Abstract
Protein acetylation is an important posttranslational modification with the recent identification of new substrates and enzymes, new links to disease, and modulators of protein acetylation for therapy. α-tubulin acetyltransferase (αTAT1) is the major α-tubulin lysine-40 (K40) acetyltransferase in mammals, nematodes, and protozoa, and its activity plays a conserved role in several microtubule-based processes. Here, we present the X-ray crystal structure of the human αTAT1/acetyl-CoA complex. Together with structure-based mutagenesis, enzymatic analysis, and functional studies in cells, we elucidate the catalytic mechanism and mode of tubulin-specific acetylation. We find that αTAT1 has an overall fold similar to the Gcn5 histone acetyltransferase but contains a relatively wide substrate binding groove and unique structural elements that play important roles in α-tubulin–specific acetylation. Conserved aspartic acid and cysteine residues play important catalytic roles through a ternary complex mechanism. αTAT1 mutations have analogous effects on tubulin acetylation in vitro and in cells, demonstrating that it is the central determining factor of α-tubulin K40 acetylation levels in vivo. Together, these studies provide general insights into distinguishing features between histone and tubulin acetyltransferases, and they have specific implications for understanding the molecular basis of tubulin acetylation and for developing small molecule modulators of microtubule acetylation for therapy.
Keywords: crystallography, enzymology
The posttranslational modification of lysine acetylation has been extensively studied in the context of gene regulation: Acetylated histone substrates have been mapped, enzymes responsible for catalysis has been characterized, function has been ascribed to specific modifications, and pathologies have been associated with improper acetylation (1–3). Although acetylation may have once been considered a modification unique to gene regulatory mechanisms, more recent studies have identified thousands of nonhistone substrates, thus placing acetylation alongside phosphorylation as an important posttranslational modification in its prevalence and wide-ranging functionality (4, 5). The tubulin subunits of microtubules are acetylated, and lysine-40 (K40) of the α-tubulin subunit has been identified as an important conserved site of microtubule acetylation (6–8). This modification is considered a hallmark of stable, long-lived microtubules (7) and α-tubulin acetyltransferase (αTAT1)/Mec-17 was recently identified to acetylate K40 on α-tubulin, with no detectable acetylation of histone substrates (9, 10). Additional studies have shown microtubule acetylation to affect the kinesin-1 motor based trafficking in mammalian cells, the rate of primary cilium assembly, and the mechanosensation ability of Caenorhabditis elegans (9, 11, 12). These findings provide unique insight into microtubule acetylation and its biological implications. Despite these advances, the molecular determinants differentiating microtubule acetylation from the more ubiquitous histone acetylation remained unclear. Here we report the molecular characterization of the αTAT1 acetyltransferase.
Results
Overall Structure of the αTAT1/Acetyl-CoA (AcCoA) Complex.
The optimal protein construct for crystallization and structure determination was guided by our previous biochemical characterization of αTAT1 (9). An expression construct encoding residues 2–236 of human αTAT1 was shown to retain almost 100% of microtubule acetyltransferase activity relative to the full-length protein, and secondary structure prediction algorithms suggested that the C terminus of the protein, residues ∼230–333, were disordered (9). We prepared selenomethionine-derivatized αTAT1(2-236) protein, obtained crystals of the protein bound to AcCoA, collected single wavelength anomalous dispersion data, phased the structure by using the selenium anomalous signal, and refined the structure to 2.2 Å resolution (Table S1).
The refined αTAT1/AcCoA structure reveals continuous electron density from amino acid 3 to 195, except for two short unresolved loops (residues 26–36 and 84–91) (Fig. 1A). Although the expression construct used for crystallization contained ∼40 residues C-terminal to the last resolved residue, and confirmed by mass spectrometry to be present in the crystals, this portion of the protein was not observed in the electron density map. Based on this result, we conclude that residues 196–236 are disordered in the crystals. Residues 3–195 of αTAT1 adopt an overall fold that shares many common features with acetyltransferases of the GNAT family, despite the low primary sequence similarity (Fig. 1 B and C). This fold includes a structurally conserved core region comprised of β-strands 1, 2, 3, 6, and 7 and α-helix 3, but more structurally variable regions flanking this core (Fig. 1B). The AcCoA cofactor is wedged in a groove between the α1 and α4 helices and against the α3 helix and β6 strand analogous to the position of the cofactor bound to other GNAT proteins; however, specific AcCoA interactions are largely mediated by residues that are not conserved within the GNAT family (Fig. 1C and Fig. S1).
Fig. 1.
Structure of the αTAT1/AcCoA complex. (A) Cartoon representation of the complex. α-helices (orange) are numbered 1–4, β-strands (blue) are numbered 1–7, loops are colored green, and the N and C termini are indicated. AcCoA is represented as sticks and colored according to element: carbon, yellow; nitrogen, blue; and oxygen, red. (B) Alignment of αTAT1 (cyan) to Tetrahymena Gcn5 (PDB ID code 1QSN; magenta) in the same orientation and AcCoA rendering for αTAT1 as in A. The functionally important α1-α2-loop, β4-β5-hairpin, and C-terminal regions of αTAT1 are labeled. (C) Sequence alignment of αTAT1 from Homo sapiens αTAT1 (Hs), Mus musculus (Mm), Rattus norvegicus (Rn), Danio rerio (Dr), C. elegans (Ce), and Tetrahymena thermophilia Gcn5 (TtGcn5). Numbering and secondary structural elements above the sequence alignment is for HsαTAT1. Magenta squares highlight residues mutated in these studies, green hexagons highlight conserved aspartic acid and cysteine residues important for catalysis, and cyan circles highlight residues that make contacts with the AcCoA cofactor through either their side chain or backbone atoms.
The structurally variable regions of αTAT1 that flank the structurally conserved core are of particular interest because corresponding regions of the Gcn5 GNAT protein mediate substrate-specific interactions with histone H3, therefore implicating these regions in α-tubulin–specific binding (13). One side of the conserved core is flanked by a 12-residue β-hairpin formed by β-strands 4 and 5 (herein called β4-β5 hairpin) that is directed away from the protein and is adjacent to a C-terminal loop that follows the α4 helix (Fig. 1A). The β-hairpin shows a high degree of sequence conservation among the αTAT1 proteins with β4 largely hydrophobic (LFVLD), the turn largely charged (DDREA) and β5 mixed hydrophobic and charged (HNEV) (Fig. 1 A and C). The corresponding region in other GNAT proteins such as Gcn5 contains a short nonconserved loop that connects β-strands of the conserved core domain (Fig. 1B) (13). Residues 180–184 at the tip of the αTAT1 C-terminal loop are wedged between the core region and the β4-β5 hairpin segment and contain one of the most highly conserved patches of sequence conservation among the αTAT1 proteins (Fig. 1C). Consistent with the potential importance of these residues for α-tubulin specific binding is the observation that it overlaps with a Gcn5 loop that plays an important role in histone H3 binding (Fig. 1B) (13).
Analysis of the α1-loop-α2 region (herein called α1-α2 loop) on the other side of the core also reveals a unique structural arrangement unlike that found in other GNAT family members. In αTAT1, α1 terminates at Gln58, which caps the pantetheine arm of AcCoA to its binding pocket and forms a seven-residue-long loop that contains αTAT1 conserved hydrophobic and small polar residues that connect to the α2-helix (Fig. 1C). Because the corresponding region of the ternary Gcn5/CoA/H3 complex mediates histone specific binding, this region of αTAT1 may also participate in tubulin-specific binding (Fig. 1B).
Implications for Catalysis.
Aspartic acid 157 was proposed to play an important role in catalysis by αTAT1 (9). This residue is located at the C terminus of β7 with its side chain oriented toward the AcCoA cofactor and approximately 6 Å away (Fig. 2A), consistent with its participation in catalysis. Interestingly, we also observe a cysteine residue, C120, approximately 8 Å away from AcCoA, suggesting that this residue might also participate in catalysis. Consistent with the proposed catalytic roles of D157 and C120 is their strict conservation among αTAT1 orthologs (Fig. 1C). Although GNAT proteins have not been shown to use cysteine residues for catalysis, they have been shown to play a catalytic role in the MYST family of acetyltransferases, which have been proposed to use a ping-pong mechanism of catalysis (14).
Fig. 2.
Catalytic site of αTAT1 and bisubstrate kinetics. (A) Cartoon representation of the catalytic site of αTAT1. The coloring scheme and labeling is the same as in Fig. 1, with the addition of sulfur colored in magenta. Residues surrounding the catalytic site are labeled for frame of reference. (B) Microtubule acetylation progress curves for WT, C120, and D157 mutant αTAT1. Curves fit to D157 mutants are shown as dashed lines because of a poor fit. (C) Bisubstrate kinetics of αTAT1 acetylation. Experiments were performed at four different AcCoA concentrations, 25, 12, 10, and 5 μM, and a double reciprocal plot of 1/velocity versus 1/[MT] (microtubule) is shown. A best fit of the plot displays a plot with the lines intersecting at a common x-intercept indicative of a ternary complex mechanism.
To confirm and probe the functional importance of D157 and C120, respectively, we mutated these residues and compared the catalytic properties of the corresponding protein mutants to the wild-type (wt) enzyme. As can be seen in Fig. 2B, whereas the wt enzyme showed kinetic parameters similar to previously reported values (kcat = 21.29 × 10−4 s−1, Km = 31.44 μM) for a microtubule substrate, the enzymes that harbored the single D157N, D157E, C120A, and C120S mutations showed significantly reduced activity. These results support the strict requirement of D157 and C120 for catalysis by αTAT1. Interestingly, another conserved aspartic acid residue, D123 (Fig. 1C), was also found to be located in close proximity to the AcCoA molecule (Fig. 2A); however, mutation of this residue to either alanine or asparagine resulted in a highly unstable protein, precluding our ability to analyze the potential catalytic role of this residue.
Although the overall fold of αTAT1 more closely resembles the GNAT proteins and the Gcn5 histone acetyltransferase in particular, which each use a ternary mechanism for catalysis, the requirement of a cysteine for catalysis shows similarity to the MYST family of histone acetyltransferases (14, 15). To probe the catalytic mechanism of αTAT1, we performed bisubstrate kinetics under steady-state conditions as a function of microtubule concentration and at different AcCoA cosubstrate concentrations. As can be seen in Fig. 2C, a plot of the data in the form of a double reciprocal plot reveals a series of straight lines that intersect at a common point, indicative of a ternary complex mechanism (16). In this mechanism, αTAT1 forms a complex with both α-tubulin and AcCoA before acetyl transfer to K40, but would not form an acetyl-Cys reaction intermediate, as has been observed with the ESA1 MYST enzyme (14). Coupled with the functional results that both D157 and C120 are required for catalysis and the structural observation that both of these residues would be in position to deprotonate the incoming lysine substrate (either directly or through a bound water molecule), we propose that both residues function as general bases for catalysis. Having two residues function as general bases for catalysis is not unprecedented because both serotonin acetyltransferase (two histidine residues) and the Naa50p amino terminal acetyltransferase (glutamate and tyrosine residues) have been proposed to use two general base residues (17, 18).
Implications for α-Tubulin–Specific Acetylation.
αTAT1 was shown to specifically acetylate K40 of α-tubulin with a strong preference for a microtubule context and to have no detectable acetylation activity toward histone substrates (9). Because the histone tails that are acetylated by histone acetyltransferases are very basic, harboring many lysine and arginine residues, it is no surprise that the histone substrate binding sites for these enzymes are largely acidic or apolar (13, 19). In contrast, the electrostatic surface of αTAT1 proximal to the tip of AcCoA, where the α-tubulin substrate must bind, is very basic (Fig. 3A). This observation is consistent with the inability of αTAT1 to acetylate histone substrates. The structure of the α/β-tubulin heterodimer bound to stathmin (PDB ID code 1FFX) is also consistent with αTAT1 acetylating K40 because K40 is located on a surface loop and flanked by residues that are largely small and apolar or acidic (..DGQMPSDK40TIGGGDD..) (20).
Fig. 3.
The αTAT1 a-tubulin substrate binding site and mutational analysis. (A) Electrostatic surface potential mapping of αTAT1 as generated by Pymol. Red, blue, and white represent acidic, basic, and hydrophobic patches, respectively. AcCoA is colored as in Fig. 1. (B and C) The ability of the αTAT1 mutants to acetylate microtubules was assessed by using an in vitro acetyltransferase assay. Experiments were performed at microtubule concentrations ranging from 0.5 μM to 50 μM to obtain kinetic parameters (kcat and Km) necessary to calculate catalytic efficiency (Table S2). Experiments were performed at least three times. Shown in A and B are the amount of acetylated microtubules formed by using 20 μM microtubules. B contains only β-hairpin mutants, and C contains mutants from the α1-α2 loop, C120, and C-terminal loop domains. Statistical analysis was performed by using an unpaired two-tailed t test in GraphPad Prism. *P < 0.05 and **P < 0.01. (D) Surface representation of αTAT1 highlighting acetylation defective (orange) and enhancing (magenta) mutants.
To probe regions of αTAT1 that might be specifically important for α-tubulin–specific binding and acetylation, we focused on the regions of αTAT1 proximal to the putative substrate-binding site that showed the most significant structural differences with the GNAT proteins and histone acetyltransferases (Fig. 1B). These regions include the β4-β5 hairpin (residues 105–117) and C-terminal loop (residues 181–184) on one side of the putative substrate binding site, and the α1-α2 loop segment (residues 58–67) on the other side (Fig. 1B). Specifically, we prepared several recombinant αTAT1 proteins bearing single alanine mutations of αTAT1 conserved residues in one of these three protein regions and purified each of the proteins to homogeneity. Only proteins that purified similarly to the wt protein, including similar elution profiles on gel filtration, were considered properly folded and used for enzyme studies using the preferred microtubule substrate.
The enzyme studies revealed that mutation of the hydrophobic residues in β-strand 4 (F105, V106, L107, and D108) or charged residues in β-strand 5 (E115 and E117) had diminished activity relative to the wt protein (Fig. 3 B and D and Table S2). A mutation of the basic residue R110 in the β-hairpin loop also showed reduced activity; however interestingly, mutation of two of the acidic residues in this loop (D109 and E111) showed slightly increased activity, with the E11A mutant showing approximately twofold more activity relative to the wt protein (Fig. 3 B and D and Table S2). A D109A/E111A double mutant, however, did not result in a synergistic increase in αTAT1 activity, a charge reversal mutation (acidic to basic) of these two residues only showed a marginal increase in activity for the D109R mutant, and no change in activity was observed for the E111R mutant (Fig. 3A and Table S2). Taken together, these results are consistent with the participation of the β4-β5 hairpin of αTAT1 in α-tubulin–specific acetylation (Fig. 3A and Table S2).
The enhanced activity of the E111A and D109A mutants for a microtubule substrate relative to wt suggested that these residues might help to regulate the enzymes access to the lumen of microtubules. To test this hypothesis, we assayed the activity of these two mutants, along with the debilitating F105A control mutant, by using GDP stabilized αβ-tubulin dimers and microtubules as substrates. We observed that the E111A and D109A mutants had increased activity, whereas the F105A mutant showed decreased activity relative to wt when microtubules or αβ-tubulin dimers were used as substrates (Fig. S2 A and B). This data demonstrates that the E111A and D109A mutants do not play a significant role in differentiating between tubulin and microtubules or regulate lumen entry.
In the C-terminal loop adjacent to the β4-β5 hairpin, N182 and F183 are oriented toward the substrate binding cleft, thus suggesting that they might also participate in α-tubulin–specific binding and acetylation. We found that mutation of these residues to alanines resulted in greatly diminished activity relative to the wt enzyme (Fig. 3 C and D and Table S2). Similarly, we found that mutation of certain residues in the α1-α2 loop region (I64A and R69A) also showed reduced microtubule acetylation (Fig. 3 B and D and Table S2). These residues are well resolved in the structure, are positioned opposite the catalytic base (D157), and have their side chains oriented toward the catalytic pocket. S61 and R74 (in the loop after α2), while surface exposed, are at a slightly greater distance from the center of the catalytic pocket and mutation of these residues had a more modest effect on enzyme activity (Fig. 3B and Table S2).
We found it interesting that residues 196–236 of αTAT1 were disordered in the crystal structure because K233 was reported to be acetylated, suggesting that K233 acetylation does not play a regulatory role for catalysis, similar to autoacetylation sites in the Rtt109 and MYST histone acetyltransferases (HATs) that are highly ordered in the corresponding crystal structures (15, 21). To address a possible regulatory role of regions of αTAT1 that are C-terminal to 236, we expressed both αTAT1 (2–236) and αTAT1-FL (residues 2–333) as N-terminal GST fusion proteins (because the untagged αTAT1-FL protein was unstable to purification) for activity measurements. As shown in Fig. S3, this comparison reveals that both protein constructs have comparable activities, demonstrating that regions C-terminal to αTAT1 do not contribute significantly to catalytic activity, which is consistent with our previous published work (9).
In Vivo Validation of αTAT1 Mutations.
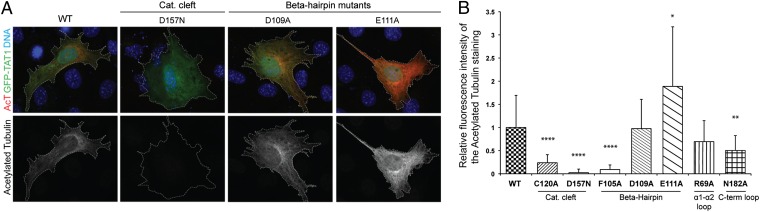
The in vitro studies suggested the existence of αTAT1 determinants specific for α-tubulin acetylation. However, acetylation in cells is the result of the action of several enzymes, and it is possible that αTAT1 is regulated through cellular partners. To determine the functional importance of the β-hairpin, α1-α2 loop, catalytic cleft, and C-term loop of αTAT1 in vivo, we transfected a key subset of αTAT1 mutants in immortalized mouse embryonic fibroblasts (MEFs) obtained from an αTAT1 knockout animal. These cells have undetectable levels of α-tubulin K40 acetylation as asserted by 611B1 immunostaining (Fig. 4). Transfection of WT αTAT1—but not of the catalytic active site mutant D157N—restored acetylation, thus demonstrating that loss of αTAT1 is the sole responsible factor for the absence of tubulin acetylation in those cells. Cells transfected with C120A, D109A, and N182A showed dramatically reduced levels of tubulin acetylation. Remarkably, the E111A mutation nearly doubled the levels of α-tubulin K40 acetylation in cells, whereas the F105A and D157N mutants were unable to acetylate tubulin in vivo (Fig. S4). Importantly, all GFP-αTAT1 variants were expressed at similar levels with tubulin acetylation levels normalized to GFP intensities in each cell (Fig. S4). The strict mirroring between in vivo and in vitro activities of αTAT1 suggests that cellular levels of α-tubulin K40 acetylation may be modulated by regulating αTAT1 activity.
Fig. 4.
Cell-based studies of selected αTAT1 mutants. (A) Representative micrographs of αTAT1−/− MEFs expressing comparable levels of GFP-αTAT1 variants. Transfected cells are outlined, GFP-αTAT1 staining is green, acetylated tubulin staining is red, and DNA is blue. Note that the untransfected αTAT1−/− MEFs display no acetylated tubulin staining. For scale, each panel is approximately 120 μm in width. (B) The ability of the αTAT1 mutants to acetylate microtubules in vivo was assessed by measuring the intensity of the acetylated tubulin immunostaining in 45 cells per mutant (for the WT, D157N, D109A, and E111A enzymes) or in 15 cells (C120A, F105A, R69A, and N182A), and normalizing the value to the intensity of the GFP-αTAT1 signal. Statistical analysis was performed by using a two-sided Wilcoxon test in R. *P < 0.05, **P < 0.01, ****P < 0.0001.
Discussion
The array of proteins with diverse biological functions that are modified by acetylation is seemingly ever expanding (5, 22). Although several acetyltransferases, such as mammalian p300/CBP and yeast Esa1, appear to modify many substrates (23, 24), most acetyltransferases such as human Gcn5 or yeast Rtt109 have a much more restricted set of substrates, as might be required to ensure their proper cellular activities (25, 26). Therefore, although it appears that protein acetyltransferases have maintained a structurally conserved core region for analogous AcCoA binding and the recognition of cognate substrate, unique structural motifs must exist to regulate substrate-specific recognition and acetylation and to prevent activity toward noncognate substrates. Our analysis here of αTAT1 has revealed several unique structural features that we have shown to be essential for its proper enzymatic activity toward K40 α-tubulin acetylation of microtubules. We have demonstrated that both D157 and C120, located in the enzyme active site, participate in catalysis through a ternary complex mechanism, where both residues likely serve as general bases for catalysis. We have also shown that regions flanking the unusually basic α-tubulin substrate-binding site that differs from other acetyltransferase proteins, the β4-β5 hairpin, C-terminal loop, and α1-α2 loop regions play particularly important roles in α-tubulin–specific binding and/or acetylation.
The importance of regions flanking the structurally conserved core region of protein acetyltransferases in mediating substrate specific binding is highlighted by comparing αTAT1 with the Gcn5 histone and Naa50p N-amino acetyltransferases bound to their respective cognate substrates (Fig. 5). The Gcn5 structure reveals a 12- to 15-Å groove flanked by N- and C-terminal regions for histone substrate to lie across one side of the protein to insert the centrally located cognate lysine side chain for acetylation (Fig. 5A) (13). The Naa50p structure reveals a more constricted groove of approximately 9 Å that is largely mediated by two flanking loop regions, that disfavor the binding of a polypeptide chain across the protein but instead favors the binding of an N-terminal amino group bound in an orientation that is nearly orthogonal to the histone peptide bound to Gcn5 (Fig. 5B) (18). αTAT1 contains a significantly wider substrate-binding groove than Gcn5 or Naa50p of approximately 20 Å (Fig. 5C). In addition, our finding that the β4-β5 hairpin region that extends away from the protein also participates in α-tubulin–specific binding and/or acetylation reveals that substrate specific determinants may extend out even further. Although we do not yet have a structure of αTAT1 bound to cognate substrate, we hypothesize that the relatively large width of the αTAT1 substrate-binding groove might use regions of microtubules that are distal to K40 of α-tubulin for substrate specific acetylation, a hypothesis that is supported by the observation that αTAT1 is unable to efficiently acetylate a peptide resembling the α-tubulin loop containing K40 (9).
Fig. 5.
Protein substrate binding sites of selected protein acetyltransferases. (A) Surface representation of TtGcn5 bound to histone H3 peptide (yellow cpk stick) and CoA (white cpk stick). (B) Surface representation of the N-terminal acetyltransferase Naa50p bound to CoA (white cpk stick) and N-terminal substrate peptide (yellow cpk stick). (C) Surface representation of αTAT1 bound to AcCoA (white cpk stick).
Although our studies do not directly address how αTAT1 gains access to the lumen of microtubules for K40 acetylation, our results together with recently published cellular (27, 28) and electron microscopy studies (29) provides important insights into how this process may occur. Specifically, modeling the αβ-tubulin dimer into an EM map of microtubules places K40 at the interface between protofilaments and suggests that the αTAT1 recognition interface may consist of the H1-S2 loop of α-tubulin combined with some elements of its β-tubulin lateral neighbor (29). This type of interface supports our observation of αTAT1 preference for microtubules over free tubulin because these lateral contacts between protofilaments would not be present in isolated tubulin dimers. Also consistent with this possibility, studies measuring the number of protofilaments in microtubule bundles in the presence and absence of αTAT1 have revealed that the number of protofilaments is regulated by the activity of αTAT1 (27, 28). Further studies will be needed to validate and elaborate on the molecular details of this hypothesis for αTAT1 entry into the lumen.
The extremely slow turnover rate of αTAT1 measured in vitro (Table S2) does not seem to correlate with the robust acetylation observed in vivo and hints that a αTAT1 binding partner in cells may facilitate K40 acetylation (6, 8). Alternatively, if one considers that only long-lived microtubules become acetylated, a slow rate of catalysis may provide the discriminatory mechanism required to differentially acetylate stable but not dynamic microtubules. Because microtubule associated proteins (MAPs) tend to accumulate on microtubules as they age, and because some MAPs send projections inside the lumen of microtubules (30), it is conceivable that such MAPs could further enhance the acetylation of long- vs. short-lived microtubules.
The microtubule binding drugs, vinca alkaloids and the taxanes, have been used as cancer treatments for hematological malignancies and solid tumors (31, 32). These two classes of drugs have been shown to either promote microtubule stability or enhance depolymerization (33, 34). Although these two mechanisms clearly represent two very different cellular states, both have been shown to function as effective cancer treatments (35). However, the ability of cancer cells to develop resistance to these therapies through multidrug resistance cellular efflux, highlights a need to identify new ways to modulate microtubules for disease treatment. The structure of αTAT1 provides a unique enzyme scaffold for designing small molecule modulators of microtubule acetylation for therapeutic use. In particular, our identification of αTAT1 mutations that either decrease or increase αTAT1 activity is a proof of principle that small molecule compounds could be identified that either increase or decrease αTAT1 activity to stabilize microtubules or to promote their depolymerization, respectively, for therapeutic purposes.
Materials and Methods
A list of chemicals used, procedures for preparing DNA constructs for cloning, and methods for protein expression and purification can be found in SI Materials and Methods. Procedures detailing crystallization, data collection, and structure determination can be found in SI Materials and Methods. Protocols for tubulin purification, microtubule assembly, and α-tubulin acetylation assays are available in SI Materials and Methods. Details of cell culture transfections, immunofluorescence, microscopy, and in vivo assay quantification are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Troy Messick and the staff of the X6A and X25 beamlines at the National Synchrotron Light Source (NSLS) for help with X-ray data collection; members of the R.M. laboratory for helpful discussions; and Toshi Shida for help with Tubulin purification. This work was funded in part by the National Institutes of Health (NIH) with a T32 Grant CA009171 (to D.R.F.) and R01 Grants GM060293 (to R.M.) and GM089933 (to M.V.N.). We also acknowledge use of the Wistar Proteomics core facility funded by NIH Grant CA 010815.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.K. is a guest editor invited by the Editorial Board.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4GS4).
See Commentary on page 19515.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209357109/-/DCSupplemental.
References
- 1.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Timmermann S, Lehrmann H, Polesskaya A, Harel-Bellan A. Histone acetylation and disease. Cell Mol Life Sci. 2001;58(5-6):728–736. doi: 10.1007/PL00000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26(37):5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 4.Kouzarides T. Acetylation: A regulatory modification to rival phosphorylation? EMBO J. 2000;19(6):1176–1179. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhary C, et al. 2009. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325(5942):834–840.
- 6.L’Hernault SW, Rosenbaum JL. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985;24(2):473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- 7.Maruta H, Greer K, Rosenbaum JL. The acetylation of alpha-tubulin and its relationship to the assembly and disassembly of microtubules. J Cell Biol. 1986;103(2):571–579. doi: 10.1083/jcb.103.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piperno G, LeDizet M, Chang XJ. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol. 1987;104(2):289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci USA. 2010;107(50):21517–21522. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akella JS, et al. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010;467(7312):218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed NA, et al. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16(21):2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Loktev AV, et al. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell. 2008;15(6):854–865. doi: 10.1016/j.devcel.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Rojas JR, et al. Structure of Tetrahymena GCN5 bound to coenzyme A and a histone H3 peptide. Nature. 1999;401(6748):93–98. doi: 10.1038/43487. [DOI] [PubMed] [Google Scholar]
- 14.Yan Y, Harper S, Speicher DW, Marmorstein R. The catalytic mechanism of the ESA1 histone acetyltransferase involves a self-acetylated intermediate. Nat Struct Biol. 2002;9(11):862–869. doi: 10.1038/nsb849. [DOI] [PubMed] [Google Scholar]
- 15.Yuan H, et al. MYST protein acetyltransferase activity requires active site lysine autoacetylation. EMBO J. 2012;31(1):58–70. doi: 10.1038/emboj.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segel IH. 1993. Enzyme kinetics: Behavior and analysis of rapid equilibrium and steady-state enzyme systems.
- 17.Hickman AB, Klein DC, Dyda F. Melatonin biosynthesis: The structure of serotonin N-acetyltransferase at 2.5 A resolution suggests a catalytic mechanism. Mol Cell. 1999;3(1):23–32. doi: 10.1016/s1097-2765(00)80171-9. [DOI] [PubMed] [Google Scholar]
- 18.Liszczak G, Arnesen T, Marmorstein R. Structure of a ternary Naa50p (NAT5/SAN) N-terminal acetyltransferase complex reveals the molecular basis for substrate-specific acetylation. J Biol Chem. 2011;286(42):37002–37010. doi: 10.1074/jbc.M111.282863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, et al. The structural basis of protein acetylation by the p300/CBP transcriptional coactivator. Nature. 2008;451(7180):846–850. doi: 10.1038/nature06546. [DOI] [PubMed] [Google Scholar]
- 20.Gigant B, et al. The 4 A X-ray structure of a tubulin:stathmin-like domain complex. Cell. 2000;102(6):809–816. doi: 10.1016/s0092-8674(00)00069-6. [DOI] [PubMed] [Google Scholar]
- 21.Tang Y, et al. Fungal Rtt109 histone acetyltransferase is an unexpected structural homolog of metazoan p300/CBP. Nat Struct Mol Biol. 2008;15(9):998. doi: 10.1038/nsmb.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spange S, Wagner T, Heinzel T, Krämer OH. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol. 2009;41(1):185–198. doi: 10.1016/j.biocel.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 23.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87(5):953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 24.Sapountzi V, Côté J. MYST-family histone acetyltransferases: Beyond chromatin. Cell Mol Life Sci. 2011;68(7):1147–1156. doi: 10.1007/s00018-010-0599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Driscoll R, Hudson A, Jackson SP. 2007. Yeast Rtt109 promotes genome stability by acetylating histone H3 on lysine 56. Science 315(5812):649–652. [DOI] [PMC free article] [PubMed]
- 26.Grant PA, et al. Expanded lysine acetylation specificity of Gcn5 in native complexes. J Biol Chem. 1999;274(9):5895–5900. doi: 10.1074/jbc.274.9.5895. [DOI] [PubMed] [Google Scholar]
- 27.Cueva JG, Hsin J, Huang KC, Goodman MB. Posttranslational acetylation of α-tubulin constrains protofilament number in native microtubules. Curr Biol. 2012;22(12):1066–1074. doi: 10.1016/j.cub.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topalidou I, et al. Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization. Curr Biol. 2012;22(12):1057–1065. doi: 10.1016/j.cub.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nogales E, Whittaker M, Milligan RA, Downing KH. High-resolution model of the microtubule. Cell. 1999;96(1):79–88. doi: 10.1016/s0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- 30.Kar S, Fan J, Smith MJ, Goedert M, Amos LA. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 2003;22(1):70–77. doi: 10.1093/emboj/cdg001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noble RL, Beer CT, Cutts JH. Role of chance observations in chemotherapy: Vinca rosea. Ann N Y Acad Sci. 1958;76(3):882–894. doi: 10.1111/j.1749-6632.1958.tb54906.x. [DOI] [PubMed] [Google Scholar]
- 32.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93(9):2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 33.Buey RM, et al. Microtubule interactions with chemically diverse stabilizing agents: Thermodynamics of binding to the paclitaxel site predicts cytotoxicity. Chem Biol. 2005;12(12):1269–1279. doi: 10.1016/j.chembiol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Lobert S, Ingram JW, Correia JJ. Additivity of dilantin and vinblastine inhibitory effects on microtubule assembly. Cancer Res. 1999;59(19):4816–4822. [PubMed] [Google Scholar]
- 35.Pajk B, et al. Anti-tumor activity of capecitabine and vinorelbine in patients with anthracycline- and taxane-pretreated metastatic breast cancer: Findings from the EORTC 10001 randomized phase II trial. Breast. 2008;17(2):180–185. doi: 10.1016/j.breast.2007.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.