Abstract
Cardiac stress responses are driven by an evolutionarily conserved gene expression program comprising dozens of microRNAs and hundreds of mRNAs. Functionalities of different individual microRNAs are being studied, but the overall purpose of interactions between stress-regulated microRNAs and mRNAs and potentially distinct roles for microRNA-mediated epigenetic and conventional transcriptional genetic reprogramming of the stressed heart are unknown. Here we used deep sequencing to interrogate microRNA and mRNA regulation in pressure-overloaded mouse hearts, and performed a genome-wide examination of microRNA–mRNA interactions during early cardiac hypertrophy. Based on abundance and regulatory patterns, cardiac microRNAs were categorized as constitutively expressed housekeeping, regulated homeostatic, or dynamic early stress-responsive microRNAs. Regulation of 62 stress-responsive cardiac microRNAs directly affected levels of only 66 mRNAs, but the global impact of microRNA-mediated epigenetic regulation was amplified by preferential targeting of mRNAs encoding transcription factors, kinases, and phosphatases exerting amplified secondary effects. Thus, an emergent cooperative property of stress-regulated microRNAs is orchestration of transcriptional and posttranslational events that help determine the stress-reactive cardiac phenotype. This global functionality explains how large end-organ effects can be induced through modest individual changes in target mRNA and protein content by microRNAs that sense and respond dynamically to a changing physiological milieu.
Keywords: microRNA-mRNA interactome, transcriptome regulation, systems biology
The heart is a muscular blood pump that delivers oxygen and nutrients to tissues. Cardiac impairment or functional overload is poorly tolerated, provoking reactive hypertrophy that is genetically programmed (1). Hundreds of hypertrophy-modulated genes have been cataloged and relevant transcriptional mechanisms elucidated (2). Additional regulatory complexity is conferred by microRNAs that adjust entire functional networks of mRNAs via mechanisms both mechanistically and physically distinct from transcriptional controls (3). Recent studies have uncovered regulation of numerous microRNAs in hypertrophied and failing hearts (4–7), and microRNAs are therapeutic targets in experimental heart disease (8).
Multiple avenues for feedback and interconnectivity between regulated microRNAs and their mRNA targets in cardiac disease can potentially engender emergent cooperative behavior. Emergent behaviors are not, however, identified by or predictable from the behavior of individual involved entities. Thus, complex microRNA–mRNA interactions are best understood through systemic interrogation of the complete microRNA–mRNA interactome. Here, through genome-wide deep sequencing of total cardiac microRNAs and mRNAs and by defining their physical and functional association within microRNA (miR) effector RNA-induced silencing complexes (RISCs) (9), we examined the global role of microRNA regulation in genetic reprogramming of hemodynamically stressed hearts. The results uncover context-specific orchestration of critical cardiac transcriptional and posttranslational pathways that is an emergent property of genome-wide mRNA regulation through what we call epitranscriptional effects of microRNAs.
Results
Compensated Cardiac Hypertrophy 1 wk After Pressure Overloading.
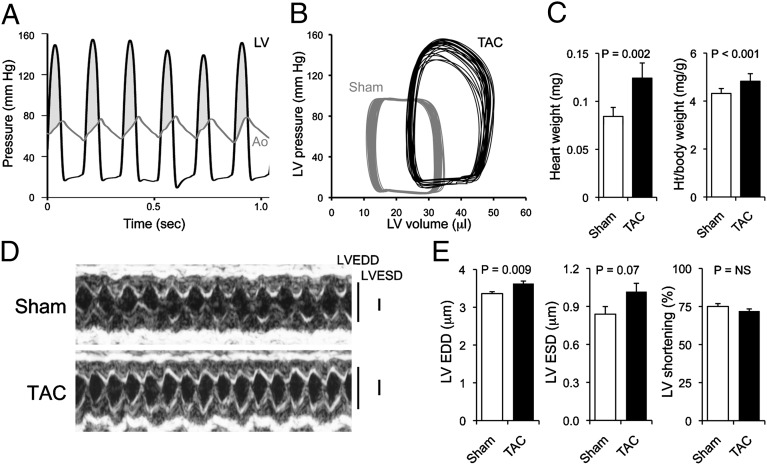
To identify genetic and microRNA-mediated events driving early cardiac hypertrophy, we studied hearts after 1 wk of pressure overloading induced by surgical transverse aortic banding. At this time, hearts are hypertrophying, the mice have recovered from surgery, and genome-wide changes in mRNA and microRNA levels reflect the primary pressure overload stimulus-hypertrophy response without confounding changes induced during later functional decompensation. Peak left ventricular systolic pressure was 131 ± 6 mmHg in aortic-banded mice, compared with 83 ± 4 mmHg after sham operation; transaortic gradients were 64 ± 7 mmHg and −3 ± 2 mmHg, respectively (Fig. 1A). Left ventricular pressure–volume relations of aortic-banded mice (Fig. 1B) showed a slightly depressed left ventricular ejection fraction with normal peak +dP/dt (positive change in pressure over time, an afterload-independent measure of myocardial contractility). Heart weight increased 1 wk after aortic banding (Fig. 1C), and echocardiography revealed early left ventricular remodeling (increased dimensions at end-diastole and end-systole; Fig. 1D) without a significant change in ejection performance (left ventricular shortening) (Fig. 1E). Molecular markers of cardiac hypertrophy were regulated as expected (SI Appendix, Fig. S1) (1, 10).
Fig. 1.
Compensated pressure-overload hypertrophy early after transverse aortic banding. (A) Simultaneous left ventricular (LV, black) and aortic (Ao, gray) pressure tracings 1 wk after transverse aortic banding (TAC). Shaded area is abnormal pressure gradient. (B) LV pressure–volume relations in a sham-operated (gray) and TAC (black) mouse heart; 12 cardiac cycles are shown for each heart. (C) Gravimetric cardiac hypertrophy (n = 9 sham, n = 5 TAC). Ht, height. Data are presented as mean ± SEM. (D) M-mode cardiac echocardiograms. (E) Mean group echo data. EDD, end-diastolic dimension; ESD, end-systolic dimension; NS, not significant.
MicroRNA Sequencing of Early Cardiac Hypertrophy.
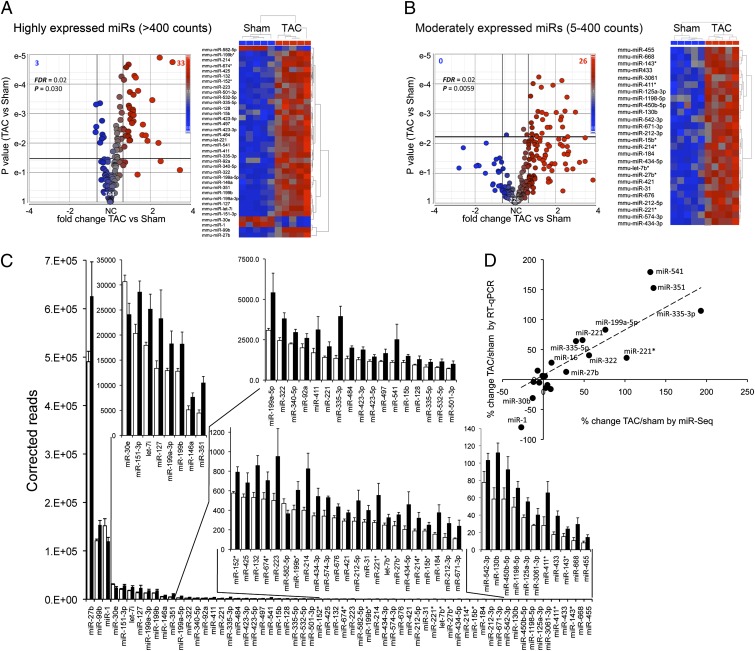
Deep microRNA sequencing of mouse hearts detected 715 microRNAs, 369 expressed at levels of at least 5 reads per 10 million aligned reads (SI Appendix, Table S1). Levels of the 50 most-abundant cardiac microRNAs in sham-operated and pressure-overloaded hearts are compared in SI Appendix, Fig. S2. Early hypertrophy significantly changed levels of 36 of 144 (25%) highly abundant (Fig. 2 A and C) and 26 of 226 (12%) moderately abundant (Fig. 2 B and C) microRNAs. Ranking according to fold change is shown in SI Appendix, Fig. S3A. Correlation of microRNA deep sequencing (miR-Seq) findings for 21 representative cardiac microRNAs with miR-specific RT-quantitative (q)PCR (r = 0.89, P = 4E-7) is shown in Fig. 2D and SI Appendix, Fig. S3B, revealing a generally greater extent of regulation for moderately abundant, compared with highly abundant, microRNAs. More microRNAs were increased than decreased in early hypertrophy, perhaps reflecting a lag period for microRNA elimination or failure of some down-regulated microRNAs to achieve genome-wide statistical significance. Hypertrophy-regulated microRNAs include miR-21, previously implicated in myocardial hypertrophy remodeling, fibrosis, and cytoprotection (11, 12); miR-127, which regulates mitochondrial bioenergetics (13); and miR-199, previously linked to cardiac hypertrophy signaling through calcineurin/NFAT (14). Concordance between regulation of microRNAs and their parent mRNAs was high (r = 0.65, P < 0.01; SI Appendix, Fig. S3C), suggesting transcriptional regulation. MicroRNA abundance from total cellular RNA and RNA extracted from Argonaute 2-containing (9) RISCs was highly correlated in both sham (r = 0.93) and early hypertrophied (r = 0.93) hearts (SI Appendix, Fig. S4 A and B, respectively), indicating that the cardiac microRNAs detected by sequencing are almost entirely in a bioactive population dedicated to mRNA targeting.
Fig. 2.
MicroRNA signature of active pressure-overload hypertrophy. (A and B) MicroRNA-Seq results for highly expressed (A) and moderately expressed (B) cardiac microRNAs. Volcano plots (Left) show fold-change TAC/sham vs. P value. Bold horizontal and vertical lines show threshold levels [±25% fold change and P value at false discovery rate (FDR) of 0.02]. Heat maps show unsupervised hierarchical clustering of normalized regulated microRNA sequence data; each column is a single mouse heart. (C) Quantitative levels for all 62 hypertrophy-regulated microRNAs. Sham (white) and TAC (black) are shown as reads per sample. Data are presented as mean ± SEM. (D) Correlation of early hypertrophy-induced changes in 21 representative microRNA levels assessed by miR-Seq vs. RT-qPCR.
Deep mRNA Sequencing of Early Cardiac Hypertrophy.
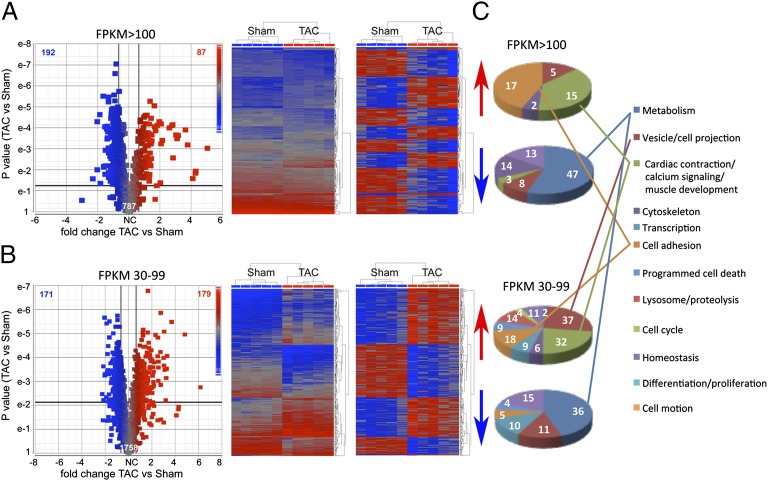
Deep mRNA sequencing identified and quantified hypertrophy-regulated mRNAs in the same hearts (SI Appendix, Table S2): ∼8,500 coding mRNAs are present in mouse hearts at three fragments per kb of exon per million mapped reads (FPKM) or greater, that is, one or more mRNA copies per cell (10, 15). Volcano plots comparing fold change and P value after aortic banding (Fig. 3 A and B and SI Appendix, Fig. S5) identify 87 significantly increased and 192 significantly decreased highly abundant (FPKM >100) mRNAs during early hypertrophy (Fig. 3A); 179 moderately abundant (FPKM 30–99) mRNAs were increased and 171 were decreased (Fig. 3B). mRNAs encoding cardiac contractile, calcium handling, muscle growth, and cell adhesion proteins were disproportionately up-regulated (P = 0.028), consistent with muscle and matrix remodeling (16), whereas metabolic genes were disproportionately down-regulated (P = 0.034), consistent with switching of cardiac metabolic substrates (17) (Fig. 3C and SI Appendix, Fig. S5 and Table S2).
Fig. 3.
Transcriptional signature of the pathologically hypertrophying mouse heart. (A) Volcano plot (Left) and heat maps (Right) of 787 highly abundant cardiac mRNAs. Heat maps show unsupervised hierarchical clustering of raw (Left) and normalized (Right) RNA-sequence data for the 279 regulated mRNAs. (B) As in A for 1,758 moderately abundant mRNAs. (C) Pie charts show functional classification of up-regulated and down-regulated mRNAs.
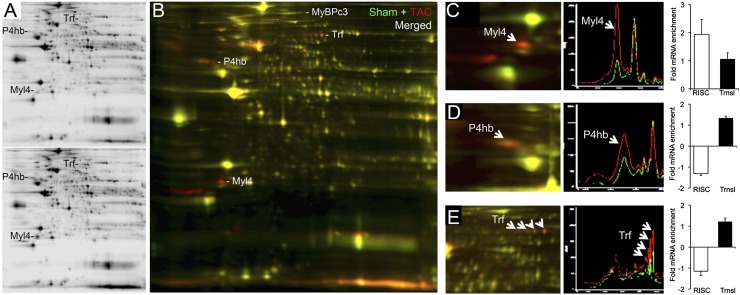
Complementary proteomics studies (18) revealed 61 regulated protein spots (>25% change in at least three of four paired studies; 45 increased and 16 decreased) (Fig. 4 A and B). Among these are myosin light-chain isoform 4 (Fig. 4C), protein disulfide isomerase (Fig. 4D), and the iron-binding protein transferrin (Fig. 4E), each of which is known to be regulated in hypertrophy (19–21). Phosphorylation of cardiac proteins such as myosin-binding protein C is also increased in pathological hypertrophy (22) (Fig. 4B). Thus, both posttranslational modification and expression of myocardial proteins are altered during cardiac hypertrophy.
Fig. 4.
Changes in the cardiac proteome 1 wk after surgical pressure overloading. (A) Representative 2D differential in-gel electrophoresis showing Cy 3-labeled sham-operated (Upper) and Cy 5-labeled 1-wk TAC (Lower) cardiac proteomes. (B) Merged Cy 3 (green) and Cy 5 (red) images. Regulated myosin-binding protein C (MyBPc3), transferrin (Trf), protein disulfide isomerase (P4hb), and myosin light-chain isoform 4 (Myl4) are labeled. (C–E) Exploded views from a separate experiment of Myl4 (C), P4hb (D), and Trf (E), with accompanying quantifications corresponding to RISC-associated versus total mRNA levels.
mRNA Recruitment to RNA-Induced Silencing Complexes Is Altered by Pressure Overload.
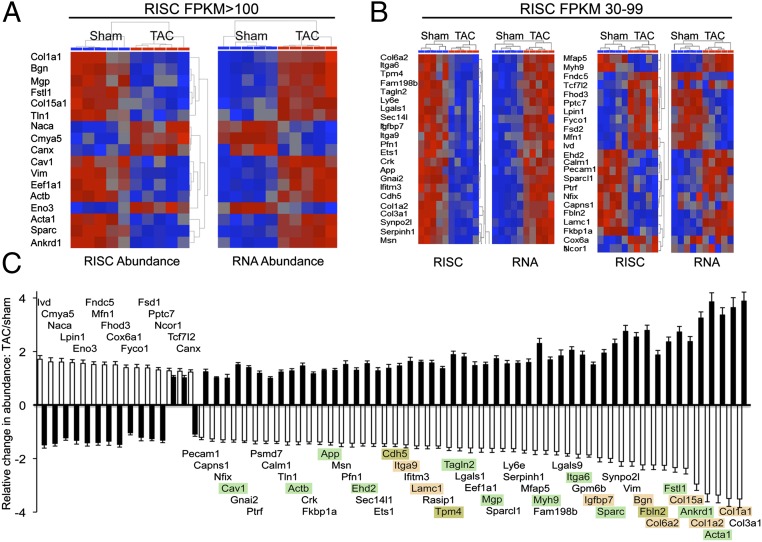
Direct effects of microRNAs accrue from destabilization or translational suppression of mRNAs within Argonaute 2-containing RISCs. We examined early hypertrophy-induced changes in this microRNA–mRNA interactome by deep sequencing mRNAs extracted from cardiac Argonaute 2 immunoprecipitates (9). mRNAs (1,228) were RISC-enriched at least twofold, defining the “RISC-associated” cardiac mRNAs (SI Appendix, Table S3). Low–RISC-abundant mRNAs showed few differences between normal and pressure-overloaded hearts (SI Appendix, Fig. S6). In contrast, 17 of 272 (∼6%) highly abundant and 46 of 596 (∼8%) moderately abundant RISC-associated mRNAs were significantly altered in hypertrophying hearts (P = 0.0064 and 0.0053, respectively; Fig. 5 A and B). As expected, there was a powerful inverse relationship (r = 0.91, P = 1.1E-25) between transcript abundance and RISC recruitment for these mRNAs (Fig. 5C).
Fig. 5.
Regulation of mRNA–RISC association during pressure-overload hypertrophy. (A) Normalized heat maps for 17 highly RISC-enriched, significantly regulated cardiac mRNAs showing RISC abundance (Left) and corresponding abundance in the transcriptome (Right). (B) As with A for 45 moderately RISC-abundant cardiac mRNAs. (C) Quantitative group mean data showing fold change after TAC for the 66 mRNAs whose abundance is regulated in the RISC (white bars) together with their respective mRNA levels in the transcriptome (black bars). Data are presented as mean ± SEM. Highlighting indicates mRNAs in gene-ontology categories of cell adhesion (orange) or cardiac contraction/calcium signaling (green) (per Fig. 3C).
Role of MicroRNA Regulation of mRNA Abundance in Developing Hypertrophy.
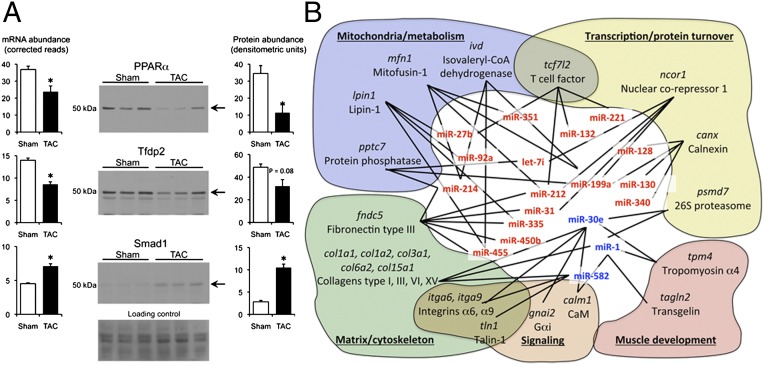
Hierarchical organization within a complex interactive system can generate coherent behavior revealed through systems analysis. We agnostically compared functional and cellular characteristics of all cardiac mRNAs targeted by microRNAs (1,228 RISC-enriched mRNAs) versus those that are regulated independently of microRNAs (1,942 RISC-independent hypertrophy-regulated mRNAs) (SI Appendix, Fig. S7 A and B, respectively). Most functional categories were equally populated by RISC-associated and -independent mRNAs. However, disproportionate microRNA-dependent regulation was notable for protein- and nucleic acid-binding transcription factors and membrane-enclosed lumen (e.g., nuclear) macromolecular complexes. Statistical comparisons of actual to predicted % occupancy within gene-ontology subcategories (SI Appendix, Table S4) revealed overrepresentation of RISC-enriched mRNAs for transcription factors (P = 2.2E-06), kinases (P = 5.3E-09), and phosphatases (P = 6.7E-07), whereas RISC-independent hypertrophy-regulated mRNAs were overrepresented only for enzymes (P = 6.3E-09) (Dataset S1). Regulation of several transcription factors was confirmed by immunoblot analysis (Fig. 6A). Target prediction within regulated microRNA–mRNAs using FastamiRs (SI Appendix, Materials and Methods) supported specific interactions affecting genes regulating metabolism, matrix/cytoskeleton, muscle development, and transcription/protein turnover (Fig. 6B).
Fig. 6.
Distinct functions of microRNA-regulated and microRNA-independent cardiac mRNAs. (A) RNA-Seq and immunoblot analyses of hypertrophy-regulated cardiac transcription factors. Ponceau stain is loading control. *P < 0.05. (B) Schematic representation of regulated microRNAs interacting with putative regulated mRNA targets within common functional networks.
Discussion
Here we used whole-genome microRNA, mRNA, and RISC sequencing to interrogate mechanisms of transcript regulation during the early cardiac response to hemodynamic overload. Levels of ∼1,200 (of ∼8,500) cardiac mRNAs and 62 (of 369) microRNAs were altered in early hypertrophy. The resulting change in the microRNA–mRNA interactome significantly altered levels of 66 microRNA-targeted cardiac transcripts. MicroRNAs are therefore not the dominant effectors of mRNA levels in stressed hearts; transcriptional controls retain that role (2). However, because microRNAs preferentially targeted mRNAs encoding kinases, phosphatases, proteases, and transcription factors, an emergent property of microRNA-mediated control of directly targeted mRNAs in early hypertrophy is modulation of transcriptional and posttranslational factors that amplify systemic changes. This microRNA mechanism of orchestrating networks of nontarget mRNAs by directly targeting transcription factors we term “epitranscriptional regulation.” We expect that this previously undescribed global stress-regulated microRNA functionality will apply broadly across organ systems.
As a truly comprehensive analysis of microRNA–mRNA levels and interactions in the same normal and stressed hearts, our results suggest three functional classes of cardiac-expressed microRNAs (SI Appendix, Fig. S8). The first consists of constitutively expressed microRNAs that regulate essential cellular housekeeping functions, including the ubiquitously expressed tumor suppressor miR-22, which targets c-Myc–dependent genes (23); miR-143, which targets transcription factors controlling vascular smooth muscle cell differentiation (24, 25); and miR-486, which is a muscle-specific regulator of Akt/PI3kinase signaling (26). The second consists of microRNAs that sustain normal cardiac homeostasis but are regulated during early cardiac hypertrophy, and include prototypical and abundant miR-1 (27, 28), which is important for striated muscle development and homeostasis (29–31). In this and other studies (32), miR-1 is down-regulated in early hypertrophy, consistent with a need to derepress its mRNA targets (histone deacetylase 4, heat-shock proteins, insulin-like growth factor 1) during cardiac remodeling. Another homeostatic microRNA is the adipogenic inhibitor miR-27b, which targets the metabolic factors PPAR-γ and C/EBPα (33, 34) and lipin-1 (current study). Our data show this to be the most-abundant up-regulated microRNA, consistent with metabolic remodeling induced by hypertrophy (16); its forced expression is sufficient to provoke pathological hypertrophy (34).
The third class of microRNAs is stress-responsive, that is, rapidly up-regulated after pressure overloading, and includes miR-21, -127, and -199. miR-21 is a widely expressed and evolutionarily conserved microRNA that targets antioncogenes, including Spry1; its expression increases in human and experimental heart disease and it is implicated in cardiac fibrosis (7, 11, 35, 36). miR-127 also regulates apoptosis/tumor signaling (37) and plays a role in lung development (38); its role in heart disease is unknown. Finally, miR-199 is increased in cardiac hypertrophy/failure and can induce cardiomyocyte hypertrophy (14, 39). Because these stress-responsive microRNAs react more dynamically to acute changes in cardiac pathophysiological status than mRNAs of the “fetal gene program” (7), they are positioned at the apex of the cardiac molecular stress-response system.
Two aspects of these results are noteworthy, independent of the systems analyses. First, we find no difference in microRNA profiles from whole hearts versus cardiac RISCs, at baseline or after stress. This indicates either that there is not a significant population of compartmentalized microRNAs sequestered for purposes other than mRNA suppression (e.g., secretion), or that such compartmentalized microRNAs have the same population makeup as those that are RISC-associated. Second, the inverse relationship between the level of regulated mRNA in the RISC versus in the translationally competent pool is incomplete (95% followed this rule), and the relationship between regulated mRNA and its encoded protein is not 1:1. These findings are similar to previous reports (40–42), reflecting the effects of multiple and variable regulatory inputs controlling steady-state levels of most factors.
We suggest that housekeeping microRNAs maintain general essential cellular functions, homeostatic microRNAs sustain normal heart-specific functions, and stress-responsive microRNAs reprogram the heart under conditions of injury or overload. Thus, stress-responsive microRNAs extract information from the environment and implement stimulus-specific transcriptional and posttranslational changes, the sum of which contributes to global molecular and proteomic remodeling. This paradigm explains indirect regulation of hundreds of cardiac mRNAs through epitranscriptional mechanisms: Individual microRNAs exert their effects via fairly subtle modulation of mRNA stability, producing only modest alterations in encoded transcription factors, kinases, phosphatases, and so forth (41, 43). However, the amplified downstream effects resulting from transcriptional activation/suppression or protein phosphorylation/dephosphorylation can induce striking changes in phenotype. In this manner, combinatorial effects of a small number of stress-responsive microRNAs on functionally related mRNA targets modify the activity of entire functional networks (44). We expect that different microRNA and mRNA signatures in decompensated heart failure and other cardiac diseases will engender distinct microRNA–mRNA interaction signatures, but that this emergent functionality will be a constant theme.
Most prior genome-wide studies of microRNA and mRNA expression used microarrays, reporting only relative expression levels between conditions. Thus, microRNAs and mRNAs that are highly regulated may nevertheless be vanishingly rare and of uncertain biological relevance (10). In contrast, deep sequencing reveals quantitative RNA amounts (reads per sample). In this regard, our results with miR-1 are remarkable. Two earlier studies reported that miR-1 was the most-abundant cardiac microRNA, comprising almost 40% of total microRNA in the adult mouse heart (45, 46). Two recent studies did not confirm this finding (47, 48). We find by deep sequencing and RT-qPCR that miR-1 is one of the most-abundant, but not the principal, microRNA in adult mouse hearts.
In Metaphysica 2 (http://classics.mit.edu//Aristotle/metaphysics.html), Aristotle opined that the whole is more than the sum of its parts. This concept applies to epitranscriptional regulation by microRNAs. Functionality of individual microRNAs is measurable, but is subordinated by the global functionality inherent in the coordinated actions of stress-responsive microRNAs and their amplified secondary and tertiary effects. This is a classic example of emergent behavior, familiar examples of which include the seemingly organized morphologies and motions exhibited by large flocks of birds or schools of fish (49). In a world where microRNAs are increasingly being investigated as disease mediators, the current findings emphasize the need for integrated genome-wide approaches to more fully understand the implications of regulated and therapeutically targeted microRNAs.
Materials and Methods
Mouse Heart Modeling and Physiological Analyses.
Methods for surgical modeling and physiological analyses of cardiac hypertrophy have been described (50, 51). All procedures were approved by the Institutional Animal Care Committees of the Washington University School of Medicine.
MicroRNA and mRNA Sequencing.
Transcriptional profiling by deep mRNA sequencing and RISC-Seq analysis have been described (9, 10). MicroRNA deep sequencing libraries were prepared with TruSeq Small RNA Sample Prep Kits (Illumina).
Myocardial Protein Expression.
Two-dimensional differential in-gel electrophoresis of paired sham-operated and 1-wk aortic-banded hearts was as described (22).
Statistical Analyses.
Analytical procedures used for RNA-Seq and RISC-Seq have been described (9, 10). Unless otherwise specified, all data are presented as mean ± SEM. P values were calculated using Student’s unpaired t test or one-way ANOVA with Newman–Keuls test as appropriate. Statistical significance was defined at P < 0.05.
Details are available in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute Grant R01 HL108943 and by the Fondation Leducq Transatlantic Network of Excellence in Cardiovascular Research Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214996109/-/DCSupplemental.
References
- 1.Dorn GW, II, Robbins J, Sugden PH. Phenotyping hypertrophy: Eschew obfuscation. Circ Res. 2003;92(11):1171–1175. doi: 10.1161/01.RES.0000077012.11088.BC. [DOI] [PubMed] [Google Scholar]
- 2.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313(5795):1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469(7330):336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Rooij E, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA. 2006;103(48):18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thum T, et al. MicroRNAs in the human heart: A clue to fetal gene reprogramming in heart failure. Circulation. 2007;116(3):258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 7.Matkovich SJ, et al. Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation. 2009;119(9):1263–1271. doi: 10.1161/CIRCULATIONAHA.108.813576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montgomery RL, et al. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124(14):1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matkovich SJ, Van Booven DJ, Eschenbacher WH, Dorn GW., II RISC RNA sequencing for context-specific identification of in vivo microRNA targets. Circ Res. 2011;108(1):18–26. doi: 10.1161/CIRCRESAHA.110.233528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matkovich SJ, Zhang Y, Van Booven DJ, Dorn GW., II Deep mRNA sequencing for in vivo functional analysis of cardiac transcriptional regulators: Application to Galphaq. Circ Res. 2010;106(9):1459–1467. doi: 10.1161/CIRCRESAHA.110.217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thum T, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 12.Dong S, et al. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284(43):29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willers IM, Martínez-Reyes I, Martínez-Diez M, Cuezva JM. miR-127-5p targets the 3′UTR of human β-F1-ATPase mRNA and inhibits its translation. Biochim Biophys Acta. 2012;1817(5):838–848. doi: 10.1016/j.bbabio.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 14.da Costa Martins PA, et al. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol. 2010;12(12):1220–1227. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- 15.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 16.Dorn GW., II The fuzzy logic of physiological cardiac hypertrophy. Hypertension. 2007;49(5):962–970. doi: 10.1161/HYPERTENSIONAHA.106.079426. [DOI] [PubMed] [Google Scholar]
- 17.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: A question of balance. J Clin Invest. 2005;115(3):547–555. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raddatz K, Albrecht D, Hochgräfe F, Hecker M, Gotthardt M. A proteome map of murine heart and skeletal muscle. Proteomics. 2008;8(9):1885–1897. doi: 10.1002/pmic.200700902. [DOI] [PubMed] [Google Scholar]
- 19.Hamelin M, et al. Proteomic analysis of ovine muscle hypertrophy. J Anim Sci. 2006;84(12):3266–3276. doi: 10.2527/jas.2006-162. [DOI] [PubMed] [Google Scholar]
- 20.Bupha-Intr T, Holmes JW, Janssen PM. Induction of hypertrophy in vitro by mechanical loading in adult rabbit myocardium. Am J Physiol Heart Circ Physiol. 2007;293(6):H3759–H3767. doi: 10.1152/ajpheart.01267.2006. [DOI] [PubMed] [Google Scholar]
- 21.Patton WF, Erdjument-Bromage H, Marks AR, Tempst P, Taubman MB. Components of the protein synthesis and folding machinery are induced in vascular smooth muscle cells by hypertrophic and hyperplastic agents. Identification by comparative protein phenotyping and microsequencing. J Biol Chem. 1995;270(36):21404–21410. doi: 10.1074/jbc.270.36.21404. [DOI] [PubMed] [Google Scholar]
- 22.Kang MY, et al. Receptor-independent cardiac protein kinase Calpha activation by calpain-mediated truncation of regulatory domains. Circ Res. 2010;107(7):903–912. doi: 10.1161/CIRCRESAHA.110.220772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong J, Du Q, Liang Z. Tumor-suppressive microRNA-22 inhibits the transcription of E-box-containing c-Myc target genes by silencing c-Myc binding protein. Oncogene. 2010;29(35):4980–4988. doi: 10.1038/onc.2010.241. [DOI] [PubMed] [Google Scholar]
- 24.Cordes KR, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elia L, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: Correlates with human disease. Cell Death Differ. 2009;16(12):1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Small EM, et al. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc Natl Acad Sci USA. 2010;107(9):4218–4223. doi: 10.1073/pnas.1000300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatsuguchi M, et al. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42(6):1137–1141. doi: 10.1016/j.yjmcc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elia L, et al. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation. 2009;120(23):2377–2385. doi: 10.1161/CIRCULATIONAHA.109.879429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436(7048):214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 30.Chen JF, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38(2):228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129(2):303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100(3):416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 33.Lin Q, Gao Z, Alarcon RM, Ye J, Yun Z. A role of miR-27 in the regulation of adipogenesis. FEBS J. 2009;276(8):2348–2358. doi: 10.1111/j.1742-4658.2009.06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, et al. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res. 2012;22(3):516–527. doi: 10.1038/cr.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy S, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82(1):21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patrick DM, et al. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120(11):3912–3916. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito Y, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9(6):435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Bhaskaran M, et al. MicroRNA-127 modulates fetal lung development. Physiol Genomics. 2009;37(3):268–278. doi: 10.1152/physiolgenomics.90268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song XW, et al. MicroRNAs are dynamically regulated in hypertrophic hearts, and miR-199a is essential for the maintenance of cell size in cardiomyocytes. J Cell Physiol. 2010;225(2):437–443. doi: 10.1002/jcp.22217. [DOI] [PubMed] [Google Scholar]
- 40.Ghaemmaghami S, et al. Global analysis of protein expression in yeast. Nature. 2003;425(6959):737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 41.Baek D, et al. The impact of microRNAs on protein output. Nature. 2008;455(7209):64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selbach M, et al. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455(7209):58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 43.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorn GW., II Decoding the cardiac message: The 2011 Thomas W. Smith Memorial Lecture. Circ Res. 2012;110(5):755–763. doi: 10.1161/CIRCRESAHA.111.256768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lagos-Quintana M, et al. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12(9):735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 46.Rao PK, et al. Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure. Circ Res. 2009;105(6):585–594. doi: 10.1161/CIRCRESAHA.109.200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rau F, et al. Misregulation of miR-1 processing is associated with heart defects in myotonic dystrophy. Nat Struct Mol Biol. 2011;18(7):840–845. doi: 10.1038/nsmb.2067. [DOI] [PubMed] [Google Scholar]
- 48.Palacín M, et al. Profile of microRNAs differentially produced in hearts from patients with hypertrophic cardiomyopathy and sarcomeric mutations. Clin Chem. 2011;57(11):1614–1616. doi: 10.1373/clinchem.2011.168005. [DOI] [PubMed] [Google Scholar]
- 49.Resnick M. Turtles, Termites, and Traffic Jams: Explorations in Massively Parallel Microworlds (Complex Adaptive Systems) Cambridge, MA: MIT Press; 1994. [Google Scholar]
- 50.Sakata Y, Hoit BD, Liggett SB, Walsh RA, Dorn GW., II Decompensation of pressure-overload hypertrophy in G alpha q-overexpressing mice. Circulation. 1998;97(15):1488–1495. doi: 10.1161/01.cir.97.15.1488. [DOI] [PubMed] [Google Scholar]
- 51.Diwan A, et al. Nix-mediated apoptosis links myocardial fibrosis, cardiac remodeling, and hypertrophy decompensation. Circulation. 2008;117(3):396–404. doi: 10.1161/CIRCULATIONAHA.107.727073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.