Abstract
Estrogen withdrawal in menopausal women leads to hot flushes, a syndrome characterized by the episodic activation of heat dissipation effectors. Despite the extraordinary number of individuals affected, the etiology of flushes remains an enigma. Because menopause is accompanied by marked alterations in hypothalamic kisspeptin/neurokinin B/dynorphin (KNDy) neurons, we hypothesized that these neurons could contribute to the generation of flushes. To determine if KNDy neurons participate in the regulation of body temperature, we evaluated the thermoregulatory effects of ablating KNDy neurons by injecting a selective toxin for neurokinin-3 expressing neurons [NK3-saporin (SAP)] into the rat arcuate nucleus. Remarkably, KNDy neuron ablation consistently reduced tail-skin temperature (TSKIN), indicating that KNDy neurons facilitate cutaneous vasodilatation, an important heat dissipation effector. Moreover, KNDy ablation blocked the reduction of TSKIN by 17β-estradiol (E2), which occurred in the environmental chamber during the light phase, but did not affect the E2 suppression of TSKIN during the dark phase. At the high ambient temperature of 33 °C, the average core temperature (TCORE) of ovariectomized (OVX) control rats was significantly elevated, and this value was reduced by E2 replacement. In contrast, the average TCORE of OVX, KNDy-ablated rats was lower than OVX control rats at 33 °C, and not altered by E2 replacement. These data provide unique evidence that KNDy neurons promote cutaneous vasodilatation and participate in the E2 modulation of body temperature. Because cutaneous vasodilatation is a cardinal sign of a hot flush, these results support the hypothesis that KNDy neurons could play a role in the generation of flushes.
Keywords: reproduction, gonadotropin-releasing hormone, thermoregulation
Estrogen withdrawal leads to hot flushes in the majority of menopausal women (1). Hot flushes are also experienced by men and women treated with tamoxifen for breast cancer, men undergoing androgen-ablation therapy for prostate cancer, young oophorectomized women, and hypogonadal men (2, 3). A hot flush is characterized by episodic activation of heat dissipation effectors, including cutaneous vasodilatation, sweating, and behavioral thermoregulation. After a flush is initiated, the activation of heat dissipation mechanisms is so effective that core temperature frequently drops (4). Despite the vast numbers of individuals affected, the etiology of flushes remains an enigma.
Hot flushes are closely timed with luteinizing hormone (LH) pulses, providing a clue that the generation of flushes is linked to the hypothalamic neural circuitry controlling pulsatile gonadotropin-releasing hormone (GnRH) secretion (5, 6). Current evidence suggests that pulsatile GnRH secretion is modulated by a subpopulation of neurons in the arcuate (infundibular) nucleus that express estrogen receptor α (ERα), neurokinin 3 receptor (NK3R), kisspeptin, neurokinin B (NKB), and dynorphin (7–11). In the hypothalamus of postmenopausal women, these kisspeptin/NKB/dynorphin (KNDy) neurons undergo an unusual somatic hypertrophy and express increased kisspeptin and NKB gene transcripts (12–15). Studies of cynomolgus monkeys indicate that the changes in KNDy neurons in postmenopausal women are secondary to withdrawal of ovarian estrogens and not due to aging per se (13, 15–17). Mutations in the genes encoding kisspeptin, NKB, or their receptors result in hypogonadotropic hypogonadism, a syndrome characterized by lack of pubertal development, impaired gonadotropin secretion, absence of secondary sex characteristics, and infertility (18–21). Thus, the hypertrophied neurons in the hypothalamus of postmenopausal women express two peptides, kisspeptin and NKB, that are essential for human reproduction.
Because ERα-expressing KNDy neurons are markedly altered in the hypothalamus of postmenopausal women, we hypothesized that these neurons could be involved in the generation of hot flushes (12). Consistent with this hypothesis, tract-tracing studies in the rodent have shown that KNDy neurons project to structures critical for the regulation of body temperature (22, 23) including the median preoptic nucleus (MnPO), an important component of a thermosensory heat-defense pathway (24). Moreover, pharmacological activation of NK3R-expressing neurons in the MnPO elicits a robust decrease in TCORE and alters tail skin vasomotion (25). Whereas these data indicate that NK3R signaling may influence body temperature at the level of the MnPO, there is no information on whether KNDy neurons modulate body temperature or mediate the effects of estrogen on the thermoregulatory system.
Studies of laboratory rats have provided useful information on the effects of estrogens on the activation of heat-dissipation effectors. Cutaneous vasodilatation of the rat's tail is a major heat-defense effector that can be evaluated indirectly by measuring changes in tail skin temperature (TSKIN) (26, 27). The vasomotor state of the tail varies across the estrous cycle, with the lowest levels of vasodilatation on proestrous night, when estrogen levels are high (28). Consistent with these findings, tail skin vasodilatation is increased by ovariectomy and decreased by treatment with estrogens (28–31). Although the increase in the average TSKIN after ovariectomy does not reflect an episodic event, like a flush, the reduction of TSKIN by a pharmacological agent has been widely used to evaluate its efficacy for reducing flushes in women (29, 32–35). Moreover, 17β-estradiol (E2) replacement in ovariectomized (OVX) rats shifts the thermoneutral zone (36), reduces Fos protein expression in the MnPO (37), and alters heat-escape behavior (38). Finally, at high ambient temperatures (TAMBIENT), OVX rats exhibit higher core temperatures (TCORE) relative to OVX rats treated with E2 (36, 39). Whereas these studies show pronounced effects of E2 on thermoregulation in the rat, the underlying neural circuitry mediating these changes is largely unknown.
In the present study, we evaluate the thermoregulatory effects of ablating KNDy neurons using stereotaxic injections of NK3-saporin (SAP), a selective neurotoxin for NK3R-expressing cells. The utility of NK3-SAP for selective ablation of KNDy neurons was recently documented (11). Circadian rhythms of TSKIN, TCORE, and activity were evaluated in KNDy-ablated and control rats that were OVX and then replaced with E2. Rats were also exposed to subneutral, neutral, and supraneutral TAMBIENT in an environmental chamber to determine if KNDy neuron ablation altered the ability to defend TCORE in response to environmental temperature challenges. Our previous studies have shown that KNDy neurons are required for tonic gonadotropin secretion, the rise in LH secretion after ovariectomy, and the E2 modulation of body weight (11). Here we determine if KNDy neurons also participate in the E2 modulation of thermoregulation.
Results
Experiment 1: KNDy Neuron Ablation Decreased Tail Skin Vasodilatation.
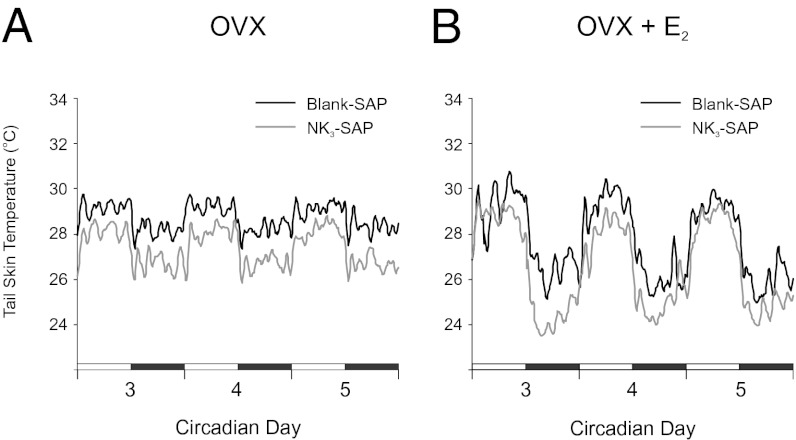
The experimental protocol is illustrated in Fig. 1. Prominent circadian rhythms of TSKIN and TCORE were observed in both NK3-SAP and Blank-SAP controls. TCORE and TSKIN exhibited a circadian periodicity of 24 h, with no significant differences in circadian rhythms between groups. However, inspection of the average circadian waveforms revealed consistently lower TSKIN in NK3-SAP rats compared with controls (Fig. 2). Statistical analysis confirmed that the average TSKIN of NK3-SAP rats was significantly lower than Blank-SAP controls in both phases of the light/dark cycle and in OVX and OVX + E2 conditions (Figs. 2 and 3B). During the dark phase of the circadian rhythm recordings, E2 treatment of OVX Blank-SAP and NK3-SAP rats reduced TSKIN (Figs. 2 and 3B). During the light phase, E2 treatment did not lower TSKIN in either Blank-SAP or NK3-SAP rats (Fig. 3B). The heat loss index (HLI), an index of tail skin vasomotion that controls for ambient and core temperatures (26), was also consistently lower in NK3-SAP rats than Blank-SAP controls (Table 1). These results indicate that KNDy-ablated rats have lower levels of tail skin vasodilatation than Blank-SAP controls.
Fig. 1.
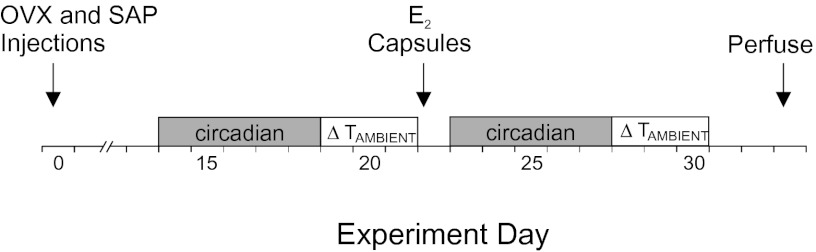
Experimental protocol. On day 0, the rats were ovariectomized, injected with Blank-SAP or NK3-SAP in the arcuate nucleus, implanted with telemetry devices and returned to their home cages. After a 12- to 15-d recovery period (shown as day 14 in this example), TCORE, TSKIN, and activity were recorded every 10 min over 5 d to evaluate changes over the light/dark cycle (circadian). The next three mornings, TCORE and TSKIN were recorded in rats exposed to TAMBIENT of 26 °C, 11 °C, and 33 °C (in that order) in an environmental chamber. One to 3 d later (shown as day 22 in this example), rats received s.c. E2 capsules. The circadian rhythm recordings were repeated, followed by the TAMBIENT exposures. The rats were killed 11 d after E2 treatment. E2,17β-estradiol; OVX, ovariectomized; SAP, saporin; TAMBIENT, ambient temperature.
Fig. 2.
Effects of KNDy neuron ablation on average TSKIN in OVX (A) and OVX + E2 rats (B). (A) Circadian rhythms of TSKIN were not different between NK3-SAP and Blank-SAP rats. However, OVX NK3-SAP rats exhibited consistently lower TSKIN than OVX Blank-SAP rats. (B) After E2 treatment, TSKIN was still consistently lower in NK3-SAP rats than Blank-SAP rats. E2 reduced TSKIN during the dark phase in both Blank-SAP and NK3-SAP rats (compare with A). These waveforms represent the average TSKIN for each group (6–11 rats per group) and were generated using a moving average of five data points. Dark bars represent the dark phase of the 24-h cycle, and numbers refer to circadian recording days 3, 4, and 5. Statistical analyses of these data are shown in Fig. 3B.
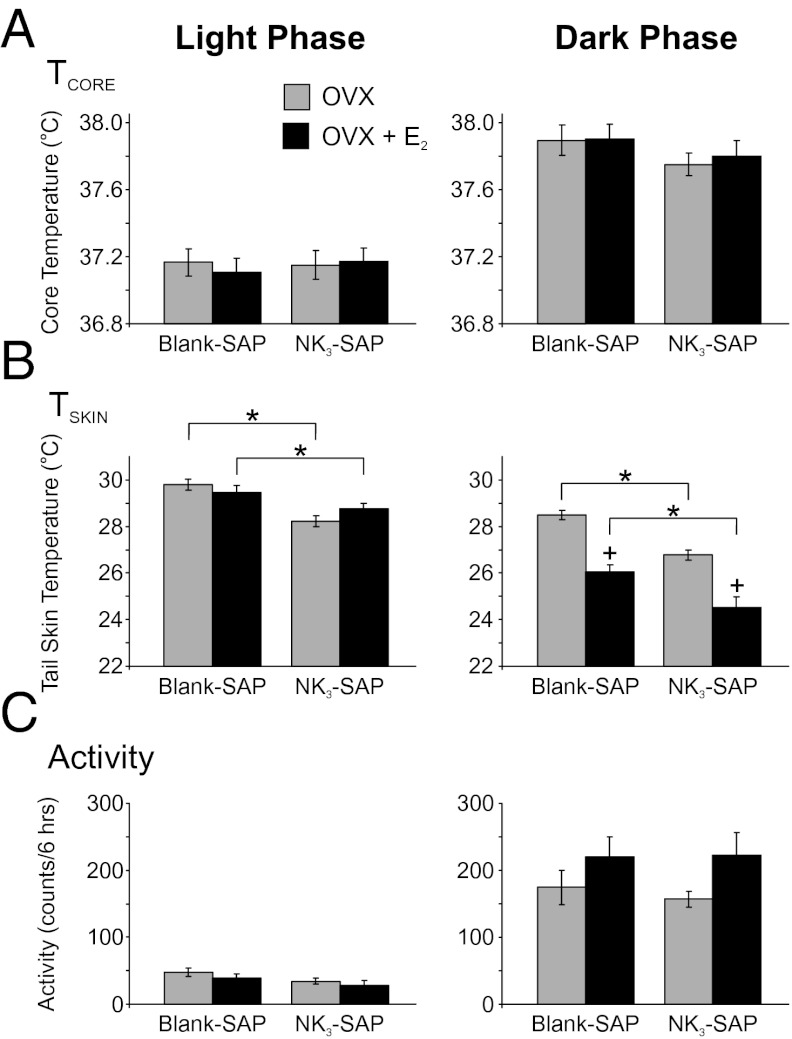
Fig. 3.
Effects of KNDy neuron ablation and E2 treatment on TCORE (A), TSKIN (B), and activity (C) during the light (Left) and dark (Right) phases. (A) Average TCORE was increased in the dark phase (compared with light) in all groups with no significant effect of NK3-SAP or E2 treatment. (B) Average TSKIN was consistently lower in NK3-SAP rats compared with Blank-SAP rats, indicative of lower levels of cutaneous vasodilatation. In the dark phase, TSKIN was decreased by E2 treatment in both Blank-SAP and NK3-SAP rats. (C) Activity (detected by the DSI telemetry probe) was markedly increased in the dark (active) phase, with no significant differences between Blank-SAP– and NK3-SAP–treated groups. There was a trend (P = 0.06) for E2 to increase activity in the dark phase in both Blank-SAP and NK3-SAP rats. Values represent mean ± SEM, n = 6–11 rats per group. *Significantly different (NK3-SAP vs. Blank-SAP, within OVX or OVX + E2). +Significantly different (OVX vs. OVX + E2, within Blank-SAP or NK3-SAP).
Table 1.
Heat loss index in control (Blank-SAP) and KNDy-ablated rats (NK3-SAP)
| Blank-SAP |
NK3-SAP |
|||
| OVX | OVX + E2 | OVX | OVX + E2 | |
| Light phase | 0.49 ± 0.01 | 0.47 ± 0.02 | 0.39 ± 0.01* | 0.42 ± 0.01* |
| Dark phase | 0.39 ± 0.01 | 0.24 ± 0.02+ | 0.29 ± 0.01* | 0.15 ± 0.02*,+ |
Values represent mean ± SEM calculated from 6-h time blocks in the middle of each phase, n = 8–11 rats per group.
*Significantly different (NK3-SAP vs. Blank-SAP, within OVX or OVX + E2).
+Significantly different (OVX + E2 vs. OVX, within Blank-SAP or NK3-SAP).
TCORE was ∼0.5 °C higher during the dark phase than in the light phase, but within each phase, there was no effect of NK3-SAP or E2 treatment on the average TCORE (Fig. 3A). The average activity of all groups of rats was markedly increased in the dark phase but there was no significant effect of NK3-SAP on activity within either the dark or the light phase (Fig. 3C). There was a trend for E2 to increase activity in the dark phase in both Blank-SAP and NK3-SAP rats (P = 0.06).
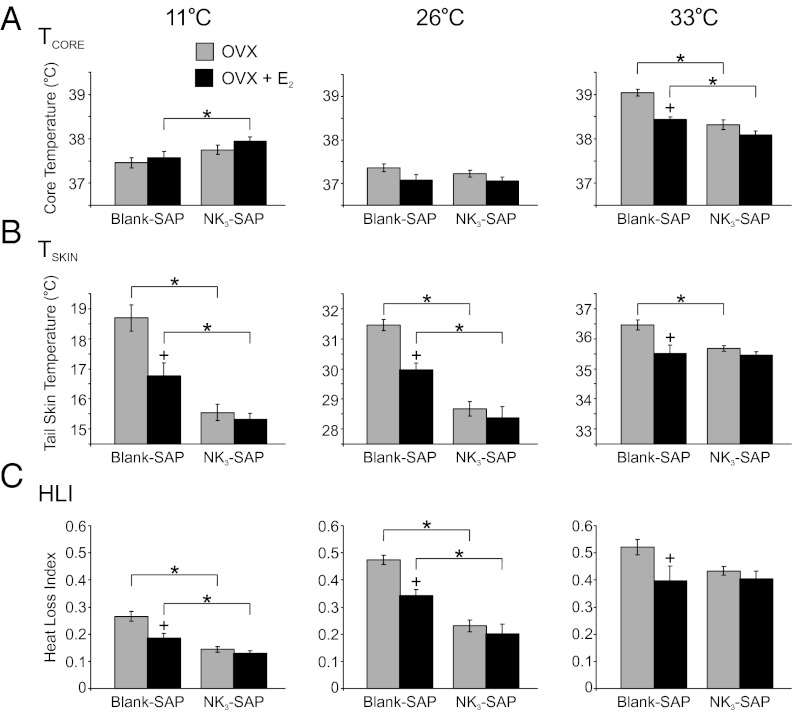
Experiment 2: At the High TAMBIENT of 33 °C, the TCORE of OVX KNDy-Ablated Rats Was Lower than in OVX Blank-SAP Rats and Unresponsive to E2 Replacement.
At the supraneutral TAMBIENT of 33 °C, the average TCORE of all groups was elevated, compared with rats exposed to the neutral TAMBIENT of 26 °C. However, the highest body temperature was observed in OVX Blank-SAP controls at the TAMBIENT of 33 °C (Fig. 4A). Consistent with previous studies (36, 37, 39), this hyperthermic TCORE was significantly reduced by E2 treatment (Fig.4A). At the TAMBIENT of 33 °C, the average TCORE of OVX NK3-SAP rats was significantly lower than that of OVX Blank-SAP rats. Moreover, unlike OVX controls, the average TCORE of OVX NK3-SAP rats was not significantly reduced by E2 treatment. At the lower TAMBIENT of 11 °C, TCORE was not altered by E2 or NK3-SAP, except for a mild increase in TCORE in OVX E2-treated NK3-SAP rats, compared with OVX E2-treated Blank-SAP controls.
Fig. 4.
Average TCORE (A), TSKIN (B), and heat loss index (HLI) (C) in Blank-SAP and NK3-SAP rats exposed to TAMBIENT of 11 °C, 26 °C, or 33 °C (Left to Right). (A) At the TAMBIENT of 33 °C the TCORE was the highest in OVX Blank-SAP rats and reduced in these rats by E2 treatment. At the TAMBIENT of 33 °C, the TCORE of NK3-SAP rats was significantly lower than Blank-SAP animals and was not affected by E2. (B) At the TAMBIENT of 11 °C and 26 °C, the TSKIN of NK3-SAP animals was significantly lower than in Blank-SAP animals. The TSKIN of the Blank-SAP rats was reduced by E2 at all TAMBIENT, but TSKIN was not lowered by E2 treatment in NK3-SAP rats at any TAMBIENT. (C) At the TAMBIENT of 11 °C and 26 °C, the average HLI of NK3-SAP rats was significantly lower than in Blank-SAP rats, indicating lower levels of vasodilatation. HLI was reduced by E2 in Blank-SAP rats but not in NK3-SAP rats. Values represent mean ± SEM, 6–11 rats per group. *Significantly different (NK3-SAP vs. Blank-SAP, within OVX or OVX + E2). +Significantly different (OVX + E2 vs. OVX, within Blank-SAP or NK3-SAP).
KNDy Neuron Ablation Decreases Tail Skin Vasodilatation in Rats Exposed to Subneutral and Neutral TAMBIENT and Prevents the Effects of E2 on TSKIN.
At all three TAMBIENT, OVX Blank-SAP rats exhibited the highest TSKIN (Fig. 4B). In agreement with previous studies (e.g., ref. 37), E2 treatment significantly decreased TSKIN and HLI in OVX Blank-SAP controls (Fig. 4 B and C). In contrast, E2 had no effect on TSKIN or HLI in NK3-SAP rats at any ambient temperature. Similar to the findings observed during the circadian recordings, at the TAMBIENT of 11 °C and 26 °C, the TSKIN and HLI were significantly lower in NK3-SAP rats than Blank-SAP rats, in both OVX and OVX + E2 conditions, indicative of lower levels of tail skin vasodilatation. NK3-SAP rats exposed to the TAMBIENT of 33 °C exhibited a higher HLI than NK3-SAP rats at the TAMBIENT of 26 °C (Fig. 4C). Thus, despite the consistently lower levels of tail vasodilatation in NK3-SAP rats at lower TAMBIENT, these rats were still capable of tail skin vasodilatation when exposed to a high TAMBIENT. At the supraneutral TAMBIENT of 33 °C, OVX NK3-SAP rats exhibited a lower TSKIN than OVX Blank-SAP controls but the HLI was not significantly different between these two groups.
Discussion
Estrogen withdrawal alters thermoregulation in rats and causes hot flushes in humans, but the neural circuits underlying these effects are unknown. In the present study, we determined the thermoregulatory effects of ablating KNDy neurons using stereotaxic injections of NK3-SAP. Remarkably, KNDy-ablated rats exhibited robust decreases in TSKIN and HLI, indicating decreased tail skin vasodilatation. This effect occurred independent of E2 treatment, throughout the light/dark cycle, and in the environmental chamber at the TAMBIENT of 11 °C and 26 °C. Although tail skin vasoconstriction is a physiological mechanism that conserves heat, KNDy-ablated rats were able to regulate TCORE. In addition, KNDy-ablated rats were still capable of tail skin vasodilatation when exposed to the high TAMBIENT of 33 °C. Under all other conditions, the HLIs of KNDy-ablated animals were lower than controls, indicating that KNDy neurons facilitate cutaneous vasodilatation.
KNDy neurons could facilitate tail skin vasodilatation via projections to rostral hypothalamic structures that control thermoregulatory effectors, such as the medial preoptic area and MnPO (22). Notably, using single-cell transcriptomics, tachykinin receptor 3 (Tacr3) gene expression (encoding NK3R) was detected in warm-sensitive, GABAergic neurons in the medial preoptic area (40). Excitation of warm-sensitive, GABAergic neurons results in tail skin vasodilatation through projections that inhibit tonic activation of sympathetic (vasoconstrictor) premotor neurons in the ventromedial medulla (41, 42). Therefore, in KNDy-ablated rats, removal of an excitatory input to NK3R-expressing warm-sensitive medial preoptic neurons could explain the lower levels of tail skin vasodilatation. KNDy neurons could also influence tail vasomotion via projections to the MnPO, a major component of a thermosensory pathway regulating heat-defense effectors (24). MnPO Fos expression is modulated by changes in TAMBIENT or chronic E2 treatment, implicating this nucleus as a site for integrating thermoregulatory and reproductive functions (37). MnPO neurons express the NK3R protein (25) and Tacr3 mRNA (43). Moreover, focal microinfusion of an NK3R agonist activates MnPO neurons (as measured by Fos), elicits a robust drop in TCORE and inhibits sympathetic vasoconstriction (25). MnPO neurons project to other thermoregulatory regions of the hypothalamus (24, 44, 45) as well as neurons in the ventromedial medulla controlling tail vasomotion (42, 46–48). Thus, removal of excitatory KNDy neuron projections to the MnPO or medial preoptic area (or both) could provide a mechanism for the lower levels of tail skin vasodilatation in KNDy-ablated rats.
Consistent with numerous studies, tail skin vasodilatation was decreased by E2 in OVX control rats (28–31, 36, 38). During the dark phase of the circadian recordings, E2 treatment of OVX controls markedly reduced TSKIN and HLI. E2 did not alter TSKIN or HLI during the light phase of the circadian recordings (3–5 d after E2 capsules), but did decrease TSKIN and HLI during the light phase in the TAMBIENT experiments (6–9 d after E2 capsules). This discrepancy is consistent with previous studies that have reported that E2 effects on TSKIN during the light phase do not appear until 7 d after E2 replacement (28). Interestingly, ablation of KNDy neurons blocked the effect of E2 on TSKIN in the light phase (during the TAMBEINT challenges), although it did not prevent the effect of E2 on TSKIN during the dark phase. Thus, KNDy neurons are essential for E2 to reduce tail skin vasodilatation during the light phase, but not during the dark phase.
At the supraneutral TAMBIENT of 33 °C, the average TCORE of OVX control rats increased to 39.0 °C and this value was reduced by E2 treatment. These findings are similar to other studies showing that E2 treatment improved the ability to maintain core temperature when exposed to high ambient temperatures (36, 39). Because the primary function of hypothalamic thermoregulation is to defend TCORE across a wide range of TAMBIENT (49), these data indicate that ovariectomy induces thermoregulatory dysfunction, which is corrected by E2 treatment. Interestingly, OVX KNDy-ablated rats exposed to the high TAMBIENT were able to maintain their body temperature closer to normal than OVX control rats. Moreover, unlike controls, E2 did not reduce TCORE in rats with KNDy neuron ablation. These results indicate that KNDy neurons mediate the E2 reduction of body temperature in OVX rats exposed to a high TAMBIENT.
We do not understand how KNDy neuron ablation improved TCORE regulation at the supraneutral TAMBIENT, just as we do not understand how E2 treatment protects against hyperthermia at this TAMBIENT. A change in metabolic activity does not explain the lower TCORE in OVX E2-treated controls because E2 treatment of OVX rodents either has no effect (38), or increases metabolic activity (50), which would result in a higher TCORE, not lower. The E2 effect on TCORE in control rats was also not secondary to increased vasodilatation, because the HLI was reduced by E2, reflecting decreased vasodilatation. However, in rats exposed to a supraneutral TAMBIENT, vasodilatation becomes maximal and insufficient to further reduce TCORE. Other heat-defense effectors are activated, including evaporative cooling from saliva spreading, postural changes, and heat escape behavior (26, 51). TCORE is also influenced by numerous other factors, such as respiratory rate, the surface to mass ratio, insulation, and integration with homeostatic systems controlling water balance and food intake (51). Therefore, many factors could potentially play a role in the E2 reduction of TCORE in a supraneutral TAMBIENT. Although the mechanism remains to be determined, these findings are relevant to understanding thermoregulatory dysfunction in menopausal women, who tolerate heat stress better after estrogen replacement therapy (52).
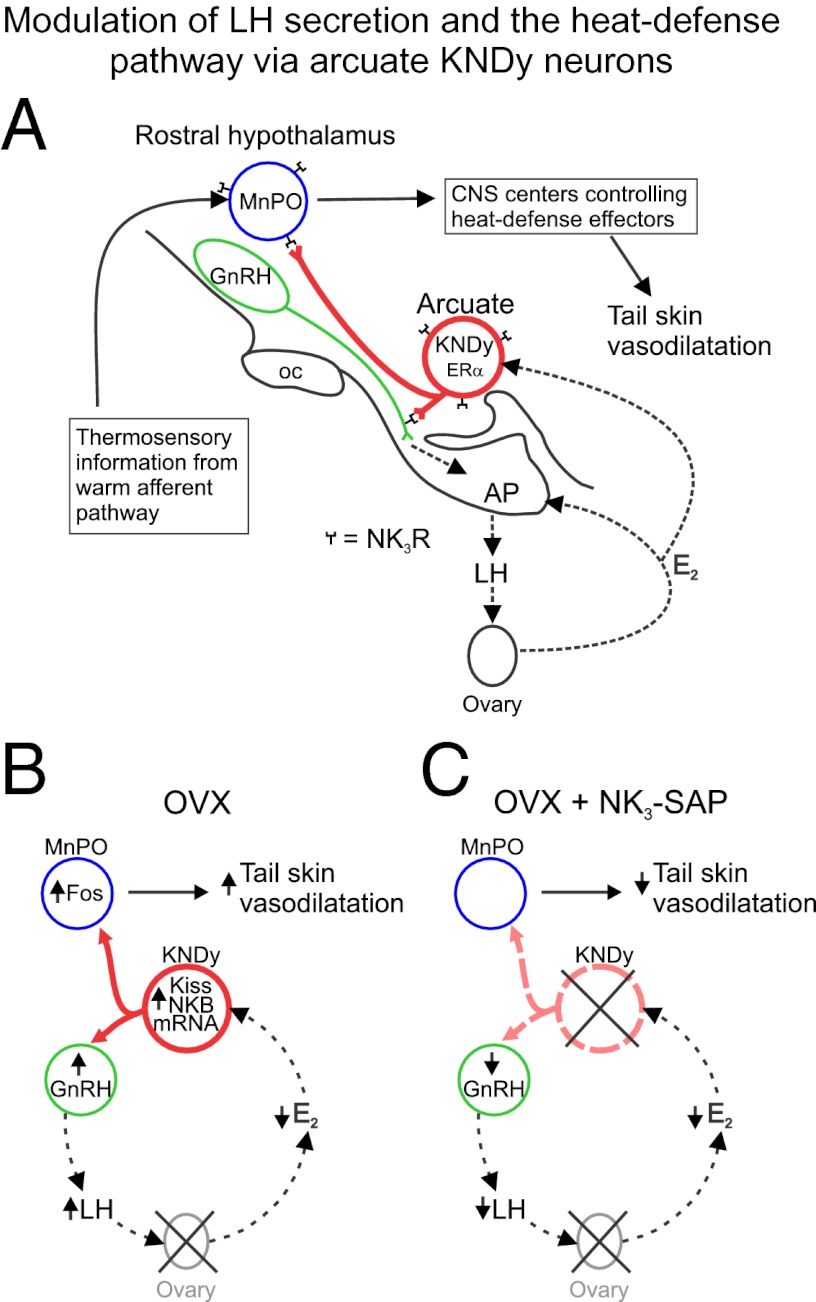
We have previously shown that KNDy neurons are required for the rise in LH secretion after the removal of E2 negative feedback (11). Fig. 5 illustrates a hypothetical circuit for KNDy neurons to mediate E2 effects on LH secretion and cutaneous vasodilatation in the rat. We hypothesized that estrogen withdrawal in humans leads to menopausal flushes by increasing the activity of KNDy neurons. This hypothesis is supported by the increased NKB and kisspeptin gene expression in postmenopausal women (12, 13), the close timing of LH pulses and flushes (5), the putative role of KNDy neurons in stimulating pulsatile LH secretion (7–10), the expression of ERα in KNDy neurons (12), and the demonstration that estrogen suppresses hot flushes (3) as well as NKB and kisspeptin gene expression in young ovariectomized monkeys (13, 16). In the present study, ablation of KNDy neurons consistently decreased tail skin vasodilatation in OVX rats, mimicking the thermoregulatory effects of E2 treatment. In addition, KNDy-neuron ablation blocked the effects of E2 on TCORE in a warm environment and TSKIN during the light phase. The demonstration that KNDy neurons facilitate tail skin vasodilatation is particularly relevant, because cutaneous vasodilatation is a cardinal feature of the menopausal hot flush. These findings provide strong support for the hypothesis that KNDy neurons could play a role in the generation of flushes.
Fig. 5.
(A) Relationship between KNDy neurons, GnRH neurons, and the heat-defense pathway in the rat. KNDy neurons branch within the arcuate nucleus and project to GnRH terminals in the median eminence (22, 56) and preoptic areas that regulate body temperature, including the MnPO (22–24, 42, 45, 48). Secretion of GnRH into portal capillaries stimulates LH secretion from the anterior pituitary gland, which stimulates the secretion of E2 from the ovaries. E2 negative feedback reduces serum LH and decreases NKB and kisspeptin mRNA in KNDy neurons (57, 58). ERα, the isoform required for estrogen negative feedback (59), is expressed in arcuate KNDy neurons (60) but not GnRH neurons (61). NK3R is expressed on arcuate KNDy neurons (60) and GnRH terminals in the median eminence (56). GnRH neurons express kisspeptin receptor mRNA (62), but the distribution of the kisspeptin receptor protein on GnRH neurons has not been described. MnPO neurons express NK3R and pharmacological activation of these neurons reduces body temperature (25). The MnPO receives information from warm-sensitive, cutaneous thermoreceptors and projects to CNS centers to modulate heat-dissipation effectors (24, 42). (B) Effects of ovariectomy on serum LH and tail skin vasodilatation: Loss of E2 after ovariectomy markedly increases NKB and kisspeptin gene expression in KNDy neurons (57, 58), increases GnRH secretion into the portal capillaries (63) and LH secretion from the anterior pituitary gland. In the MnPO, at neutral TAMBIENT, Fos is increased in OVX rats, compared with OVX + E2 rats (37). Tail skin vasodilatation is increased by ovariectomy and decreased in OVX rats by E2 replacement (28–31). (C) Effects of KNDy neuron ablation in OVX rats: KNDy neuron ablation lowers serum LH and prevents the rise in LH secretion after ovariectomy (11). Because KNDy neurons do not project to the portal capillary system to directly influence pituitary gonadotrophs (56), this effect is likely mediated via lower levels of GnRH secretion. Ablation of KNDy neurons (with loss of their projections to the rostral hypothalamus) also reduces tail skin vasodilatation. These findings suggest that withdrawal of E2 in OVX rats increases LH secretion and tail skin vasodilatation by increasing the activity of KNDy neurons. AP, anterior pituitary gland; ERα, estrogen receptor α; E2, estradiol-17β; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; Kiss, kisspeptin; KNDy, kisspeptin, neurokinin B, and dynorphin-expressing neurons; MnPO, median preoptic nucleus; NKB, neurokinin B; NK3R, neurokinin 3 receptors; oc, optic chiasm.
Methods
Young adult, female Sprague-Dawley rats (approximately 12 wk old, 200–250 g; Harlan Laboratories) were individually housed in a quiet, temperature- and humidity-controlled room (ambient temperature of 21.1–22.5 °C, humidity set at 50%) in the University of Arizona Animal Care Facility with a 12:12 h light:dark cycle (lights on at 0700 h). Rats had ad libitum access to water and a low-phytoestrogen diet (Harlan Teklad 2014; Harlan Laboratories). All protocols were approved by the University of Arizona Animal Care and Use Committee and conformed to National Institutes of Health (Bethesda, MD) guidelines. The serum hormone levels, body weights, and the brain histology from rats used in the present study have been previously described (11). Please refer to ref. 11 for detailed methods and documentation of the selectivity of KNDy neuron ablation.
Rats (n = 24) were OVX under general anesthesia using a mixture (1.0 mL/kg i.m.) containing ketamine (33.3 mg/mL), xylazine (10.7 mg/mL), and acepromazine (1.3 mg/mL). A PhysioTel transmitter (TA10-F40; Data Sciences International, DSI) was inserted into the peritoneal cavity for measurements of TCORE and activity. Stereotaxic surgery was used to make bilateral injections of 10 ng NK3-SAP (Advanced Targeting Systems; n = 14) in 100 nL PBS into the arcuate nucleus (two on each side). Control rats (n = 10) received 4 arcuate injections of 10 ng of Blank-SAP (11-aa peptide conjugated to saporin, Advanced Targeting Systems) in 100 nl PBS. Twenty to 23 d after the initial surgery, under isoflurane anesthesia, rats were implanted with two s.c. capsules (each 20 mm effective length, 1.57 mm inner diameter, 3.18 mm outer diameter; Dow Corning) containing 360 μg/mL 17β-estradiol dissolved in sesame oil. RIA showed that this regimen produced physiological levels of serum E2 that were similar in Blank-SAP– and NK3-SAP–treated animals (11).
Temperature Recordings.
TCORE and gross motor activity were measured via telemetry using the implanted DSI transmitter. A previous study showed that activity detected by telemetry is comparable to the activity detected from infrared motion sensors (53). Individual cages were placed on an RPC-1 Physiotel receiver that was connected by a Data Exchange Matrix (DSI) to a computer equipped with Dataquest A.R.T. software (DSI). TSKIN was recorded with a SubCue Mini datalogger (SubCue Dataloggers) as previously described (28). The dataloggers were housed in a protective nylon casing taped to the lateral surface of the tail (4.0 cm from the base) under brief (<5 min) isoflurane anesthesia. The magnitude of E2 effects on TSKIN recorded by these probes is similar to that obtained by surgically implanted telemetry devises (28, 32, 34). TAMBIENT was recorded with an IT-18 thermocouple (Physitemp) inserted into a QuadTemp datalogger (Madgetech). Temperature measurement devices were calibrated according to manufacturer specifications and validated against a National Institute of Standards and Technology certified TC4000 thermocouple datalogger (Madgetech).
Experiment 1: Effects of KNDy Neuron Ablation on Circadian Rhythms of TCORE, TSKIN, and Activity in OVX and OVX + E2 Rats.
Twelve to 15 d after the initial surgery, TCORE, TSKIN, activity, and TAMBIENT were recorded every 10 min for five consecutive 24-h light/dark cycles (Fig. 1). One day after E2 capsule implantation, these recordings were repeated for another five 24-h cycles. For these 5-d recording sessions, the rats were housed in their home cages in a dedicated room in the animal facility that was relatively isolated from noise and had access allowed only to the investigators. Cage cleaning was conducted before and after the 5-d recordings. The rats were placed in transparent, plastic shoebox-style cages containing wood-shaving bedding and the cages were placed on individual telemetry receivers. Although individual cages were needed for telemetry, the rats maintained visual, auditory, and olfactory contact with other animals.
Experiment 2: Effects of KNDy Neuron Ablation on the E2 Modulation of TCORE, TSKIN, and HLI in Rats Exposed to TAMBIENT of 26 °C, 11 °C, or 33 °C.
To determine if KNDy neuron ablation altered the ability of the rats to defend TCORE in response to environmental temperature challenges, rats were exposed to temperatures that were within the thermoneutral zone (26 °C), subneutral (11 °C), or supraneutral (33 °C) TAMBIENT. The thermoneutral zone is defined as the range of TAMBIENT in which thermoregulation is achieved only by sensible (dry) heat loss, without regulatory changes in metabolic heat production or evaporative cooling (54). Within the thermoneutral zone, TCORE is regulated primarily by skin vasomotion. The selection of TAMBIENT was based on our previous analysis of the thermoneutral zone in OVX and OVX + E2 rats (36).
After the completion of the circadian rhythm recordings, animals were brought to the laboratory for three consecutive mornings and exposed to one of three TAMBIENT in an environmental chamber. The experiments were conducted in the morning to avoid the confounding influence of E2 positive feedback. Animals were habituated to the experimental procedure on three occasions within the first 14 d after surgery. The environmental chamber (Forma model 3940; Thermo Scientific) was equilibrated to the required temperature with humidity set at 50%. Each rat was placed in a 6 inch × 6 inch × 4 inch plastic grid cage, which allowed free movement and ad libitum access to food and water as described previously (36). The cages were placed on telemetry receivers in the environmental chamber and TCORE, TSKIN, and TAMBIENT were recorded every 10 min for 3 h. This procedure was repeated after the OVX rats were implanted with E2 and the second set of circadian rhythm recordings were obtained (Fig. 1).
For sacrifice, animals were injected with a lethal dose of sodium pentobarbital (100 mg/kg i.p.) and perfused through the ascending aorta with 200 mL of 0.1 M phosphate buffered heparinized saline followed by 400 mL of 4% (wt/vol) paraformaldehyde in 0.1 M PBS, pH 7.4. Brains were extracted and immunohistochemical methods were used to characterize the effects of NK3-SAP injections. Three NK3-SAP rats with preservation of KNDy neurons were considered to be “missed” injections and excluded from further analysis. As reported previously, the rats injected with NK3-SAP in the arcuate nucleus exhibited near-complete degeneration of KNDy neurons with variable, incomplete loss of lateral hypothalamic NK3R neurons adjacent to the injection site (11). Selectivity was demonstrated by intact Nissl architecture and preservation of proopiomelanocortin, neuropeptide Y, and GnRH immunoreactive elements in the arcuate nucleus and adjacent median eminence (11).
Data Analysis.
A temperature probe on the tail skin surface will reflect not only active changes in vasomotor tone, but also passive changes in TAMBIENT and TCORE. To provide a more accurate assessment of tail skin vasomotion, we calculated the heat loss index [HLI = (TSKIN − TAMBIENT)/(TCORE − TAMBIENT)], which removes the passive influences of ambient and core temperatures and is strongly correlated with blood flow (26). The theoretical range of the HLI is between 0 (corresponding to maximal skin vasoconstriction) and 1 (maximal skin vasodilatation).
Circadian rhythms were evaluated over the 5-d period using circadian physiology software (55). Averages of TSKIN, TCORE, HLI, and activity were calculated for each animal during light (inactive) or dark (active) phases. Six-hour time blocks were used from the middle of each phase (1000 h to 1600 h and 2200 h to 0400 h for light and dark, respectively) to avoid the lights on/off transition. Because E2 effects on TSKIN during the dark phase are not significant until 3 d after capsule implantation (28), days 3–5 of the circadian recordings were used for statistical analysis. Group averages of TSKIN, TCORE, activity (counts/6 h), and HLI were generated from the means of individual rats. Group means were compared using a two-way ANOVA with Tukey’s post hoc analysis (α = 0.05).
For the temperature challenges, the mean TCORE and TSKIN for each animal was calculated from the second and third hour of recording in the environmental chamber. The averages from each animal were used to calculate group averages. Data were analyzed using a two-way ANOVA and Tukey’s post hoc tests (α = 0.05). Normothermia (37.2 ± 0.3 °C) was considered to be the average TCORE (±1 SD) during the light phase of all rats exposed to a TAMBIENT of 26 °C, which is within the thermoneutral zone of both OVX and OVX + E2 rats (36).
Acknowledgments
We thank Ms. Cheryl Johnson and her staff for facilitating the circadian rhythm recordings in the University of Arizona Animal Care Facility. This work was supported by National Institutes of Health, National Institute on Aging Grant R01 AG032315.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Kronenberg F. Hot flashes: Epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86, discussion 123–133. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- 2.Stearns V, et al. Hot flushes. Lancet. 2002;360(9348):1851–1861. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 3.Santoro N. Symptoms of menopause: Hot flushes. Clin Obstet Gynecol. 2008;51(3):539–548. doi: 10.1097/GRF.0b013e31818093f6. [DOI] [PubMed] [Google Scholar]
- 4.Freedman RR. Physiology of hot flashes. Am J Hum Biol. 2001;13(4):453–464. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- 5.Casper RF, Yen SSC, Wilkes MM. Menopausal flushes: A neuroendocrine link with pulsatile luteninizing hormone secreation. Science. 1979;205(4408):823–825. doi: 10.1126/science.462193. [DOI] [PubMed] [Google Scholar]
- 6.Tataryn IV, Meldrum DR, Lu KH, Frumar AM, Judd HL. LH, FSH and skin temperaure during the menopausal hot flash. J Clin Endocrinol Metab. 1979;49(1):152–154. doi: 10.1210/jcem-49-1-152. [DOI] [PubMed] [Google Scholar]
- 7.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. doi: 10.1016/j.brainres.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehman MN, Coolen LM, Goodman RL. Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakabayashi Y, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinsey-Jones JS, et al. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153(1):307–315. doi: 10.1210/en.2011-1641. [DOI] [PubMed] [Google Scholar]
- 11.Mittelman-Smith MA, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–2812. doi: 10.1210/en.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239–2247. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- 13.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92(7):2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- 14.Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20(12):1376–1381. doi: 10.1111/j.1365-2826.2008.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rance NE. Menopause and the human hypothalamus: Evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30(1):111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84(6):2111–2118. doi: 10.1210/jcem.84.6.5689. [DOI] [PubMed] [Google Scholar]
- 17.Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16(2):146–153. doi: 10.1111/j.0953-8194.2004.01143.x. [DOI] [PubMed] [Google Scholar]
- 18.Seminara SB, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 19.de Roux N, et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topaloglu AK, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topaloglu AK, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629–635. doi: 10.1056/NEJMoa1111184. [DOI] [PubMed] [Google Scholar]
- 22.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166(2):680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeo S-H, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152(6):2387–2399. doi: 10.1210/en.2011-0164. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA. 2010;107(19):8848–8853. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011;152(12):4894–4905. doi: 10.1210/en.2011-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: Ambient temperature for experiments in rats: A new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92(6):2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- 27.Gordon CJ, Puckett E, Padnos B. Rat tail skin temperature monitored noninvasively by radiotelemetry: Characterization by examination of vasomotor responses to thermomodulatory agents. J Pharmacol Toxicol Methods. 2002;47(2):107–114. doi: 10.1016/s1056-8719(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 28.Williams H, Dacks PA, Rance NE. An improved method for recording tail skin temperature in the rat reveals changes during the estrous cycle and effects of ovarian steroids. Endocrinology. 2010;151(11):5389–5394. doi: 10.1210/en.2010-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berendsen HHG, Kloosterboer HJ. Oestradiol and mirtazapine restore the disturbed tail-temperature of oestrogen-deficient rats. Eur J Pharmacol. 2003;482(1–3):329–333. doi: 10.1016/j.ejphar.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 30.Opas EE, Rutledge SJ, Vogel RL, Rodan GA, Schmidt A. Rat tail skin temperature regulation by estrogen, phytoestrogens and tamoxifen. Maturitas. 2004;48(4):463–471. doi: 10.1016/j.maturitas.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Cosmi S, et al. Simultaneous telemetric monitoring of tail-skin and core body temperature in a rat model of thermoregulatory dysfunction. J Neurosci Methods. 2009;178(2):270–275. doi: 10.1016/j.jneumeth.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Berendsen HHG, Weekers AHJ, Kloosterboer HJ. Effect of tibolone and raloxifene on the tail temperature of oestrogen-deficient rats. Eur J Pharmacol. 2001;419(1):47–54. doi: 10.1016/s0014-2999(01)00966-9. [DOI] [PubMed] [Google Scholar]
- 33.Alfinito PD, Huselton C, Chen X, Deecher DC. Pharmacokinetic and pharmacodynamic profiles of the novel serotonin and norepinephrine reuptake inhibitor desvenlafaxine succinate in ovariectomized Sprague-Dawley rats. Brain Res. 2006;1098(1):71–78. doi: 10.1016/j.brainres.2006.04.057. [DOI] [PubMed] [Google Scholar]
- 34.Bowe J, et al. The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J Endocrinol. 2006;191(2):399–405. doi: 10.1677/joe.1.06919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deecher DC, et al. Alleviation of thermoregulatory dysfunction with the new serotonin and norepinephrine reuptake inhibitor desvenlafaxine succinate in ovariectomized rodent models. Endocrinology. 2007;148(3):1376–1383. doi: 10.1210/en.2006-1163. [DOI] [PubMed] [Google Scholar]
- 36.Dacks PA, Rance NE. Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology. 2010;151(3):1187–1193. doi: 10.1210/en.2009-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dacks PA, Krajewski SJ, Rance NE. Ambient temperature and 17β-estradiol modify Fos immunoreactivity in the median preoptic nucleus, a putative regulator of skin vasomotion. Endocrinology. 2011;152(7):2750–2759. doi: 10.1210/en.2010-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosono T, et al. Effects of estrogen on thermoregulatory tail vasomotion and heat-escape behavior in freely moving female rats. Am J Physiol Regul Integr Comp Physiol. 2001;280(5):R1341–R1347. doi: 10.1152/ajpregu.2001.280.5.R1341. [DOI] [PubMed] [Google Scholar]
- 39.Baker MA, Dawson DD, Peters CE, Walker AM. Effects of estrogen on thermoregulatory evaporation in rats exposed to heat. Am J Physiol. 1994;267(3 Pt 2):R673–R677. doi: 10.1152/ajpregu.1994.267.3.R673. [DOI] [PubMed] [Google Scholar]
- 40.Eberwine J, Bartfai T. Single cell transcriptomics of hypothalamic warm sensitive neurons that control core body temperature and fever response signaling asymmetry and an extension of chemical neuroanatomy. Pharmacol Ther. 2011;129(3):241–259. doi: 10.1016/j.pharmthera.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romanovsky AA. Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29(38):11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J Comp Neurol. 1996;372(3):395–414. doi: 10.1002/(SICI)1096-9861(19960826)372:3<395::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11(1):62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romanovsky AA, et al. The transient receptor potential vanilloid-1 channel in thermoregulation: A thermosensor it is not. Pharmacol Rev. 2009;61(3):228–261. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura Y, Nakamura K, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience. 2009;161(2):614–620. doi: 10.1016/j.neuroscience.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura K, et al. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22(11):4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka M, McKinley MJ, McAllen RM. Roles of two preoptic cell groups in tonic and febrile control of rat tail sympathetic fibers. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R1248–R1257. doi: 10.1152/ajpregu.91010.2008. [DOI] [PubMed] [Google Scholar]
- 49.Kanosue K, Crawshaw LI, Nagashima K, Yoda T. Concepts to utilize in describing thermoregulation and neurophysiological evidence for how the system works. Eur J Appl Physiol. 2010;109(1):5–11. doi: 10.1007/s00421-009-1256-6. [DOI] [PubMed] [Google Scholar]
- 50.Xu Y, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordon CJ. Thermal biology of the laboratory rat. Physiol Behav. 1990;47(5):963–991. doi: 10.1016/0031-9384(90)90025-y. [DOI] [PubMed] [Google Scholar]
- 52.Tankersley CG, Nicholas WC, Deaver DR, Mikita D, Kenney WL. Estrogen replacement in middle-aged women: Thermoregulatory responses to exercise in the heat. J Appl Physiol. 1992;73(4):1238–1245. doi: 10.1152/jappl.1992.73.4.1238. [DOI] [PubMed] [Google Scholar]
- 53.Garami A, et al. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci. 2011;31(5):1721–1733. doi: 10.1523/JNEUROSCI.4671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The Commission for Thermal Physiology of the International Union of Physiological Sciences Glossary of terms for thermal physiology: Third edition. Jpn J Physiology. 2001;51:245–280. [Google Scholar]
- 55.Refinetti R. Circadian Physiology. 2nd Ed. Boca Raton, FL: CRC/Taylor and Francis Group; 2006. [Google Scholar]
- 56.Krajewski SJ, et al. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- 57.Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60(4):337–345. doi: 10.1159/000126768. [DOI] [PubMed] [Google Scholar]
- 58.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 59.Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78(4):204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- 60.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- 61.Hrabovszky E, et al. Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142(7):3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- 62.Han S-K, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarkar DK, Fink G. Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: Effects of steroids. J Endocrinol. 1980;86(3):511–524. doi: 10.1677/joe.0.0860511. [DOI] [PubMed] [Google Scholar]