Abstract
Interbacterial interaction pathways play an important role in defining the structure and complexity of bacterial associations. A quantitative description of such pathways offers promise for understanding the forces that contribute to community composition. We developed time-lapse fluorescence microscopy methods for quantitation of interbacterial interactions and applied these to the characterization of type VI secretion (T6S) in Pseudomonas aeruginosa. Our analyses allowed a direct determination of the efficiency of recipient cell lysis catalyzed by this intercellular toxin delivery pathway and provided evidence that its arsenal extends beyond known effector proteins. Measurement of T6S apparatus localization revealed correlated activation among neighboring cells, which, taken together with genetic data, implicate the elaboration of a functional T6S apparatus with a marked increase in susceptibility to intoxication. This possibility was supported by the identification of T6S-inactivating mutations in a genome-wide screen for resistance to T6S-mediated intoxication and by time-lapse fluorescence microscopy analyses showing a decreased lysis rate of recipient cells lacking T6S function. Our discoveries highlight the utility of single-cell approaches for measuring interbacterial phenomena and provide a foundation for studying the contribution of a widespread bacterial interaction pathway to community structure.
Keywords: imaging, peptidoglycan, polymicrobial, protein secretion
Although bacteria are often considered single-celled organisms, most possess complex mechanisms to facilitate intercellular information exchange. Some of these, such as quorum sensing, operate at a distance and coordinate the production of diffusible commodities (1, 2). Recent work has revealed evolutionarily distinct pathways present in Gram-negative bacteria that are predicted to function at much closer length scales (3, 4), including Tra-mediated lipoprotein exchange in Myxococcus xanthus (5) and the widely distributed contact-dependent inhibition and type VI secretion systems (T6SSs) (6, 7). These pathways can exert dramatic phenotypic changes in neighboring cells, and thus they likely play important roles in the development, composition, and homeostasis of bacterial communities.
The T6SS is a complex secretory apparatus with the ability to translocate effector proteins between Gram-negative bacterial cells (8, 9). Our knowledge of T6S effector protein function derives primarily from studies of the Pseudomonas aeruginosa Hcp Secretion Island I-encoded T6SS (H1-T6SS). This pathway delivers at least three effector proteins, Tse1–3 (type VI secretion exported 1–3), to the periplasm of recipient Gram-negative bacteria. Two of these proteins, Tse1 and Tse3, act within this compartment by hydrolyzing peptidoglycan, whereas Tse2 accesses the cytoplasm where it induces stasis (8, 10, 11). In addition to targeting other bacteria, cells can deliver effectors to their own kind in a process termed “intercellular self-intoxication.” To avert the harmful consequences of this process, cells possess specific immunity proteins, Tsi1–3 (type VI secretion immunity 1–3), which bind to and inactivate cognate effectors.
The T6SS displays a broad target range; however, there are limits to its activity. As cell wall-degrading type VI secretion (T6S) effectors require compartmentalization in the periplasm to exert effects on recipient cells, their action is restricted to Gram-negative organisms (12). There is also evidence for disparate susceptibilities to T6S-dependent intoxication among Gram-negative species (13–15), though the basis for this has remained unknown.
Quantitative time-lapse microscopy has revolutionized the way in which bacterial populations can be characterized (16, 17). By measuring phenomena at the single-cell level, cell subpopulations not discernable in traditional population studies can be resolved. Here we developed quantitative time-lapse fluorescence microscopy (TLFM) tools for the study of T6S-mediated interactions between P. aeruginosa and two Gram-negative species, Burkholderia thailandensis and Salmonella enterica serovar Typhimurium. Using this technique, we resolve key questions pertaining to spatiotemporal characteristics and potency of T6S-mediated intercellular lysis. We further show that coordinated T6S apparatus assembly between neighboring cells leads to a “double-edged sword” phenomenon: T6S activation within a cell leaves it more susceptible to T6S-based intoxication by its competitor.
Results
Quantitative Single-Cell Analysis of Interbacterial Interactions.
We developed a customized Matlab-based software package for quantitative single-cell analyses of interspecies bacterial interactions. Like related tools, this package identifies bacteria from phase images using a watershed algorithm, performs cell tracking, links cells between frames (allowing lineage analysis), and can identify and track fluorescent subcellular foci representing punctate protein localization patterns (17, 18). Fig. 1 provides an overview of our analysis methods. We added to these capabilities a number of analysis modules tailored to the unique questions that we sought to address in this study. Specifically, we endeavored to quantitatively describe T6S-dependent interactions between two species of bacteria in a spatially and temporally resolved manner.
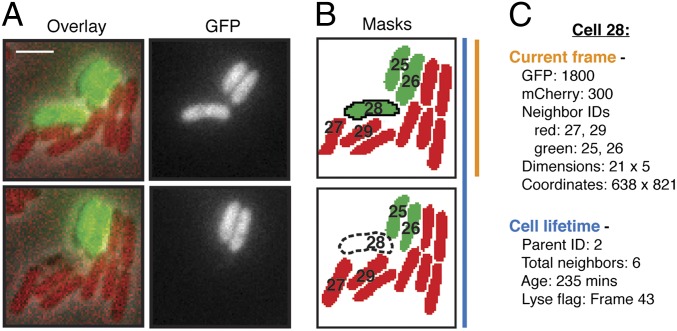
Fig. 1.
Overview of TLFM-based intercellular interaction analyses. (A) Consecutive cropped frames from a typical TLFM experiment in which two populations of differentially labeled cells (B. thailandensis attTn7::gfp and P. aeruginosa attTn7::mCherry) were cocultured and imaged. The overlay includes phase, GFP, and mCherry channels. (Scale bar, 2 μm.) (B) Cell masks generated by image segmentation. Red and green coloring corresponds to distinct subpopulations identified by gating on fluorescence intensity. (Lower) Dashed outline depicts a software-identified lysis event. Hypothetical cell identification numbers are shown. (C) Example of cell-specific values measured during automated analysis. Certain data are recorded once and pertain to the cell lifetime as a whole (blue); other parameters vary between frames (orange).
Cell lysis is a known consequence of intoxication by the H1-T6SS (12); thus, we used this readily observable process as a means of tracking T6S activity. Given that cells undergoing lysis release their cytoplasmic contents, we defined a lytic event as a precipitous drop of cellular fluorescence between frames. Using an iterative approach, we defined the optimal fluorescence decrease (minimizing false positive and negative observations) at 80% and required that this drop be maintained beyond a single frame (Fig. 1A). We implement the lysis detection module post segmentation so that information including the identity and cellular neighborhood of lysed cells can be analyzed. A second functionality that we introduced to the software is the capacity to distinguish cells of a population on the basis of fluorescence intensity (Fig. 1B). Following segmentation, the average fluorescence intensity in each channel within each cell mask is calculated and background is subtracted. This feature allows for gating of donor and recipient cells on the basis of the expression of a unique fluorescent protein—green and monomeric cherry fluorescent proteins (GFP and mCherry, respectively) in this study. The combination of recorded cell-specific measurements with frame-to-frame cell linking, lysis detection, and donor/recipient identification allows measurement of T6S-dependent effects between mixed populations of bacteria in a spatially and temporally resolved manner (Fig. 1C).
Spatiotemporal Analysis of T6S-Dependent Interactions.
To our knowledge, there are no reports describing the direct visualization of T6S-dependent interactions between two species of bacteria. We initially chose to investigate the P. aeruginosa–B. thailandensis donor–recipient pair, as both organisms are genetically tractable, thrive in similar temperature and nutrient conditions, and serve as models for understanding the T6SS in our laboratory. To increase the number of measurable T6S-dependent interactions occurring under the short timescale of our studies, we used the P. aeruginosa ∆retS parental background in all experiments (19, 20). In this background, which has been extensively used for the study of the H1-T6SS, Tse1–3 export is constitutive and the expression of genes associated with sessile P. aeruginosa is stimulated at the posttranscriptional level (10, 21, 22). Homogeneous mixtures of P. aeruginosa attTn7::mCherry and B. thailandensis attTn7::gfp were inoculated onto growth medium-infused agarose pads and observed using TLFM. Control experiments were performed with P. aeruginosa bearing a mutation known to inactivate the H1-T6SS (∆tssM1) (23). Initial inspection of image sequences revealed apparent phenomenology attributable to T6SS activity. Most strikingly, we observed extensive lysis of B. thailandensis grown with P. aeruginosa, but not with P. aeruginosa ∆tssM1 (Fig. 2A; Fig. S1; Movies S1 and S2).
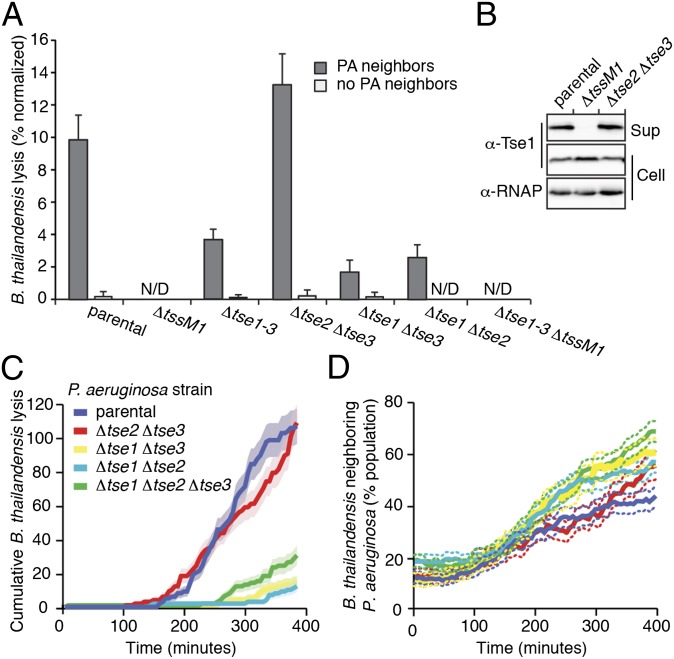
Fig. 2.
The P. aeruginosa H1-T6SS causes dose- and contact-dependent lysis of recipient cells. (A) TLFM image sequence of a P. aeruginosa–B. thailandensis coculture. B. thailandensis and P. aeruginosa are GFP- and mCherry-labeled, respectively. Outlined blue cells on masks lyse in subsequent frame. Arrowheads point to B. thailandensis cells that lyse following P. aeruginosa contact. Fig. S1 shows the corresponding ΔtssM1 control. (Scale bar, 6 μM.) (B and C) Lysis quantification from TLFM experiment depicted in A. Either percentage of lysis of total cellular population (B) or distinct lysis counts for indicated bacterial populations (C). Error bars depict standard counting error. N/D, none detected. (D and E) B. thailandensis (D) or S. Typhimurium (E) lysis increases linearly with increased contact to P. aeruginosa. Red dashed and solid black lines depict total cells and percentage lysis, respectively. Results represent data pooled from two independent experiments.
Next, we quantitatively analyzed TLFM sequences of P. aeruginosa–B. thailandensis cocultures using our custom image analysis software. Consistent with our initial visual inspection, automated analyses indicated significantly higher cellular lysis in cocultures containing the parental P. aeruginosa strain than in those bearing the tssM1 deletion (Fig. 2 A and B). To determine which of the two species was undergoing lysis, we gated cells using green and red fluorescence levels to identify the constituents of the lysed cell population. This analysis revealed that the P. aeruginosa parental and ∆tssM1 strains experience a consistently low basal lysis rate, whereas B. thailandensis undergoes lysis only in the presence of P. aeruginosa with a functional T6SS (Fig. 2C; Movies S3 and S4). Importantly, switching the fluorescent proteins used to mark the two organisms did not significantly impact the number of detected lysis events (Fig. S2).
The T6SS has been suggested to function as a cell contact-dependent puncturing device (24, 25). Data supporting this contention derive from studies wherein donor and recipient populations are separated by a synthetic membrane of many microns in thickness (13); thus, whether the T6SS includes an appendage that allows it to act at a distance between cells—as is often speculated and depicted—has not been addressed experimentally. To investigate this question with higher resolution, we measured the lysis of individual B. thailandensis cells as a function of contact with P. aeruginosa in a TLFM sequence. Of the 116 B. thailandensis lysis events recorded, 114 occurred within cells in immediate contact (<200 nm) with P. aeruginosa (SI Materials and Methods). This observation strongly suggests that the T6S apparatus does not function via an extended extracellular appendage.
The efficiency of T6S intoxication has also not been measured directly. Population-level studies provide a sensitive means to assess the contribution of T6S to fitness; however, without resolving cell contact and its duration, the potency of the system cannot be directly ascertained from these experiments. To probe the efficiency of H1–mediated intoxication, we measured the occurrence of recipient cell lysis as a function of contact duration with P. aeruginosa. We calculated contact duration such that 10 min of contact with a single P. aeruginosa donor cell would be equivalent to 5 min of contact with two cells. B. thailandensis lysis displayed a linear (R2 = 0.96) dose dependence over a large range of P. aeruginosa contact (0–150 contact minutes), suggesting that successful delivery of effectors is a rare event, that cells require multiple payloads to lyse, or both (Fig. 2D). On the basis of our analysis, we determined that P. aeruginosa lyses B. thailandensis cell populations at a rate of 5.2%/h of contact. To understand how this rate compares with the efficiency of H1-T6S–mediated lysis of other bacteria, we performed similar TLFM analyses of P. aeruginosa–S. Typhimurium cocultures (Movies S5 and S6). P. aeruginosa stimulated lysis of S. Typhimurium in a contact-, H1-T6SS-, and dose-dependent manner (R2 = 0.97); however, the efficiency of lysis was significantly lower against this organism (0.44%/h) (Fig. 2E). This may in part be due to a prolonged spheroplast intermediate that precedes H1-T6SS–catalyzed lysis of S. Typhimurium (Movie S5).
The H1-T6SS is thought to act on recipient cells through the combined actions of its known effectors, Tse1–3 (10). We measured the contribution of the individual H1-T6SS effectors to the observed interspecies cell lysis phenotype. To circumvent the potential complications of synergy, we generated a panel of P. aeruginosa single effector strains. B. thailandensis lysis incurred during growth with the single effector strains was compared with that promoted by the parental strain, a strain lacking all three known effectors, and the ∆tssM1 strain using our TLFM analysis method. This revealed that Tse1 is responsible for the majority (80%) of H1-T6SS–dependent lysis of B. thailandensis and S. Typhimurium (Fig. 3A and Fig. S3). Comparison of Tse1 levels in parental and ∆tse2 ∆tse3 culture supernatants indicated that the high lytic activity of the ∆tse2 ∆tse3 background is not the result of compensatory effector release (Fig. 3B). Surprisingly, our results showed that neither Tse2 nor Tse3 contribute to Tse1-independent H1-T6SS–catalyzed B. thailandensis lysis. Indeed, P. aeruginosa cells lacking all known H1-T6SS effectors caused lysis of B. thailandensis with slightly greater efficiency than strains possessing only Tse2 or Tse3 (Fig. 3A). Introduction of the tssM1 deletion to the ∆tse1–3 background abrogated recipient lysis, ruling out the possibility that this strain acquired H1-T6SS–independent lytic activity. The lysis rate of B. thailandensis catalyzed by each strain was also examined. Tse1-dependent and -independent lysis mechanisms displayed temporal separation, with Tse1 inducing recipient cell lysis earlier (Fig. 3 C and D). Together, our data conclusively demonstrate the occurrence of interspecies antagonistic interactions mediated by the T6SS under conditions suitable for single-cell study. Our analyses show that H1-T6SS–dependent lysis requires direct donor–recipient cell contact and that the efficiency of recipient cell intoxication varies significantly between two species of proteobacteria. Finally, we discovered a late-acting, Tse1–3-independent mechanism of the H1-T6SS that promotes lysis of recipient cells.
Fig. 3.
Tse1 acts early to promote the majority of H1-T6SS–catalyzed B. thailandensis lysis. (A) Lysis quantification from TLFM sequences of B. thailandensis coculture with the indicated P. aeruginosa strains. Error bars, ± counting error (C.E.) (B) Western blot analysis of Tse1 expression in supernatant (Sup) and cell-associated (Cell) fractions of the indicated P. aeruginosa strains. RNA polymerase (RNAP) was used as a loading control. (C) Running total of frame-by-frame B. thailandensis lysis events observed in coculture experiments with the indicated P. aeruginosa strains. Shading corresponds to C.E. (D) Neighbor-cell analysis of cocultures in C indicating similar neighbor density for each experiment. Dotted lines of corresponding color depict C.E.
Adjacent Cells Influence T6S Apparatus Assembly and Localization.
Our ability to quantitatively measure T6S-dependent cellular interactions under TLFM conditions motivated us to extend our analyses to include the localization properties of the T6S apparatus. To visualize the secretory apparatus, we examined a P. aeruginosa strain expressing clpV1–gfp from the native clpV1 locus (23). As reported previously, we observed that localization of this conserved AAA+ family ATPase is highly dynamic—oscillating between a diffuse cytoplasmic pattern and filamentous and punctate structures, representing active and inactive forms of the T6S apparatus, respectively (Movie S7) (21).
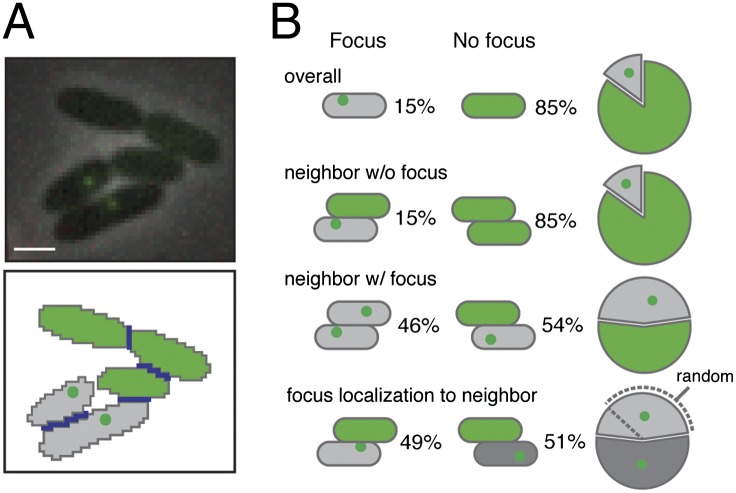
It has been proposed that nondiffuse ClpV1–GFP structures represent conformational states in the assembled phage tail-like T6S apparatus (21, 26). As a first step toward determining the functional significance of ClpV1 localization properties, we measured the occurrence and subcellular localization of ClpV1–GFP puncta within cells as a function of neighbor cell characteristics (Fig. 4A). Because T6S-dependent self-intoxication is well documented and occurs efficiently, we performed this analysis in P. aeruginosa monocultures. On the basis of automated focus identification within 12,233 cells, we determined the probability of a given cell displaying a focus to be 15.3 ± 0.27% (Fig. 4B and SI Materials and Methods). The probability of observing a focus in the subpopulation of cells with neighbors was similar (14.9 ± 0.37%, P value = 0.38), suggesting that apparatus assembly is not activated by cell–cell contact under the conditions of our assay. However, when we examined the subpopulation of cells with observed foci, the probability of neighboring cells displaying foci increased dramatically to 46 ± 2.4% (P value < 0.0001). We also searched for spatial correlations in T6S assembly within neighboring cells. Although the presence of a neighboring cell did not influence assembly of the apparatus, we detected a significant bias in the subcellular localization of the apparatus—a bias favoring localization to cell–cell interfaces (Pcalc, 32 ± 7.1 × 10−2%, Pobs, 49 ± 3.6%, P value < 0.0001). In summary, our data indicate that H1-T6SS activation is correlated among neighboring cells and suggest that neighbor cells recruit the T6S apparatus to cell interfaces. Our results are in alignment with the recent observation that T6S assembly between adjacent cells is spatially and temporally linked (21).
Fig. 4.
Assembly and localization of the T6S apparatus is influenced by neighboring cells. (A) Overlay of phase and GFP channels of P. aeruginosa cells expressing clpV1-gfp (Upper) with corresponding cell masks (Lower). (Scale bar, 1 μm.) Cell–cell interfaces (blue lines) and foci (green dots) were identified and quantified to determine observed and predicted conditional probabilities (SI Materials and Methods). (B) Schematic depicting conditions for which probabilities of T6S apparatus localization were determined with paired results. Gray cells with green dots and filled green cells represent observations of localized or diffuse ClpV1-GFP, respectively.
Efficient Intoxication by the T6SS Requires a Functional T6S Apparatus in Recipient Cells.
One explanation for the heightened probability of observing concurrent T6SS assembly in neighboring cells is that assembly proceeds more efficiently in neighbor A if neighbor B itself possesses an assembled apparatus. We term this the “sensing model.” An alternative explanation is that T6SS activation is highly sensitive to undetermined microenvironmental fluctuations that cells A and B experience concomitantly. Given our earlier observations linking T6SS assembly to activity, a paradoxical prediction of the sensing model is that T6SS activity should contribute both to intoxication of neighbors and to apparent susceptibility to intoxication: the lack of a functional T6SS in a recipient cell would lead to a failure to stimulate T6S activation in a donor, thereby decreasing the extent of recipient targeting.
To test this prediction of the sensing model, we measured the effect of an HSI-I gene cluster deletion on susceptibility to T6S-mediated intoxication using interbacterial competition assays. Interestingly, inactivation of the H1-T6SS dramatically improved the competitive fitness of a Tse2-susceptible background of P. aeruginosa (∆tse2 ∆tsi2) (Fig. 5A and Fig. S4) (10). According to the sensing model, the protection afforded by T6S inactivation should not be specific to a particular effector. Indeed, we found that the HSI-I deletion also granted increased fitness to a strain susceptible to Tse3-dependent intoxication (Fig. 5B) (8). Many mechanisms could explain the apparent T6S resistance accompanied by an HSI-I deletion. To determine whether it is specifically the activity of the T6S apparatus in the recipient that increases targeting efficiency, we next examined the effect of the clpV1 deletion on recipient fitness. The ClpV1 protein stimulates T6S activity by promoting turnover of the apparatus (25, 27). As anticipated, clpV1 deletion increased the fitness of the Tse2-susceptible strain, and, furthermore, susceptibility of the strain was returned to parental levels by genetic complementation of the clpV1 gene. Although the clpV1 deletion provided a statistically significant increase in fitness to the ∆tse2 ∆tsi2 strain, our data indicate that it does so to a lesser extent than a deletion of the entirety of HSI-I, and neither of these deletions restores fitness to that of a fully immune strain. Notably, clpV1 deletions are known to retain partial T6SS activity (8, 14, 15); thus, we find that cellular targeting efficiency correlates to T6S activity in recipient cells.
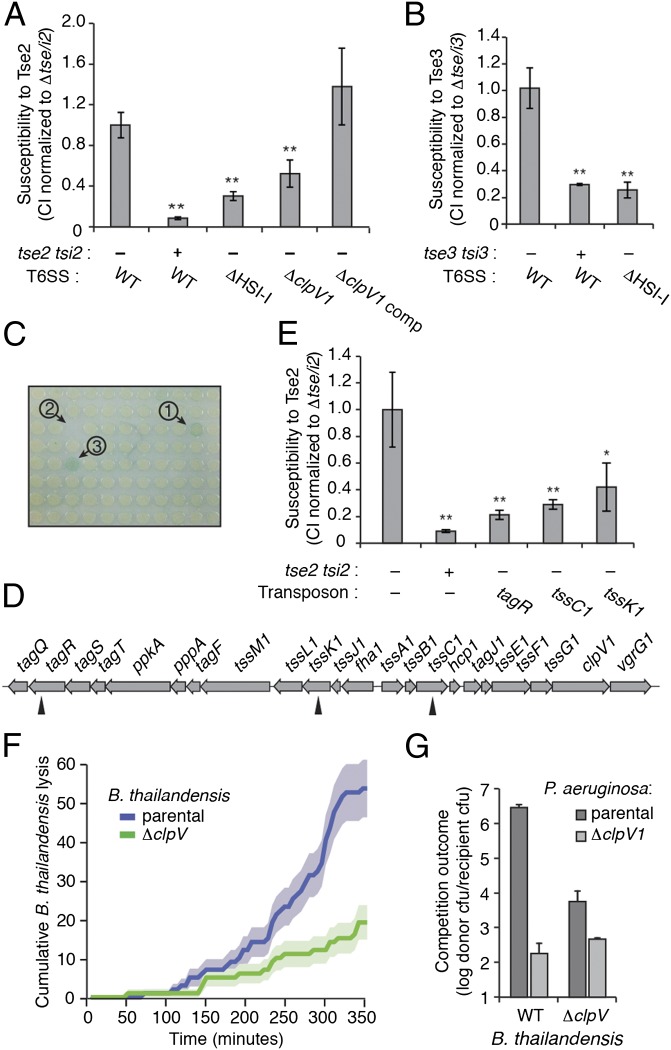
Fig. 5.
Efficient intoxication by the T6SS requires a functional T6S apparatus in recipient cells. (A and B) Outcome of growth competition experiments between the parental and P. aeruginosa recipient strains. The presence (+) or absence (–) of tse2 tsi2 (A) and tse3 tsi3 (B) in the recipient strain and the genetic status of the H1-T6SS cluster (WT, wild-type) are indicated. Fitness differences were not observed in control experiments performed under T6S nonconducive conditions (Fig. S4). Competitive index (CI) is defined as the change (final/initial) in ratio of donor and recipient colony-forming units normalized to the susceptible control (A, ∆tse/i2; B, ∆tse/i3). (C) Representative 96-well plate used in screen for H1-T6SS resistance. Each plate contained a positive control (1) and a no-growth control (2). This plate also includes a positive hit (3). (D) Schematic of the H1-T6SS gene cluster with identified Tn insertion sites indicated (arrowheads). A complete list of mutants is provided in Table S1. (E) Outcome of growth competition experiments between the parental and indicated P. aeruginosa strains normalized as described for A. (F) Frame-by-frame running total of lysis events from a TLFM sequence in which the indicated B. thailandensis strain was cocultured with parental P. aeruginosa. Shading indicates counting error. (G) Outcome of growth competition experiments between the indicated strains. Asterisks mark competition outcomes significantly different from the corresponding effector immunity-deficient strain (*P < 0.05; **P < 0.01). Error bars, ±SD. n = 3.
We also interrogated the sensing model in a less biased fashion by performing a genome-wide screen for transposon (Tn) insertions within P. aeruginosa that lead to resistance to T6S-based intoxication. Using a 96-well interbacterial competition assay format, we screened 2,748 random Tn insertions in the P. aeruginosa ∆retS ∆tse2 ∆tsi2 attB::lacZ background (recipient) for resistance to intoxication by P. aeruginosa ∆retS (donor). Positive clones were selected visually on the basis of LacZ activity (Fig. 5C). We identified and mapped 14 independent Tn insertions (hit rate, 0.5%) that conferred T6S resistance in our screen (Table S1). Remarkably, three of these were located within the HSI-I gene cluster (Fig. 5D). Seven additional insertions mapped to the gacA and gacS genes, which encode known positive regulators of the H1-T6SS (19). The genes immediately disrupted by Tn insertions within HSI-I include tagR, tssC1, and tssK1. Prior studies indicate that each of these is essential to T6S function (13, 28, 29). Quantitative growth competition assays with the Tn insertion strains verified that they display significant increased fitness relative to the parental (Fig. 5E).
Having demonstrated that efficient T6S-dependent intercellular self-intoxication requires an active T6SS within recipient cells, we next probed whether the requirement for T6S in recipient cells extended to interactions between bacterial species. Our laboratory previously found that T6SS-1 of B. thailandensis targets bacterial cells (13). Furthermore, under standard laboratory conditions, T6SS-1 is the only one of five T6SSs in B. thailandensis that exports effectors to levels detectable using a sensitive mass spectrometry assay used by our laboratory. Given these data, we reasoned that T6SS-1 inactivation might provide B. thailandensis resistance to intoxication by the H1-T6SS of P. aeruginosa. To test this, we compared the lysis rates of wild-type and T6SS-1–inactive (∆clpV) B. thailandensis strains grown with P. aeruginosa using our single-cell TLFM analysis method. Similar to our findings in self-intoxication studies, T6S inactivation in B. thailandensis provided significant resistance to T6S-dependent lysis by P. aeruginosa (Fig. 5F). We also performed population-level lysis and growth competition assays, which further confirmed that T6SS-1 inactivation protects B. thailandensis against the H1-T6SS of P. aeruginosa (Fig. 5G and Fig. S5). Overall, our TLFM analyses and genetic data support the hypothesis that T6S activation is transmitted between neighboring bacterial cells. The consequence of this positive feedback loop is that T6S apparatus activation exposes a cell to heightened intoxication.
Discussion
Our data lead us to hypothesize that T6S is a double-edged sword. The activity of T6S within a bacterial cell exposes that cell to increased T6S-mediated intoxication by its neighbors. This effect could be widespread, as we find that inactivating mutations in the system confer apparent resistance to both intra- and interspecies H1-T6SS–mediated attack. Interestingly, a similar phenomenon has been observed with type IV secretion (T4S). Binns and colleagues showed that the transfer efficiency of an IncQ plasmid between Agrobacterium tumefaciens cells is increased dramatically when both donor and recipient possess the Ti plasmid (30), which encodes the T4S apparatus. As observed in our study, this effect required the function of many essential components of the secretion system within the recipient cell.
The molecular mechanisms underlying the double-edged sword effect of T6S remain incompletely understood; however, our quantitative analysis of the effect of neighboring cells on T6S activation and localization, combined with the observation of “T6SS dueling” made recently by the Mekalanos laboratory, support the hypothesis that an activated T6SS within a cell triggers T6S activation in its neighbors (21). Further consistent with this hypothesis, we do not observe complete resistance by T6S inactivation, mutations impact resistance in a manner proportional to their effect on T6S apparatus function, and resistance is not effector specific. It is important to emphasize that our data do not predict that bacteria lacking a T6SS are immune to T6S-mediated attack. The potency of T6S is also likely determined by other factors such as the general suitability of the organism for T6S apparatus-mediated protein transfer and its inherent susceptibility to the particular cargo of effectors. For example, we have shown that the H1-T6SS promotes relatively efficient lysis of an Escherichia coli K-12 derivative that lacks T6S (12).
What is the mechanism of coupled T6S activation? We observe that susceptibility to T6S intoxication of a strain lacking tse1–3 remains T6S-dependent, suggesting that effector proteins, or at least those currently known, are not required for sensing. We speculate that coupling is achieved by homo- or heterotypic interactions between extracellular apparatus components of adjacent cells. These may either induce a signaling cascade leading to T6S activation or simply act as a nucleation point for the further assembly of the apparatus.
Whether coupled T6S activation has an adaptive role, and whether this benefits the donor or recipient, remains to be determined. It is noteworthy that the net consequence of T6S inactivation in B. thailandensis is unfavorable to the organism. This implies that the offensive capacity of B. thailandensis T6SS-1, at least against P. aeruginosa, is greater in magnitude under the conditions of our assay than the detrimental consequences of additional exposure to the effectors of the P. aeruginosa H1-T6SS. Coupled T6S activation could provide a means for cells to avoid wasteful T6S activation. In this scenario, an exogenous T6SS may act as a cue to the donor that a susceptible cell is in its vicinity. This is conceivable given the broad distribution of T6S among Gram-negative bacteria.
The unexpected finding that ∆tse1–3 promotes T6S-dependent B. thailandensis lysis suggests that either there are lytic H1-T6S effector(s) in addition to Tse1–3 that act on B. thailandensis or that the H1-T6S apparatus itself can induce lysis. If the apparatus were sufficient to cause lysis, we would predict extensive lysis—regardless of effector immunity—occurring among cells undergoing intercellular self-intoxication. However, we did not find evidence of T6S-mediated lysis between adjacent P. aeruginosa cells in our experiments. Furthermore, we did not observe an increase in lysis of P. aeruginosa cells that neighbored B. thailandensis, despite the latter also possessing a functional T6SS. Thus, our results suggest that Tse1–3 do not encompass the complete effector repertoire of the H1-T6SS. The delayed kinetics of Tse1-independent lysis may explain why previous studies failed to identify H1-T6SS effectors beyond Tse1–3 (10). The temporal regulation of such effector(s) may be distinct from that of Tse1–3, or these substrates may be present at reduced levels.
To our knowledge, a quantitative single-cell analysis of interspecies bacterial interactions has not yet been reported. Here we used this method to characterize T6S-dependent processes; however, the technology could be applicable to many important questions in the field of microbiology. For example, it could be used to examine the spatiotemporal regulation of phenotypes modulated by the presence of other bacteria including motility, growth rate, and the production of virulence factors. With increased automation, the method could also be implemented as a discovery tool for identifying processes influenced by neighboring cells, including novel interbacterial interaction mechanisms. There is growing recognition of the health, agricultural, and environmental consequences of emergent properties within bacterial communities. Single-cell analyses of mixed populations offer a means to decipher the complex interactions that underlie these traits.
Materials and Methods
DNA Manipulation and Growth Conditions.
P. aeruginosa, B. thailandensis, and S. Typhimurium strains were generated using PAO1 (31), E264, and LT2 backgrounds, respectively. Gene deletions within P. aeruginosa and B. thailandensis were generated in frame using allelic exchange. A detailed description of growth conditions and strain construction can be found in SI Materials and Methods.
Microscopy.
Microscopy images were acquired using a Nikon Ti-E inverted microscope fitted with a 60× oil objective, automated focusing (Perfect Focus System, Nikon), a Xenon light source (Sutter Instruments), a CCD camera (Clara series, Andor), a custom environmental chamber, and image acquisition software (NIS Elements, Nikon). For TLFM experiments, P. aeruginosa cells cultivated overnight were washed in a Luria broth (LB) formulation low in NaCl (8) and mixed with either B. thailandensis or S. Typhimurium at a ratio of 1:1 or 1:2, respectively. The bacterial suspension (1 μL) was spotted onto growth pads prepared from Vogel Bonner minimal media containing 2.5% agarose wt/vol, 0.2% wt/vol sodium nitrate, and a 19-amino-acid mixture defined in ref. 32. For snapshot images, log-phase bacteria were spotted. Inoculated growth pads were hermetically sealed using rubber gaskets coated with vaseline, paraffin, and lanolin at 1:1:1. For time-lapse series, automated image acquisition was performed at 5-min intervals for 4–8 h.
TLFM Analysis.
Cells were identified using a watershed segmentation algorithm and linked between frames using custom Matlab software (2012a, Mathworks). Distinct populations within an experiment were identified using empirically defined fluorescence intensity gates. Debris (noncells) was size-excluded and fluorescence-excluded. Automated lysis detection flagged cells in which a decrease in mean cellular fluorescence of >80% occurred between consecutive frames. Contact duration was calculated by summing the number of donor cells in contact with a recipient in each frame over the lifetime of that cell. Unless otherwise indicated, results represent a single experiment typically using two fields of view (10,000–20,000 cells). Each experiment was repeated at least three times independently. Focus probability analysis and additional details regarding lysis detection and neighbor identification are located in SI Materials and Methods.
Screen for T6SS Susceptibility Determinants.
P. aeruginosa ∆retS ∆tse2 ∆tsi2 attB::lacZ was mutagenized using Tn5 as described in ref. 33. Mutants (recipients) were inoculated into 96-well plates containing 200 μL LB and propagated overnight at 37 °C. An equal volume of P. aeruginosa ∆retS (donor) overnight culture was added to each well, and the mixture was spotted onto LB 3% wt/vol indicator (40 μg/mL 5-bromo-4-chloro-indolyl-β-d-galactopyranoside) plates. Each plate contained a positive control (recipient, ∆retS attB::lacZ). Strains containing transposon insertions that conferred resistance to the donor generated a blue spot. These were isolated from the competition spot, and arbitrary PCR was performed as described previously (34) to determine transposon insertion sites.
Supplementary Material
Acknowledgments
We thank M. Stewart, B. Cookson and H. Schweizer for plasmids and G. Davis and B. Kulasekara for help with microscopy. This work was supported by grants to J.D.M. from the National Institutes of Health (NIH) (Grants AI080609 and AI057141) and the Cystic Fibrosis Foundation (Grant CFR565-CR07); and to P.A.W. from the University of Washington Royalty Research Fund, National Science Foundation (NSF) (Grants PHY-084845 and MCB-1151043), and the Sloan Foundation. M.L. was supported by NIH Cellular and Molecular Training Grant GM07270 and A.B.R. by NSF Grant DGE-0718124. J.A.D.L. was a Mary Gates Scholar. J.D.M. holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213963109/-/DCSupplemental.
References
- 1.Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 2.Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 3.Blango MG, Mulvey MA. Bacterial landlines: Contact-dependent signaling in bacterial populations. Curr Opin Microbiol. 2009;12(2):177–181. doi: 10.1016/j.mib.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes CS, Aoki SK, Low DA. Bacterial contact-dependent delivery systems. Annu Rev Genet. 2010;44:71–90. doi: 10.1146/annurev.genet.42.110807.091449. [DOI] [PubMed] [Google Scholar]
- 5.Pathak DT, et al. Cell contact-dependent outer membrane exchange in myxobacteria: Genetic determinants and mechanism. PLoS Genet. 2012;8(4):e1002626. doi: 10.1371/journal.pgen.1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoki SK, et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature. 2010;468(7322):439–442. doi: 10.1038/nature09490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell AB, et al. A widespread bacterial type VI secretion effector superfamily identified using a heuristic approach. Cell Host Microbe. 2012;11(5):538–549. doi: 10.1016/j.chom.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell AB, et al. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475(7356):343–347. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverman JM, Brunet YR, Cascales E, Mougous JD. Structure and regulation of the type VI secretion system. Annu Rev Microbiol. 2012;66:453–472. doi: 10.1146/annurev-micro-121809-151619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hood RD, et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe. 2010;7(1):25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, et al. Structural basis for type VI secretion effector recognition by a cognate immunity protein. PLoS Pathog. 2012;8(4):e1002613. doi: 10.1371/journal.ppat.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou S, et al. Structure of a peptidoglycan amidase effector targeted to Gram-negative bacteria by the type VI secretion system. Cell Rep. 2012;1(6):656–664. doi: 10.1016/j.celrep.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz S, et al. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog. 2010;6(8):e1001068. doi: 10.1371/journal.ppat.1001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci USA. 2010;107(45):19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murdoch SL, et al. The opportunistic pathogen Serratia marcescens utilises type VI secretion to target bacterial competitors. J Bacteriol. 2011;193(21):6057–6069. doi: 10.1128/JB.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locke JC, Elowitz MB. Using movies to analyse gene circuit dynamics in single cells. Nat Rev Microbiol. 2009;7(5):383–392. doi: 10.1038/nrmicro2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sliusarenko O, Heinritz J, Emonet T, Jacobs-Wagner C. High-throughput, subpixel precision analysis of bacterial morphogenesis and intracellular spatio-temporal dynamics. Mol Microbiol. 2011;80(3):612–627. doi: 10.1111/j.1365-2958.2011.07579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein J, et al. TLM-Tracker: Software for cell segmentation, tracking and lineage analysis in time-lapse microscopy movies. Bioinformatics. 2012;28(17):2276–2277. doi: 10.1093/bioinformatics/bts424. [DOI] [PubMed] [Google Scholar]
- 19.Brencic A, Lory S. Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Mol Microbiol. 2009;72(3):612–632. doi: 10.1111/j.1365-2958.2009.06670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman AL, et al. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev Cell. 2004;7(5):745–754. doi: 10.1016/j.devcel.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Basler M, Mekalanos JJ. Type 6 secretion dynamics within and between bacterial cells. Science. 2012;337(6096):815. doi: 10.1126/science.1222901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hachani A, et al. Type VI secretion system in Pseudomonas aeruginosa: Secretion and multimerization of VgrG proteins. J Biol Chem. 2011;286(14):12317–12327. doi: 10.1074/jbc.M110.193045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mougous JD, et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006;312(5779):1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leiman PG, et al. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci USA. 2009;106(11):4154–4159. doi: 10.1073/pnas.0813360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basler M, Pilhofer M, Henderson GP, Jensen GJ, Mekalanos JJ. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature. 2012;483(7388):182–186. doi: 10.1038/nature10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mougous JD, Gifford CA, Ramsdell TL, Mekalanos JJ. Threonine phosphorylation post-translationally regulates protein secretion in Pseudomonas aeruginosa. Nat Cell Biol. 2007;9(7):797–803. doi: 10.1038/ncb1605. [DOI] [PubMed] [Google Scholar]
- 27.Bönemann G, Pietrosiuk A, Diemand A, Zentgraf H, Mogk A. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. EMBO J. 2009;28(4):315–325. doi: 10.1038/emboj.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu F, Schwarz S, Mougous JD. TagR promotes PpkA-catalysed type VI secretion activation in Pseudomonas aeruginosa. Mol Microbiol. 2009;72(5):1111–1125. doi: 10.1111/j.1365-2958.2009.06701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng J, Leung KY. Dissection of a type VI secretion system in Edwardsiella tarda. Mol Microbiol. 2007;66(5):1192–1206. doi: 10.1111/j.1365-2958.2007.05993.x. [DOI] [PubMed] [Google Scholar]
- 30.Bohne J, Yim A, Binns AN. The Ti plasmid increases the efficiency of Agrobacterium tumefaciens as a recipient in virB-mediated conjugal transfer of an IncQ plasmid. Proc Natl Acad Sci USA. 1998;95(12):7057–7062. doi: 10.1073/pnas.95.12.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stover CK, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406(6799):959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 32.Palmer KL, Aye LM, Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol. 2007;189(22):8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez-García E, Calles B, Arévalo-Rodríguez M, de Lorenzo V. pBAM1: An all-synthetic genetic tool for analysis and construction of complex bacterial phenotypes. BMC Microbiol. 2011;11:38. doi: 10.1186/1471-2180-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castang S, Dove SL. Basis for the essentiality of H-NS family members in Pseudomonas aeruginosa. J Bacteriol. 2012;194(18):5101–5109. doi: 10.1128/JB.00932-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.