with 10% (vol/vol) FBS. Data represent means from triplicates with values normalized by GADPH content at indicated time intervals. (C) Anti-AC IgM significantly inhibits LPS-induced TGF-β activation in DCs cultured under indicated conditions for 18 h (n = 6). Error bars indicate SD. **P < 0.01.
Due to its known regulatory role in TLR signaling (18), we next examined the expression of MKP-1. Confirming an earlier report (19), MKP-1 protein was only modestly induced by LPS at early time points (Fig. 2A), whereas at much later time points (i.e., 180 min) higher levels were associated with late negative feedback resolution of MAPK activation (20). In striking contrast, the addition of anti-AC IgM and C1q induced high levels of MKP-1 at early time points, peaking at ∼30 min (Figs. 1A and 2A).
Fig. 2.
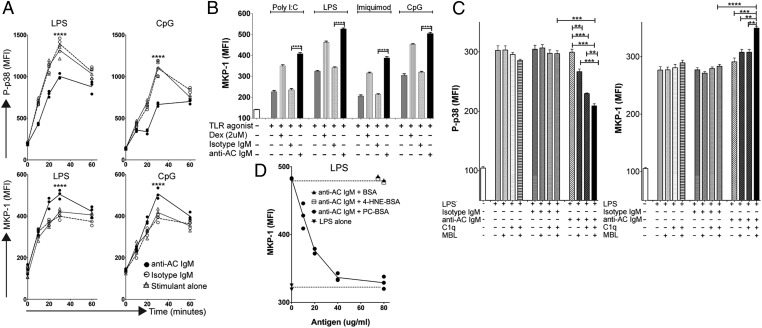
Kinetics and antigen specificity of anti-AC IgM inhibition of TLR-mediated p38 MAP kinase activation and MKP-1 induction. (A) Kinetic studies of intracellular levels of P-p38 (Upper panels) or MKP-1 (Lower panels) in DCs following exposure to anti-AC IgM and LPS (Left panels) or CpG (Right panels). Stimulations were performed in triplicates in serum-free media supplemented with C1q. Geometric mean fluorescence intensity (MFI) for MHCII-high DCs is shown (see Fig. S2 for gating). (B) MKP-1 induction by anti-AC IgM in response to agonists for diverse TLR. Results represent MFI mean ± SD for MHCII-high DCs from triplicate cultures after 30 min. (C) Inhibitory effects of anti-AC IgM on LPS-stimulated DCs requires C1q or MBL. Conditions are indicated for triplicate cultures at 30 min. (D) Concentration-dependent PC-antigen–specific inhibition of anti-AC IgM-mediated MKP-1 induction. DCs in serum-free media were cultured for 30 min without or with LPS under indicated conditions. Depicted values represent MFI from individual replicate cultures, with each condition performed in triplicate for MHCII-high DCs. C1q was used at 80 μg/mL and MBL at 20 μg/mL, based on pilot titration studies. Error bars indicate SD. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Upon further investigation, we found that costimulation using LPS and anti-AC antibody resulted in rapid early induction of MKP-1 transcripts with similar kinetics as protein expression (Fig. 1). This was highly selective as transcripts for MKP-5 (DUSP10), a related family member, and TLR4 were unaffected (Fig. 1B). Anti-AC IgM costimulation inhibited LPS-mediated induction of TNF-α and IL-10, as previously reported (12), and although TGF-β transcripts were not altered (Fig. 1B), levels of activated TGF-β protein were decreased (Fig. 1C). These findings suggested that anti-AC IgM inhibition of LPS stimulation is mediated by MKP-1 without involvement of IL-10 or TGF-β.
TLRs and Complement in NAb-Mediated MKP-1 Induction and p38 Inhibition.
To extend our findings to other TLRs, we used intracellular flow cytometry assays to identify individual cell changes in levels of P-p38 and MKP-1. After validating this approach using TLR4 (LPS) stimulation (Fig. 2 and Fig. S2), we examined MKP-1 responses for several other TLRs, including TLR9 (CpG), TLR3 [poly(I:C)], and TLR7 (imiquimod). In all instances, significant NAb-mediated inhibition was documented similar to what was observed for TLR4 (LPS) (Fig. 2 A and B). Furthermore, we could demonstrate a dose–response relationship for modulation of responses to the TLR agonists, as increasing concentrations of IgM anti-AC were associated with greater MKP-1 levels and less p38 activation (Fig. S3).
We next studied the contribution of the early complement recognition components, C1q and mannose-binding lectin (MBL), which themselves can enhance AC phagocytosis and play immunomodulatory roles (21). In flow cytometry assays, however, the addition of either MBL or C1q alone to LPS-stimulated DC cultures did not significantly affect P-p38 activation or translocation (Fig. 2C). In contrast, when combined with anti-AC IgM, the addition of MBL significantly reduced intracellular P-p38 kinase levels (P = 0.005), and the addition of C1q induced slightly more inhibition (P = 0.003), whereas the addition of both MBL and C1q resulted in even further reduction (P = 0.0001; Fig. 2C). A similar pattern of MKP-1 induction was also observed with the addition of MBL, C1q, or both (P = 0.008, P = 0.003, P < 0.0001, respectively; Fig. 2C). These findings support the dependence of this central signaling pathway on C1q or MBL.
Antigen-Specific Inhibition of Anti-AC IgM-Mediated Effects on MKP-1 Signaling.
The anti-AC antibody used in our studies was shown to bind PC-antigenic determinants exposed on ACs (7). To establish that the anti-AC IgM effects on signaling pathways were mediated by this antigen-specific interaction, LPS stimulation studies were performed at concentrations of anti-AC IgM that could be inhibited by soluble antigen (Fig. 2D). When PC–albumin was added, a concentration-dependent inhibition of anti-AC IgM-mediated MKP-1 was observed that plateaued at ∼40 μg/mL whereas no inhibition was obtained with control ligands (Fig. 2D). Hence, these data supported the hypothesis that the effects of this IgM antibody on MAPK signaling and MKP-1 are directly linked to the recognition of PC determinants.
Regulatory NAb Affects Spatiotemporal Localization of Primary MAPKs and MKP-1.
Phosphorylation of specific residues on primary MAPKs activates the kinases and induces translocation to nuclear sites where gene transcription is regulated (22). Accordingly, immunofluorescence studies confirmed that LPS stimulation of DCs dramatically increased both phosphorylation of p38 kinase and nuclear localization (Fig. 3). Strikingly, the addition of anti-AC IgM antibody and C1q, MBL, or C1q plus MBL significantly inhibited these changes in P-p38 concordant with the induction of elevated MKP-1 in the nuclei (Fig. 3). Again, MKP-1 levels were highest with the addition of both C1q and MBL (Fig. 3). By contrast, similar cocultures of LPS-stimulated DCs with anti-AC NAb, but without complement recognition molecules, had no inhibitory effect (Fig. 3). These findings were confirmed in quantitative immunofluorescence surveys of larger numbers of cells (Fig. S4). Anti-AC IgM in the presence of complement also significantly inhibited TLR-mediated nuclear accumulation of P-ERK1/2 and P-JNK (Fig. S4). Importantly, inhibition of antichromatin IgG immune complex-induced DC activation by anti-AC IgM/complement factor also correlated with MKP-1 induction (Fig. S5) and, as previously reported, with the inhibition of P-p38 (16).
Fig. 3.
Anti-AC IgM requires C1q and/or MBL for induction of nuclear MKP-1 and inhibition of nuclear localized P-p38 in LPS-stimulated DCs. Immunofluorescence studies for MKP-1 and P-p38 were performed on LPS-stimulated DCs cultured in serum-free media for 30 min under the indicated conditions. Staining used mouse anti–P-p38-Alexa488, rabbit anti–MKP-1 counterstained with anti-rabbit IgG-PE, and Hoechst dye. Data are representative of six or more independent experiments.
On the basis of earlier reports showing that anti-inflammatory corticosteroids induce sustained expression of MKP-1 (23), we investigated the effects of the potent glucocorticoid dexamethasone on DC costimulation. Our studies confirmed that LPS plus dexamethasone also induced higher levels and promoted nuclear localization of MKP-1, as well as reduced levels of p38 phosphorylation (Fig. 3; Fig. S6). Interestingly, we found that coexposure of LPS-stimulated DCs to glucocorticoid and anti-AC IgM antibody additively both increased MKP-1 levels and inhibited MAPK cascade activation (Fig. S6). Taken together, these findings demonstrated that, akin to the effects of dexamethasone, anti-AC in the presence of C1q and MBL increases levels of nuclear MKP-1 and blocks TLR-induced nuclear localization of the activated forms of primary MAPKs.
Serum Antibodies to AC-Associated Determinants Modulate TLR Responses.
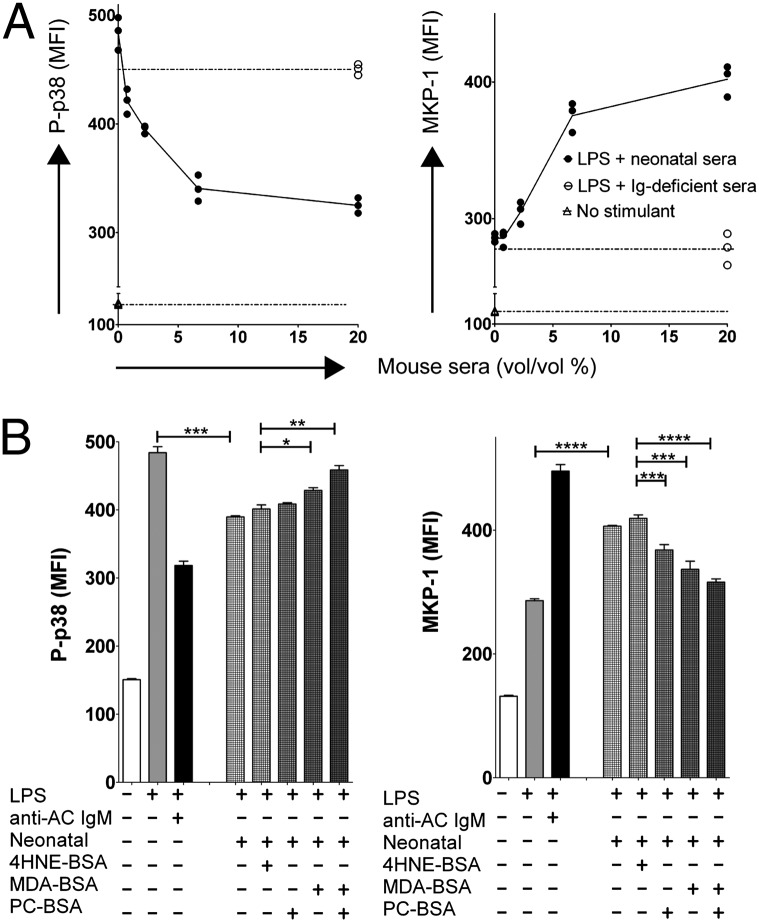
Anti-AC B-cells and antibodies expressing the T15 clonotype are highly represented even in the neonatal repertoire (24). To determine the activity of such serum NAbs on cell signaling, we compared the effects of sera from either immunocompetent or Ig-deficient muMT mice (Fig. 4 and Fig. S7). Similar to anti-AC IgM, both normal neonatal and adult sera demonstrated a dose-dependent inhibition of LPS-induced intracellular-activated P-p38 concordant with induction of MKP-1 levels, which reached a plateau at ∼7.5% (vol/vol) of sera (Fig. 4A and Fig. S7B). Sera from mice previously treated with infusions of ACs, which specifically raise levels of anti-AC IgM (9), displayed further induction of MKP-1 and reduction of P-p38 (Fig. S7). In contrast, sera from muMT mice had no effect.
Fig. 4.
Natural IgM antibodies with PC and MDA specificities in sera induce MKP-1 and inhibit P-p38 in LPS-stimulated BM-DCs. (A) Sera from neonatal mice inhibit P-p38 and induce MKP-1 in LPS-stimulated DCs. Coculture of LPS-stimulated DCs in serum-free media with C1q (80 μg/mL) for 30 min without or with the addition of titrated percentages of sera (% vol/vol) from 3-wk-old C57BL/6 neonates. (B) PC and MDA specificities in neonatal sera promote induction of MKP-1 and inhibition of P-p38. DCs in serum-free media were cultured for 30 min under indicated conditions. Replicates included serum (20%), after incubation for 1 h at 4 °C with PC-BSA, MDA-BSA, or 4HNE-BSA at 50 μg/mL or with T15-IgM, isotype control (20 μg/mL), or C1q (80 μg/mL). Intracellular flow cytometric mean fluorescence intensity (MFI) values are depicted for individual replicate cultures (n = 3). Error bars indicate SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To document that these effects were specifically mediated by serum IgM, we passed sera over anti-IgM Sepharose-conjugated columns before testing. IgM depletion reduced the P-p38 suppression observed with unfractionated sera (20% vol/vol) by 85 ± 6%, and the induction of MKP-1 was correspondingly reduced by 90 ± 3%. In contrast, addition of IgG-depleted neonatal sera minimally altered suppression of P-p38 induction (∼8% less), or the induction of MKP-1 (∼11% less) at the peak of response, compared with unfractionated neonatal sera or sera passed through a control Sepharose column.
In additional antigen-inhibition studies, we found that preincubation with either PC– albumin or MDA–albumin, but not with the control 4HNE–albumin, diminished NAb-mediated suppression and that coincubation with both PC– and MDA–albumin had an additive effect (Fig. 4 and Fig. S7B). Taken together, these findings suggested that IgM NAbs in sera are the predominant factors responsible for the observed modulation of inflammatory signaling pathways in DCs and that both PC- and MDA-reactive NAbs have this capacity.
MKP-1 Is Essential for Anti-AC IgM-Mediated Suppression of Inflammatory Responses.
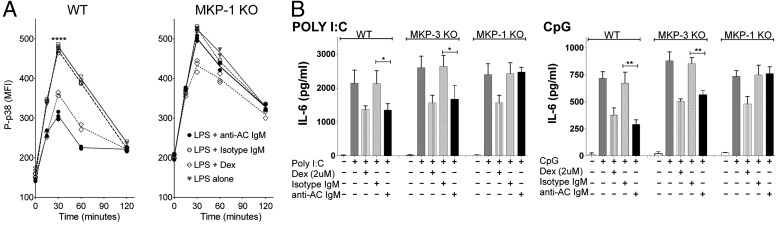
To investigate the relative contribution of MKP-1 to NAb-mediated suppression, we compared responses of DCs from wild-type or MKP-1–deficient mice. Such MKP-1–deficient mice are reported to exhibit overexuberant inflammatory responses, but no other immune developmental abnormalities (25, 26). Accordingly, MKP-1–deficient DCs demonstrated a slight elevation in baseline levels of P-p38 and a significant delay in the resolution of LPS responses at 120 min, but overall kinetics following stimulation with LPS were similar to wild-type DCs (Fig. 5A). Strikingly, the inhibitory effect of anti-AC IgM on P-p38 induction was essentially absent in MKP-1–deficient DCs, representing a ∼90% impairment (Fig. 5A and Fig. S8A). Immunofluorescence studies also showed a minimal increase in the expression and nuclear localization of P-p38 in MKP-1–deficient DCs after costimulation with LPS and anti-AC IgM (Fig. 3 and Fig. S8). Interestingly, dexamethasone inhibition of LPS induction of P-p38 was also reduced ∼50% in MKP-1–deficient DCs (Fig. 5A).
Fig. 5.
MKP-1 is required for anti-AC IgM-mediated inhibition of TLR signaling and inflammatory cytokine production. (A) Intracellular flow cytometric studies of the kinetics of LPS-induced P-p38 induction in wild-type (Left) and MKP-1–deficient DCs (Right). Standard conditions were used, as indicated in Fig. 2. (B) IL-6 production after 48 h in cultures without or with poly(I:C) (Left) or CpG DNA (Right) of wild-type, MKP-1, and MKP-3–deficient DCs in serum-free media under indicated conditions (n = 3). Error bars indicate SEM. *P < 0.05, **P < 0.01, ****P < 0.0001.
We also examined the effects of MKP-1 deficiency on induced cytokine responses. Compared with poly(I:C) or CpG alone, the addition of C1q and anti-AC IgM but not isotype control, significantly inhibited IL-6 cytokine production in DCs from wild-type or MKP-3 (DUSP6) knockout mice. By contrast, MKP-1–deficient DCs had complete loss of this anti-AC IgM-mediated inhibition (Fig. 5B). In additional studies in MKP-1–deficient DCs, even high concentrations of anti-AC IgM (i.e., 60 μg/mL) failed to inhibit imiquimod or LPS induction of IL-6 or IL-12p70 (Fig. S8). Dexamethasone also inhibited TLR-stimulated cytokine production in DCs from control mice, consistent with reports in other cell types (27), but, similar to its effect on P-p38, here inhibition was only partially dependent on MKP-1 (Fig. 5). These findings demonstrate the central role of MKP-1 in natural antibody-mediated suppression of TLR-mediated responses in DCs.
Discussion
Here we show that anti-AC IgM inhibits TLR activation of DCs by limiting the activation of the primary MAPKs that have been implicated in many pathologic inflammatory responses. We further observed that this NAb-induced inhibition is applicable to diverse TLRs, including TLR3, -4, -7, and -9. This NAb-mediated suppression requires C1q or MBL, and these functional properties are representative of the anti-inflammatory influences of circulating natural IgM antibodies. We did not find essential roles for the anti-inflammatory factors IL-10 and TGF-β. Importantly, this NAb-mediated process inhibits TLR signaling, thereby suppressing the potent activating effects of DNA, RNA, and other endogenous cellular TLR ligands present in ACs.
The most striking finding was that the regulatory influence of anti-AC IgM had an absolute requirement for MKP-1. MKP-1 is well known for its many counter-regulatory roles, which include the late negative feedback of responses to LPS stimulation, the blunting of responses after rapid re-exposure to a TLR agonist such as LPS tolerance (reviewed in ref. 17), as well as contributing to the anti-inflammatory properties of glucocorticoids (23). Accordingly, the anti-AC NAb induced earlier and enhanced expression of MKP-1 after TLR engagement, which appeared to effectively attenuate MAPK phosphorylation, such that the full activation and nuclear accumulation of the primary MAPKs were then never attained. This increase in MKP-1 protein level, which was associated with anti-AC IgM exposure, also correlated with the induction of higher levels of MKP-1 transcripts. Other studies have reported that the regulation of MKP-1 can be mediated through increased transcript half-life and ribosomal stabilization (28). However, the regulation of MKP-1 can also be affected by posttranslational modifications, by phosphorylation (29), or by acetylation that may increase phosphatase activity (30), each of which could be relevant to the current findings. Our studies also documented an additive effect of anti-AC IgM NAb and dexamethasone for early MKP-1 induction and inhibition of LPS-induced p38 MAPK activation that, when combined, exceeded the maximum effects of either agent alone. In part, this is likely explained by the additive integration of separate signals received via distinct cell membrane-associated receptors triggered by dexamethasone (i.e., glucocorticoid receptor) or by anti-AC IgM complexes (discussed below). As glucocorticoids are among the most widely prescribed treatments for inflammatory and autoimmune diseases, it is indeed intriguing that actively inhibitory signal transduction pathways of glucocorticoids are also evoked by complexes coordinated by IgM autoantibodies to oxidation-associated neo-determinants.
Our studies therefore characterize a previously unknown NAb regulatory pathway, which integrates and coordinates the influence of innate immune factors, which are much more ancient than immunoglobulins, on myeloid cell function. Indeed, orthologs of TLR were first characterized in insects (31), and the MAPK-signaling system may represent one of the earliest evolutionarily conserved pathways of immunity, being present in plants and mammals (32). Likewise, MKP-1 orthologs have also been described in protozoans (33), and in mice MKP-1 deficiency is associated with severe defects in the control of innate responses (34) and exacerbation of collagen-induced arthritis (26).
Fundamental to the inhibitory effects of regulatory NAbs, polymeric IgMs express both multiple sites for C1q interaction and high mannose glycoconjugates that bind MBL (35), which can greatly enhance the recruitment of C1q and MBL to AC complexes (9, 12), but these do not bind activating FcRs. Mice deficient in C1q, MBL, or secreted IgM have impaired control of inflammatory responses and, in some cases, autoimmune predisposition (36–39). The regulatory inhibitory signaling properties of the anti-AC IgM were shown to be dependent on the presence of C1q or MBL, two soluble macromolecules of the innate immune system that share a common primordial genetic ancestor (40). When deposited on the surface of an AC, these represent “eat me” signals that enhance apoptotic clearance (41); however, we found that by themselves these recognition molecules demonstrated no significant activity in our assays. Furthermore, we have previously shown that the complement-dependent immunomodulatory properties of anti-AC do not have an absolute requirement for downstream activation of the complement cascade (12). Yet, a number of candidate receptors for C1q and MBL themselves have been implicated in innate immune cell interactions (21), and determining whether IgM recruited C1q/MBL on the AC can interact with one or more of these receptors will be investigated in the future.
We postulate that the herein described NAb anti-inflammatory regulatory pathway provides advantages for the control of excess and chronic inflammatory responses that could otherwise result in tissue injury, including those from pathologic autoimmune responses. Any condition that leads to an increased burden of ACs may then induce a focused B-cell response and production of higher anti-AC IgM levels, which then act to re-establish a homeostatic balance. Indeed, an increase in the production, or an impairment of phagocytic clearance, of ACs is a common component of many chronic inflammatory conditions, including murine models of accelerated atherogenesis in which anti-AC natural antibodies have been implicated in protective responses (42, 43). With the current elucidation of a direct anti-inflammatory effect of this type of natural antibody, our studies provide a plausible explanation for the recent finding that high levels of PC-specific IgM in humans correlate with protection from myocardial infarction and stroke in at-risk clinical populations (44, 45). In addition, as nucleic acid-containing immune complexes are postulated to contribute to the pathogenesis of autoimmune diseases, such as systemic lupus erythematosus (SLE), our findings that these responses are modulated by anti-AC IgM may explain recent evidence from surveys of SLE patients that higher anti-AC NAb levels correlated with lower disease activity and organ injury (44). The cellular source of these protective IgM antibodies may have been conserved during immune evolution, as IgM anti-PC antibodies have recently been reported to also be predominantly produced by human B-1 cells (46).
In conclusion, our findings therefore provide a molecular rationalization for the mechanism by which anti-AC natural antibodies can trigger an inhibitory pathway that can dampen pathogenic inflammatory responses that include TLR responses. On the basis of the cumulative data, this natural-antibody system appears to act at a fundamental choke point in the MAPK system that also contributes to resetting and normalization of innate responses. We speculate that this results from the organization by the IgM–NAb of a complex synapse on phagocytes involving cell membrane receptors for ACs and for MBL/C1q, integrins, and, potentially, other molecules (Fig. 6) (discussed in ref. 8). Taken together, our studies therefore provide evidence that anti-AC IgM, which is a prominent component from the primitive B-1 repertoire within the adaptive immune system (2), has the capacity to co-opt and amplify counter-regulatory pathways that control the much more ancient MAPK system. This regulatory natural-antibody system may also provide therapeutic opportunities for the treatment of a broad range of common diseases.
Fig. 6.
Model of anti-AC IgM-mediated suppression of TLR responses. IgM antibodies to determinants on ACs recognize and form complexes with ACs (or AC microparticles), which recruit high levels of C1q and MBL. This AC–immune complex forms an interaction with DCs that may involve integrins, receptors for C1q and MBL, and other AC receptors. In quiescent DCs, these complexes may result in little or no effects on MAPK signaling. However, in the context of stimulation by agonists for endosomal (TLR3, -7, and -9) or membrane-associated (TLR4) innate immune receptors, there is an integration of signals with those from NAb–MBL/C1q–AC complexes that result in high nuclear expression of the anti-inflammatory phosphatase MKP-1. This pathway contributes to inhibition of the activation and nuclear localization of the primary MAP kinases and their downstream proinflammatory substrates.
Materials and Methods
Antibodies and Complement.
T15-IgM (from the EO6 hybridoma) (42) and the IgM isotype control have previously been described (9, 12). Purified IgMs were documented to be low or undetectable for endotoxin (<0.5 EU/mg), and aliquots were stored at −80 °C. Purified C1q was from Quidel and MBL from S. Thiel (University of Aarhus, Aarhus, Denmark).
Mice.
Age and sex-matched adult C57BL/6, congenic B-cell–deficient muMT mice were provided by the Jackson Laboratory, and MKP-1−/− were the kind gift of Charles Lowenstein (University of Rochester, Rochester, NY) (30). Mice were bred under specific pathogen-free conditions as supervised by the University of California San Diego and New York University Animal Care Program under approved protocols by the Institutional Animal Care and Use Committees of those institutions.
Immunoblot.
BM-DC (SI Materials and Methods) lysates were prepared in RIPA buffer with proteinase inhibitor and phosphatase inhibitor mixture (Roche), run on 4–12% precast gels (Invitrogen), and transferred onto PVDF membrane (Invitrogen).
Intracellular Flow Cytometry Studies.
BM-DCs were cultured in replicates (SI Materials and Methods). After cell stimulation, the cells were centrifuged and resuspended in Cytofix/Cytoperm solution (Becton Dickinson), followed by PermWash solution (Becton Dickinson) as per manufacturer’s protocol. Cells were stained for P-p38, MKP-1 and costained with anti-MHCII to identify high-TLR-responsive cells as described (16).
Statistical analysis used the two-tailed t test using Welsh correction when appropriate; P < 0.05 was considered significant (Instat; GraphPad).
Studies were repeated 3–12 times. Additional information is in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Paraskevi Briassouli for TGF-β analysis. This work was supported by National Institutes of Health Grants R01AI090118, R01 AI068063, American Recovery and Reinvestment Act supplement, and R01AI090118; the American College of Rheumatology Research Education Foundation Within Our Reach campaign; the Alliance for Lupus Research; the Arthritis Foundation; and The P. Robert Majumder Charitable Trust.
Footnotes
Conflict of interest statement: G.J.S. is the inventor on a patent application submitted by the University of California at San Diego. C.G., Y.C., J.V., S.K., S.T., M.C., and D.H.K. declare that they have no competing interests.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211868109/-/DCSupplemental.
References
- 1.Ehrenstein MR, Notley CA. The importance of natural IgM: Scavenger, protector and regulator. Nat Rev Immunol. 2010;10(11):778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 2.Chou MY, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119(5):1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: A key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26(4):347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 4.Cancro MP, Sigal NH, Klinman NR. Differential expression of an equivalent clonotype among BALB/c and C57BL/6 mice. J Exp Med. 1978;147(1):1–12. doi: 10.1084/jem.147.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satow Y, Cohen GH, Padlan EA, Davies DR. Phosphocholine binding immunoglobulin Fab McPC603: An X-ray diffraction study at 2.7 A. J Mol Biol. 1986;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- 6.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26(4):385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 7.Shaw PX, Goodyear CS, Chang MK, Witztum JL, Silverman GJ. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J Immunol. 2003;170(12):6151–6157. doi: 10.4049/jimmunol.170.12.6151. [DOI] [PubMed] [Google Scholar]
- 8.Silverman GJ. Regulatory natural autoantibodies to apoptotic cells: Pallbearers and protectors. Arthritis Rheum. 2011;63(3):597–602. doi: 10.1002/art.30140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182(10):6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38(4):259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 11.Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur J Immunol. 2005;35(1):252–260. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol. 2009;183(2):1346–1359. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21(5):643–653. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Baccala R, et al. Sensors of the innate immune system: Their mode of action. Nat Rev Rheumatol. 2009;5(8):448–456. doi: 10.1038/nrrheum.2009.136. [DOI] [PubMed] [Google Scholar]
- 16.Vas J, Grönwall C, Marshak-Rothstein A, Silverman GJ. Natural antibody to apoptotic cell membranes inhibits the proinflammatory properties of lupus autoantibody immune complexes. Arthritis Rheum. 2012;64(10):3388–3398. doi: 10.1002/art.34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases: Regulating the immune response. Nat Rev Immunol. 2007;7(3):202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 18.Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75(3):487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 19.Chen P, et al. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169(11):6408–6416. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- 20.Hu JH, et al. Feedback control of MKP-1 expression by p38. Cell Signal. 2007;19(2):393–400. doi: 10.1016/j.cellsig.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44(1–3):33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Reiser V, Ammerer G, Ruis H. Nucleocytoplasmic traffic of MAP kinases. Gene Expr. 1999;7(4–6):247–254. [PMC free article] [PubMed] [Google Scholar]
- 23.Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol. 2002;22(22):7802–7811. doi: 10.1128/MCB.22.22.7802-7811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sigal NH, Pickard AR, Metcalf ES, Gearhart PJ, Klinman NR. Expression of phosphorylcholine-specific B cells during murine development. J Exp Med. 1977;146(4):933–948. doi: 10.1084/jem.146.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorfman K, et al. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene. 1996;13(5):925–931. [PubMed] [Google Scholar]
- 26.Salojin KV, et al. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176(3):1899–1907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- 27.Maier JV, et al. Dual specificity phosphatase 1 knockout mice show enhanced susceptibility to anaphylaxis but are sensitive to glucocorticoids. Mol Endocrinol. 2007;21(11):2663–2671. doi: 10.1210/me.2007-0067. [DOI] [PubMed] [Google Scholar]
- 28.Kuwano Y, et al. MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol Cell Biol. 2008;28(14):4562–4575. doi: 10.1128/MCB.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brondello JM, Pouysségur J, McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286(5449):2514–2517. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- 30.Cao W, Bao C, Padalko E, Lowenstein CJ. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med. 2008;205(6):1491–1503. doi: 10.1084/jem.20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 32.Asai T, et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415(6875):977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 33.Moncho-Amor V, Galardi-Castilla M, Perona R, Sastre L. The dual-specificity protein phosphatase MkpB, homologous to mammalian MKP phosphatases, is required for D. discoideum post-aggregative development and cisplatin response. Differentiation. 2011;81(3):199–207. doi: 10.1016/j.diff.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Q, et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203(1):131–140. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnold JN, Dwek RA, Rudd PM, Sim RB. Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol Lett. 2006;106(2):103–110. doi: 10.1016/j.imlet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Boes M, et al. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci USA. 2000;97(3):1184–1189. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Botto M, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19(1):56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 38.Ehrenstein MR, O’Keefe TL, Davies SL, Neuberger MS. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc Natl Acad Sci USA. 1998;95(17):10089–10093. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174(6):3220–3226. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 40.Matsushita M, et al. Origin of the classical complement pathway: Lamprey orthologue of mammalian C1q acts as a lectin. Proc Natl Acad Sci USA. 2004;101(27):10127–10131. doi: 10.1073/pnas.0402180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogden CA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194(6):781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw PX, et al. Natural antibodies with the T15 idiotype may act in athero-sclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105(12):1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binder CJ, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9(6):736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 44.Grönwall C, et al. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin Immunol. 2012;142(3):390–398. doi: 10.1016/j.clim.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su J, et al. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology (Oxford) 2008;47(8):1144–1150. doi: 10.1093/rheumatology/ken120. [DOI] [PubMed] [Google Scholar]
- 46.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70−. J Exp Med. 2011;208(1):67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.