Abstract
A critical challenge for physicians facing patients presenting with signs and symptoms of acute heart failure (AHF) is how and where to best manage them. Currently, most patients evaluated for AHF are admitted to the hospital, yet not all warrant inpatient care. Up to 50% of admissions could be potentially avoided and many admitted patients could be discharged after a short period of observation and treatment. Methods for identifying patients that can be sent home early are lacking. Improving the physician’s ability to identify and safely manage low-risk patients is essential to avoiding unnecessary use of hospital beds.
Two studies (STRATIFY and DECIDE) have been funded by the National Heart Lung and Blood Institute with the goal of developing prediction rules to facilitate early decision making in AHF. Using prospectively gathered evaluation and treatment data from the acute setting (STRATIFY) and early inpatient stay (DECIDE), rules will be generated to predict risk for death and serious complications. Subsequent studies will be designed to test the external validity, utility, generalizability and cost-effectiveness of these prediction rules in different acute care environments representing racially and socioeconomically diverse patient populations.
A major innovation is prediction of 5-day as well as 30-day outcomes, overcoming the limitation that 30-day outcomes are highly dependent on unpredictable, post-visit patient and provider behavior. A novel aspect of the proposed project is the use of a comprehensive cardiology review to correctly assign post-treatment outcomes to the acute presentation. Finally, a rigorous analysis plan has been developed to construct the prediction rules that will maximally extract both the statistical and clinical properties of every data element. Upon completion of this study we will subsequently externally test the prediction rules in a heterogeneous patient cohort.
A. Introduction
Nearly all acute heart failure (AHF) patients who present for emergency department (ED) evaluation are admitted for inpatient care. Up to half of these admissions could be avoided, with the lower risk patients being discharged and monitored on an outpatient basis after a brief period of observation.1–3 A critical challenge for physicians is determining who is a candidate for outpatient management and who should be admitted for more extensive and costly treatment. Currently, there are no generalizable or effective methods to assist the physician working in an acute care setting; the decision to admit the patient is notoriously conservative. There is also a lack of evidence to facilitate an early decision to discharge after a brief inpatient treatment period. Every avoidable admission places an unnecessary burden on the health care system. Thirty day readmissions are also common, especially in those discharged prematurely, and may be costly to hospitals due to lack of reimbursement. Improving the physician’s ability to appropriately manage the flow of AHF patients into and out of hospital is fundamental to reducing unnecessary inpatient days.
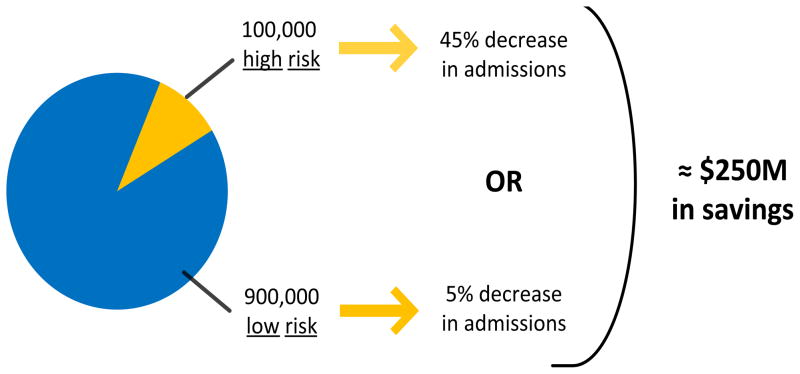
Studies of risk factors in patients with AHF have shown it is possible to identify variables associated with poor outcomes, such as inpatient complications, repeat admission for AHF, and death.4–19 While providing valuable information and suggesting the need for a prediction rule, these studies are limited in their clinical applicability and translation to the acute care setting. Data from inpatient sources have been combined with data from outpatient sources,4,5,7–11,14,16,17,20 retrospective chart review methodology has been employed6–12,14–17, and large databases designed for other purposes have been analyzed in an attempt to understand risk for poor outcomes.16 Moreover, the impact of decision making in the acute setting on outcomes measured at 30-days and beyond is blurred by outpatient management decisions and patient health behavior in the time between hospitalization and outcomes assessment. Further, when the majority of patients are being admitted to the hospital, identifying additional markers of high risk is not likely to impact acute decision making. We believe targeting “non-high-risk” AHF patients is most appropriate because of the potential return on investment and improvement in quality of life.21 Patients who are at high-risk for in-hospital events account for less than 10% (100,000) of the nearly 1,000,000 patients hospitalized annually with AHF.22,23 In order to impact 45,000 of these admissions, disposition decision making would have to change in 45% of the high-risk group. That contrasts starkly with the 900,000 “non-high-risk” patients. If disposition decision making could identify 5% of these 900,000 “non-high-risk” patients who are currently admitted but who could be eligible for safe, early ED or hospital discharge, it would impact up to 45,000 patients and conserve significant resources.(Figure 1)
Figure 1.
Percent of AHF admissions generally described as low and high risk, as well as projected resource savings after changes in admission rate. Beyond the clinical challenge of identifying high-risk versus low-risk patients safe for discharge, the much larger volume of those at low-risk allows for significantly smaller changes to have the same impact in resource savings.
The objective of the STRATIFY and DECIDE studies is to develop two multivariable AHF models based on established clinical and statistical standards24–32 that accurately estimate risk for adverse outcomes. The first rule (STRATIFY) will identify ED patients that do not need to be admitted to the inpatient setting, while the second (DECIDE) will facilitate discharge decisions during the early phase of an inpatient admission. These studies are designed to provide information that will conserve a significant amount of healthcare resources and improve quality of life by facilitating the decision to not admit a patient with signs or symptoms of AHF who does not require inpatient management, as well as shortening the length-of-stay for those patients who require admission.
B. Rationale for the STRATIFY and DECIDE Trials
Heart failure is a worldwide problem of epidemic proportions33 and a tremendous burden to overall healthcare costs. Nearly 6 million Americans have heart failure and about 670,000 new cases are diagnosed each year in the US alone.34 The incidence is expected to increase dramatically due to an aging population, improved survival from acute coronary syndromes, and advances in the management of cardiovascular diseases.35–37 Hospitalization for AHF accounts for the largest expenditure for care of these patients; it is estimated to be about $29.6 billion per year or, for Medicare patients, $5912 per discharge. This is more than double any cancer diagnosis,38–40 and represents about 3% of the total national health care budget.41 If innovative approaches are not developed to reduce these staggering costs, the economic burden will become unmanageable35:
1. STRATIFY Rationale
One-third of known heart failure patients receive inpatient care each year, and at least 80% of AHF presentations to an emergency department are admitted to the hospital.2,38 Patients seen in an emergency department (ED), admitted, and treated in an inpatient bed for AHF account for the majority of expenditures.42 Up to 80% of patients discharged from the hospital with a primary diagnosis of AHF come from the ED, thus decision making in this acute care environment is an ideal target for intervention. This target is the focus of STRATIFY.2,43,44 Improving the ability of the emergency physician to safely discharge a subset of ED patients with AHF would save significant healthcare resources. Based on ACC/AHA and AHCPR guidelines, it has been suggested up to 50% of admitted patients are low-risk and may be candidates for outpatient management in lieu of admission.2,20 Conversely, some patients may be discharged prematurely, as 20–30% of AHF inpatients are readmitted to the hospital in the subsequent 30 days.45,46 Thus, STRATIFY aims to find a balance between safe discharge and necessary inpatient care.
2. DECIDE Rationale
Poor risk-stratification in the acute setting, particularly overestimation of disease severity, is a major cause of over use of limited in-hospital resources for this rapidly growing patient population.44,47 Physicians’ tolerance for adverse events following discharge is low. Without evidence-based guidance the default decision is to admit, often for prolonged periods of treatment and observation.48 While STRATIFY aims to improve the ability of the physician to decide whether to admit patients with AHF, DECIDE is designed to help the physician identify the earliest time point after initial therapy where safe discharge may occur. This is critical to optimizing the allocation of in-hospital resources for those patients who are truly ill and require intensive management and therapy. Current decision guidelines are based on either little evidence, or are provided without evidence. 49–52 The 2009 ACC/AHA guidelines included a section on the “hospitalized patient” with AHF. There were 18 Class I recommendations, but only two of which related to acute management: 1) use natriuretic peptides if “contribution of heart failure is unknown” in the current presentation; and 2) if there is significant volume overload use a diuretic and begin in the acute setting.53 In a similar vein the Heart Failure Society of America published detailed guidelines on AHF management and factors that should prompt admission, but did not characterize patients safe for discharge.50 If only a small portion of AHF patients could be safely cared for in environments less costly than inpatient settings, or once hospitalized the length of stay could be reduced from the current 4–5 days, this would translate into substantial savings. We also seek to address this specific economic target.
3. Previous Risk Models: a Foundation for STRATIFY and DECIDE
Over the last twenty years, several studies have derived risk models for AHF patients.51,7,16 In 2005, a risk model predicting mortality was derived using data from a registry of 65,275 hospitalized patients with heart failure. 22 The model predicts a risk for mortality as low as 2.1% and demonstrates that data from the acute setting can be used to identify low-, moderate-, and high-risk patient groups. This is perhaps the most elegant tool to help decision making to date, yet its use remains limited since only admitted patients were included, only 39 of more than 100 variables available to the ED physician were considered, and the model was designed to predict mortality only. This model has particular utility for triaging patients to a specific level of care in the hospital, but it is not designed to help decide who should be admitted.
One major limitation that has yet to be addressed when evaluating risk of subsequent events is the relationship between outcomes and the acute presentation for heart failure. A mechanistic link between the acute event and subsequent outcomes is assumed, but rarely validated. More comprehensive assessment of the likely association between the adverse outcome and the acute event is required.
Other existing risk models for AHF suggest this area of research will prove successful, although they are limited for decision making in the acute setting. They tend to be developed from retrospective reviews of inpatient charts using convenience samples, only consider mortality as an outcome54, or consider outcomes remote from the presentation.4–19,55,56 These studies provide a useful starting point for our studies, namely a relatively comprehensive list of variables that have been identified as potentially useful for early decision making.
C. Design of STRATIFY and DECIDE
STRATIFY (www.clinicaltrials.gov identifier: NCT00508638) and DECIDE (www.clinicaltrials.gov identifier: NCT00911703) are prospective, observational studies of patients presenting to an acute setting with signs and symptoms of AHF. The studies are designed to develop prediction rules at complementary time points:
1. The rule to be derived from the STRATIFY dataset will be constructed to determine, in the ED setting, which patients are at low-risk of inpatient or outpatient death or serious in-hospital or out-of-hospital complications in AHF, and could thus be considered for outpatient management. 2. The rule to be derived from DECIDE will be constructed to identify patients who are at low-risk for inpatient or outpatient death and serious in-hospital or out-of-hospital complications after a short (1–3 day) hospital stay.
The STRATIFY prediction rule will be based on data obtained while a patient is being evaluated for AHF in the acute setting. The DECIDE prediction rule will be based on the use of data from both the acute care and inpatient setting, including measures of response to therapy. Our conceptual hypothesis is that these prediction rules will increase the number of patients for whom physicians can safely avoid admission or discharge rapidly after a short period of inpatient observation.
To develop the prediction rule for supporting ED disposition decisions (STRATIFY), we included variables typically available within the first three hours of presentation to an acute care setting. To develop the prediction rule for supporting decisions about early discharge of admitted patients (DECIDE), we also included treatments received and test results from the early inpatient stay, as well as patient and family interviews regarding access to care and adherence to medications and health care plans. We used trained research staff to prospectively collect data at the time a patient presents to the ED with symptoms of AHF and, for admitted patients, one day and three days into their inpatient stay. Relying on chart review or existing registry data often leads to missing, inconsistent or irrelevant information. Because of the prospective design and setting, our patient population is representative of clinical practice. By including all patients being evaluated for AHF regardless of final hospital diagnosis, we avoided selection bias. Outcome data were recorded and are being evaluated for association with the acute event. The primary analysis is to model 5-day outcomes; a secondary analysis will model 30-day outcomes.
We will use statistical methods, combined with clinical relevance, to simplify the complex of predictor variables into a useable mathematical model to predict risk of outcomes. Predicted probabilities will be used as the measure of patient risk. Our hypothesis is that the predicted probabilities can be used to assist physicians as they decide who can be managed as an outpatient and, once admitted, who can be discharged early, after a brief observation period.
D. STRATIFY Protocol
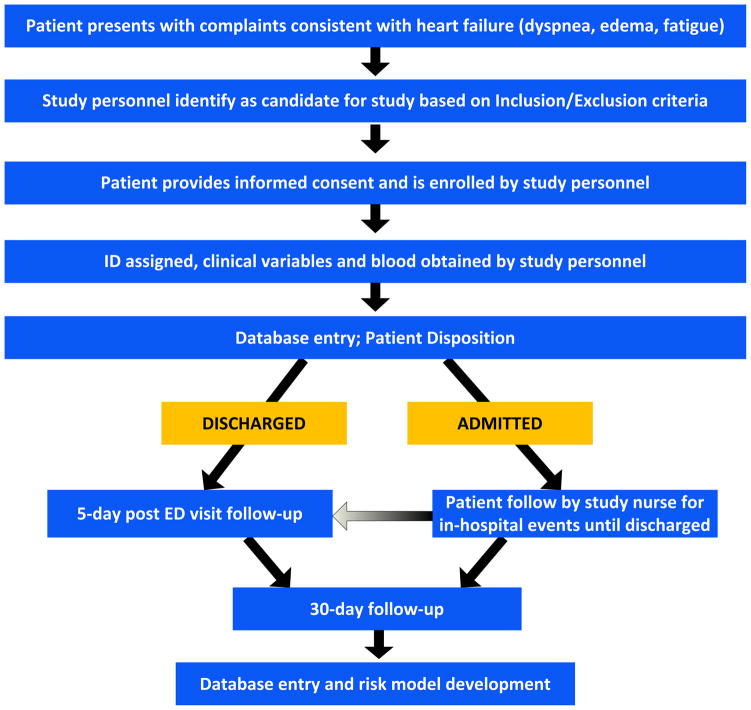
The study design is summarized in Figure 2. Briefly, trained clinical research assistants prospectively gathered data from patients with signs and symptoms of AHF presenting to one of 4 participating EDs. Potential predictors available in the first 3 hours after presentation were gathered. Outcomes were measured using a combination of chart review, patient phone call and death registry review. A panel of cardiologists then ascertains the relationship between the outcome and the acute presentation (see cardiology oversight below). On completion of data cleaning, the prediction rules will be derived. Both emergency physicians and cardiologists will advise on model development to ensure clinically valid, broadly applicable, risk stratification and prediction rules.
Figure 2.
Enrollment from ED treatment through 72–96 hours after treatment
1. Cardiology Oversight
Heart failure is a complex condition, so it is necessary to include appropriate expertise for deciding a true diagnosis for enrolled patients and for understanding how various treatments and comorbidities influence outcomes. We formed a cardiology oversight group (COG) that provides comprehensive support for both STRATIFY and DECIDE. The group consists of three active clinical academic cardiologists. The COG offers: 1) consensus agreement on the diagnosis for all cases, 2) updates on current cardiology opinion, the state of heart failure care, and the potential impacts this might have on outcomes monitored in this study, and lastly, 3) consensus agreement on the probability that observed outcomes at 5 and 30 days are directly related to the AHF presentation. A fourth cardiologist was available to adjudicate any discordance in outcome determination.
2. Setting and Subjects
Our study recruited patients at two emergency departments in Nashville, Tennessee and three in Cincinnati, Ohio. These emergency departments represent demographically and socioeconomically diverse patient populations. Selecting multiple contrasting settings to conduct this project allowed us to overcome a primary concern of risk assessment strategies; the diverse patient population increases the applicability of our prediction rules.
3. Inclusion Criteria
For the purposes of this study, we used a modification of the Framingham Criteria. These criteria are accepted for establishing an etiology of dyspnea before definitive studies have been performed. Our modification reflects contemporary practice for making a preliminary diagnosis of AHF in the acute setting. Use of BNP > 100 pg/ml to support an AHF diagnosis is also standard at our institutions, but this was not be used for inclusion in the proposed study because lack of BNP elevation was expected to exclude some low-risk patients. These inclusion criteria were tested in our preliminary studies and found to be representative of the ED AHF patient in whom a prediction rule would assist in disposition decision-making. Patients included in STRATIFY were required to:
a) Fulfill the Modified Framingham Criteria (Table 1)
Table 1.
Modified Framingham Criteria
| Major | Minor |
|---|---|
|
|
The Framingham Criteria, reported in 1971.57,58 use history, physical exam and ancillary tests to categorize patients as definite, probable and questionable heart failure.(Table 1) In this study, four of the Framingham Criteria were not used: i) circulation time, ii) vital capacity, iii) weight loss in response to treatment, and iv) autopsy findings. Vital capacity and circulation time are two parameters that are not typically available in the acute setting, and weight loss in response to treatment would only help with a retrospective diagnosis of AHF. Inclusion of patients in the STRATIFY cohort required two major, or one major and two minor Framingham Criteria to be met.
b) Be willing and able to give informed consent
4. Exclusion Criteria
Patients less than 18 years of age were not study candidates. High-risk patients were not excluded. While high-risk patients are relatively easy to identify in the ED, objective criteria for defining high-risk obtained from our model will help to avoid errors when deciding the approach to managing these patients. Excluding high-risk patients such as those with severe respiratory distress, hypoxia, or new onset heart failure would limit the generalizability of our model. While we focus on data collected in the first 3 hours of evaluation in our primary analysis, for complete data collection and exploration in secondary analyses, all data available in the ED as part of a standard work-up were collected.
E. The DECIDE Protocol
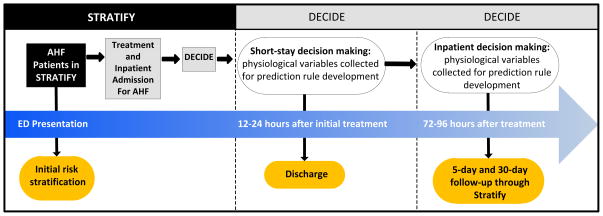
The DECIDE protocol was designed to enroll a subset of STRATIFY subjects that were determined to have AHF and underwent treatment for AHF in the acute setting. The setting and subjects and use of the COG were identical to STRATIFY. Data were prospectively collected at 12–24 and 72–96 hours after ED therapy in patients who were treated for AHF in the ED with diuretics or vasodilators and subsequently admitted. (Figure 3) In addition to the inclusion and exclusion criteria listed for STRATIFY, those patients enrolled in DECIDE were also required to have:
Figure 3.
Patient enrollment and flow from STRATIFY (RO1) to DECIDE (K23).
a) Baseline data available within 3 hours of initial ED therapy
For STRATIFY, baseline data were collected within 3 hours of ED presentation regardless of whether therapy had begun. DECIDE updated index ED visit variables with data from follow-up time points to capture the impact of AHF therapy and to evaluate their ability to predict subsequent adverse events. Thus, timing of therapy relative to changes in serial variables (i.e. laboratory data, vital signs) was an important consideration for baseline data collection. Bias is possible if high-risk patients were treated earlier than those less acutely ill and the study assistants failed to identify those patients rapidly. This affected very few patients in our prior studies. 59,60 Similar to STRATIFY, all baseline ED variables were collected in DECIDE regardless of time from therapy. This allows for exploratory secondary analyses examining the impact of time of therapy on predictive ability of the variable.
F. STRATIFY AND DECIDE PROTOCOL- Candidate Predictor Variables
Predictor variables for an AHF prediction rule must be readily available to physicians in the routine management of patients presenting with signs and symptoms of AHF, and they should enter the model in the same temporal manner with which the predictor would be available in the clinical setting.24,28,30–32,61,62 The most important predictors must have clear, clinically sensible definitions, and have minimal missing values among the participants.24,35 Candidate predictor variables should be predetermined based on clinical expertise and an exhaustive review of the related literature.31,62 They need to be biologically plausible for the predictive rules to maintain face validity and be realistically available. We are limiting the first AHF prediction rule (STRATIFY) to include only candidate predictors whose information is available within the first 3 hours from presentation. For DECIDE we will limit the data to that which is available at the time of decision making in the ED or either at the 12–24 hour or 72–96 hour time point. Incorporating data not available at these time points would result in a rule not easily incorporated into routine practice.
We pre-selected the primary candidate predictor variables for the studies (Table 2), in accordance with these standards. We based our selections on the results of our own analysis from an AHF cohort and review of the literature60. Because of the dynamic nature of this research area and the length of time over which patients were being recruited, we also collected data on additional variables as well. Their inclusion in the final prediction rules depends on biological plausibility and availability to evaluating physicians within the allotted time windows, as well as sufficient lack of missingness to be evaluable.
Table 2.
Example Candidate Predictor Covariates
| Predictor | Ascertainment & Definition | Operational Definition | Type | d.f. |
|---|---|---|---|---|
| * Troponin I | Core Lab Test | Laboratory Value | c | 2 |
| * Sodium | Core Lab Test | Laboratory Value | c | 3 |
| * Vital Sign(s) | EMR | Vital Sign Value | c | 2 |
| * Creatinine | Core Lab Test | Laboratory value | c | 2 |
| * BNP | Core Lab Test | Laboratory value | c | 1 |
| * Hemoglobin | Standard Lab Test | Laboratory value | c | 1 |
| * Moderate dyspnea after Treatment | Interview | Yes/no | d | 1 |
| * Urinary Output | EMR | Milliliters | c | 1 |
| $White Blood Count | Standard Lab Test | Laboratory value | c | 1 |
| $BUN | Core Lab Test | Laboratory value | c | 1 |
| $Literacy | Interview | Yes/no | d | 1 |
| ≥ 2 HF admits in last 6 months | Interview and EMR | Yes/no | d | 1 |
| Overall DF | 20 | |||
= Significant Predictor in predictive instruments $ = historical risk predictors c=continuous (modeled without assuming linearity using restricted cubic splines), d=dichotomous d.f. = degrees of freedom = # knots −1, 1 for linear
G. STRATIFY AND DECIDE PROTOCOL - Study Outcomes
The primary outcome being modeled in the STRATIFY and DECIDE studies is the occurrence of adverse events at 5 days after the initial evaluation. Adverse events at 30 days are also being modeled as a secondary outcome. A hierarchical listing of these specific adverse events with an a priori ordinal scale severity values is presented in Table 3. This hierarchy was created by the study investigators, comprised of both emergency physicians and cardiologists. The outcome to be predicted must be clearly defined in order to eliminate potential misclassification, and be clinically relevant.31 The most well defined outcome is all-cause death. Additional outcomes with potential for subjectivity, and thus requiring explicit definitions, include: 5-day and 30-day ED return visits and hospital admissions for AHF-related complaints, as well as AHF-related cardiovascular complications within 5 and 30 days of initial ED visit. The accurate determination of whether the adverse events are related to AHF is of utmost importance for this study. As previously described, the COG is reviewing each reported 5-day and 30-day adverse event and making a consensus determination on whether it was AHF-related. This consensus outcome determination will be made prior to any analysis of the candidate predictor variables.
Table 3.
Adverse Event Outcomes and Bisk Modifiers in Predictive Instrument with Ordinal Scale Severitv Value Assignments
| Outcomes | |||
|---|---|---|---|
| Clinical conditions | Inpatient Procedures | Risk Modifiers | |
|
Death, all cause [10] | Sudden Death/Defibrillation/CPR [9] | ICU care |
| ACS [5] | Mechanical cardiac support [8] | Treatment with vasopressors | |
| Unscheduled HF Hospital Admission [4] | Intubation/Mechanical Ventilation [7] | Hospitalization greater than 48 hours | |
| Unscheduled Non-HF Hospital Admission [3] | Emergent Dialysis [6] | ||
| Return ED visit for HF related complaint not requiring admission [2] | PCI/CABG [5] | ||
| Return clinic visit for HF related complaint not requiring admission [2] | None of the above [0] | ||
| Unscheduled ED non-HF visit not requiring admission [1] | |||
| None of the above [0] | |||
ACS= acute coronary syndrome; HF= heart failure; CPR= cardiopulmonary resuscitation; PCI= percutaneous coronary intervention; CABG= coronary artery bypass surgery; ICU= intensive care unit
H. STRATIFY AND DECIDE PROTOCOL - Analytic Approach
The final product of the statistical analysis will be predictive models with multiple variables (with appropriate transformations) that have good discrimination, are easy to measure, and have straightforward interpretations. To this end, the analysis consists of three stages: data reduction and highly limited variable selection from the a priori selected individual- and location-specific potential predictors; model development; and model checking and validation following established detailed methodology for the development and validation of prediction rules.24,27–32,61,62
With more than 100 potential predictors identified a priori, over-fitting the model with colinear predictors is a realistic danger. Standard sample size requirements, supported by simulation studies and expert opinion, conclude that there must be 15 subjects or events per degree of freedom, (i.e. per regression coefficient examined or estimated) for the rule to be reliable and not at risk of overfitting.28,63 Based on these sample size restrictions, it is even more important to limit the number of predictors. Popular approaches to data reduction and variable selection include univariate screening with p-values and stepwise selection. While these methods are convenient, they violate many principles of statistical estimation and hypothesis testing. Specifically, stepwise methods lead to instability of predictor selection, biased estimates of coefficients, exaggeration of p-values and worse predictive quality than using the full model without selection.32,62
We will employ variable selection based upon physiologic relevance, lack of redundancy, and feasibility of reliable data collection. Variables that are easy to obtain in the acute setting will be given more emphasis than those more difficult to obtain. A panel of specialists will review the potential predictors and group those that could be highly correlated with the goal of summarizing them into a single score using data reduction techniques such as principle component analysis and hierarchical clustering. 24 To avoid overfitting and ensure a reliable prediction rule, we will adhere to the accepted formula for an ordinal outcome of approximately a 15:1 ratio of effective sample size to predictor degree of freedom, where the effective sample size is derived from the true sample size and the frequency of the outcome.24,64,65 The composite outcome variables (incidence of 5-day and 30-day adverse events) are structured as a hierarchy of ordinal outcomes (Table 3) and proportional odds logistic regression will be used for analysis. 24,66
We will internally validate the calibration and discrimination of the rule using bootstrap resampling in order to estimate the likely performance of the rule on a new sample of patients from the same patient stream. We will use bootstrapping to perform the internal validation, as it offers advantages over data-splitting and cross-validation for internal validation, especially by preserving the sample size.24 The R statistical computing language will be preferentially for modeling analyses, along with the R rms package.67,68
We will review the prediction rule with clinicians (both emergency physicians and cardiologists) who are masked to the rule’s predictive discrimination (c-index). We also intend to derive multiple scoring systems based on the regression coefficients, and test these systems with clinicians to choose the most “sensible” rule. Future prospective, multicenter investigations will be planned to externally validate our prediction rules. These investigations will externally test the model across a more heterogeneous group of patients at a number of different institutions.
This work was supported by National Heart, Lung and Blood Institute grants K23HL085387 and R01HL088459, and by the National Center for Advancing Translational Sciences of the National Institute of Health under Award Number UL1 TR000445. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors are solely responsible for the design and conduct of this study, all study analyses and drafting and editing of the paper.
Conclusions
Development of prediction rules to identify ED patients with AHF at low-risk for adverse events has largely been unsuccessful. As a result, over 80% of ED presentations for AHF are admitted to the hospital. Similarly, once hospitalized, there are no validated objective endpoints of therapy. Guidance from evidence-based prediction rules will reduce unnecessary admissions of low-risk patients and shorten inpatient length-of-stay for those who are admitted. We proposed to develop two prediction rules: one that will assist in ED decision making and one that will facilitate early, safe hospital discharge. Our results will be translated into algorithms that will be disseminated worldwide. This is the first step toward achieving our broad objective of improving care for patients with AHF in synchrony with appropriate allocation of hospital resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher J Lindsell, University of Cincinnati.
Cathy Jenkins, Vanderbilt University.
Frank Harrell, Vanderbilt University.
Gregory J Fermann, University of Cincinnati.
Karen F Miller, Vanderbilt University.
Sue Roll, University of Cincinnati.
Sue Roll, University of Cincinnati.
Matthew I Sperling, University of Cincinnati.
David J Maron, Vanderbilt University.
Allen J Naftilan, Vanderbilt University.
John A McPherson, Vanderbilt University.
Neal L Weintraub, University of Cincinnati.
Douglas B Sawyer, Vanderbilt University.
Alan B Storrow, Vanderbilt University.
References
- 1.Collins SP, Lindsell CJ, Naftilan AJ, et al. Low-risk acute heart failure patients: external validation of the Society of Chest Pain Center’s recommendations. Critical pathways in cardiology. 2009;8:99–103. doi: 10.1097/HPC.0b013e3181b5a534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graff L, Orledge J, Radford MJ, Wang Y, Petrillo M, Maag R. Correlation of the Agency for Health Care Policy and Research congestive heart failure admission guideline with mortality: peer review organization voluntary hospital association initiative to decrease events (PROVIDE) for congestive heart failure. Annals of emergency medicine. 1999;34:429–37. doi: 10.1016/s0196-0644(99)80043-2. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh M, Auble TE, Yealy DM. Validation of the Acute Heart Failure Index. Annals of emergency medicine. 2008;51:37–44. doi: 10.1016/j.annemergmed.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 4.Brophy JM, Deslauriers G, Boucher B, Rouleau JL. The hospital course and short term prognosis of patients presenting to the emergency room with decompensated congestive heart failure. The Canadian journal of cardiology. 1993;9:219–24. [PubMed] [Google Scholar]
- 5.Brophy JM, Deslauriers G, Rouleau JL. Long-term prognosis of patients presenting to the emergency room with decompensated congestive heart failure. The Canadian journal of cardiology. 1994;10:543–7. [PubMed] [Google Scholar]
- 6.Burkhardt J, Peacock WF, Emerman CL. Predictors of emergency department observation unit outcomes. Acad Emerg Med. 2005;12:869–74. doi: 10.1197/j.aem.2005.03.534. [DOI] [PubMed] [Google Scholar]
- 7.Chin MH, Goldman L. Correlates of major complications or death in patients admitted to the hospital with congestive heart failure. Archives of internal medicine. 1996;156:1814–20. [PubMed] [Google Scholar]
- 8.Chin MH, Goldman L. Correlates of early hospital readmission or death in patients with congestive heart failure. Am J Cardiol. 1997;79:1640–4. doi: 10.1016/s0002-9149(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 9.Cowie MR, Wood DA, Coats AJ, et al. Survival of patients with a new diagnosis of heart failure: a population based study. Heart (British Cardiac Society) 2000;83:505–10. doi: 10.1136/heart.83.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esdaile JM, Horwitz RI, Levinton C, Clemens JD, Amatruda JG, Feinstein AR. Response to initial therapy and new onset as predictors of prognosis in patients hospitalized with congestive heart failure. Clin Invest Med. 1992;15:122–31. [PubMed] [Google Scholar]
- 11.Harjai KJ, Thompson HW, Turgut T, Shah M. Simple clinical variables are markers of the propensity for readmission in patients hospitalized with heart failure. Am J Cardiol. 2001;87:234–7. A9. doi: 10.1016/s0002-9149(00)01328-x. [DOI] [PubMed] [Google Scholar]
- 12.Katz MH, Nicholson BW, Singer DE, Kelleher PA, Mulley AG, Thibault GE. The triage decision in pulmonary edema. J Gen Intern Med. 1988;3:533–9. doi: 10.1007/BF02596094. [DOI] [PubMed] [Google Scholar]
- 13.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–7. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 14.Plotnick GD, Kelemen MH, Garrett RB, Randall W, Fisher ML. Acute cardiogenic pulmonary edema in the elderly: factors predicting in-hospital and one-year mortality. South Med J. 1982;75:565–9. doi: 10.1097/00007611-198205000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Rame JE, Sheffield MA, Dries DL, et al. Outcomes after emergency department discharge with a primary diagnosis of heart failure. American heart journal. 2001;142:714–9. doi: 10.1067/mhj.2001.118473. [DOI] [PubMed] [Google Scholar]
- 16.Selker HP, Griffith JL, D’Agostino RB. A time-insensitive predictive instrument for acute hospital mortality due to congestive heart failure: development, testing, and use for comparing hospitals: a multicenter study. Med Care. 1994;32:1040–52. doi: 10.1097/00005650-199410000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Villacorta H, Rocha N, Cardoso R, et al. Hospital outcome and short-term follow-up of elderly patients presenting to the emergency unit with congestive heart failure. Arq Bras Cardiol. 1998;70:167–71. doi: 10.1590/s0066-782x1998000300005. [DOI] [PubMed] [Google Scholar]
- 18.Arenja N, Breidthardt T, Socrates T, et al. Risk stratification for 1-year mortality in acute heart failure: classification and regression tree analysis. Swiss medical weekly. 2011;141:w13259. doi: 10.4414/smw.2011.13259. [DOI] [PubMed] [Google Scholar]
- 19.Van Spall HG, Atzema C, Schull MJ, et al. Prediction of emergent heart failure death by semi-quantitative triage risk stratification. PloS one. 2011;6:e23065. doi: 10.1371/journal.pone.0023065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler J, Hanumanthu S, Chomsky D, Wilson JR. Frequency of low-risk hospital admissions for heart failure. Am J Cardiol. 1998;81:41–4. doi: 10.1016/s0002-9149(97)00851-5. [DOI] [PubMed] [Google Scholar]
- 21.Collins S, Schauer D, Gupta A, Brunner H, Storrow A, Eckman M. Cost-Effectiveness Analysis of Emergency Department Decision Making in Non-High Risk Heart Failure Patients. Am J Emerg Med. 2009 doi: 10.1016/j.ajem.2008.02.025. In Press. [DOI] [PubMed] [Google Scholar]
- 22.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for inhospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–80. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 23.Adams JKF, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) American heart journal. 2005;149:209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Harrell F. Regression Modeling Strategies with Applications to Linear Models, Survival Analysis and Logistic Regression. New York: Springer; 2001. [Google Scholar]
- 25.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–52. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Harrell FE, Jr, Lee KL, Matchar DB, Reichert TA. Regression models for prognostic prediction: advantages, problems, and suggested solutions. Cancer treatment reports. 1985;69:1071–77. [PubMed] [Google Scholar]
- 28.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. Journal of the National Cancer Institute. 1988;80:1198–202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 29.Harrell FE, Jr, Margolis PA, Gove S, et al. Development of a clinical prediction model for an ordinal outcome: the World Health Organization Multicentre Study of Clinical Signs and Etiological agents of Pneumonia, Sepsis and Meningitis in Young Infants. WHO/ARI Young Infant Multicentre Study Group. Stat Med. 1998;17:909–44. doi: 10.1002/(sici)1097-0258(19980430)17:8<909::aid-sim753>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. Jama. 1997;277:488–94. [PubMed] [Google Scholar]
- 31.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059–79. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Making. 2001;21:45–56. doi: 10.1177/0272989X0102100106. [DOI] [PubMed] [Google Scholar]
- 33.Tsao L, Gibson C. Heart failure: An epidemic of the 21st century. Crit Pathways in Cardiol. 2004;3:194–204. doi: 10.1097/01.hpc.0000146867.90558.ca. [DOI] [PubMed] [Google Scholar]
- 34.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connell JB. The economic burden of heart failure. Clin Cardiol. 2000;23:III6–10. doi: 10.1002/clc.4960231503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croft JB, Giles WH, Pollard RA, Keenan NL, Casper ML, Anda RF. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Archives of internal medicine. 1999;159:505–10. doi: 10.1001/archinte.159.5.505. [DOI] [PubMed] [Google Scholar]
- 37.McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. Journal of the American College of Cardiology. 2002;39:60–9. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 38.AHA. Heart Disease and Stroke Statistics-2007 Update. Dallas, TX: 2006. [Google Scholar]
- 39.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult-Summary Article A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) Journal of the American College of Cardiology. 2005;46:1116–43. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 40.Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? Jama. 2005;294:1944–56. doi: 10.1001/jama.294.15.1944. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson LW, Braunwald E. Recognition and Management of Patients With Heart Failure. In: Goldman L, Braunwald E, editors. Primary Cardiology. Philadelphia: WB Saunders; 1998. pp. 310–29. [Google Scholar]
- 42.Institute NHLaB. Morbidity and Mortality: 2002 Chart Book on Cardiovascular, Lung, and Blood Diseases. National Institutes of Health; May, 2002. [Google Scholar]
- 43.Polanczyk CA, Rohde LE, Philbin EA, Di Salvo TG. A new casemix adjustment index for hospital mortality among patients with congestive heart failure. Med Care. 1998;36:1489–99. doi: 10.1097/00005650-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Smith WR, Poses RM, McClish DK, et al. Prognostic judgments and triage decisions for patients with acute congestive heart failure. Chest. 2002;121:1610–7. doi: 10.1378/chest.121.5.1610. [DOI] [PubMed] [Google Scholar]
- 45.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. The New England journal of medicine. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 46.Lee DS, Schull MJ, Alter DA, et al. Early deaths in patients with heart failure discharged from the emergency department: a population-based analysis. Circ Heart Fail. 2010;3:228–35. doi: 10.1161/CIRCHEARTFAILURE.109.885285. [DOI] [PubMed] [Google Scholar]
- 47.Poses RM, Smith WR, McClish DK, et al. Physicians’ survival predictions for patients with acute congestive heart failure. Archives of internal medicine. 1997;157:1001–7. [PubMed] [Google Scholar]
- 48.McCausland JB, Machi MS, Yealy DM. Emergency physicians’ risk attitudes in acute decompensated heart failure patients. Acad Emerg Med. 2010;17:108–10. doi: 10.1111/j.1553-2712.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 49.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 50.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. Journal of cardiac failure. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Hsieh M, Auble TE, Yealy DM. Predicting the future: Can this patient with acute congestive heart failure be safely discharged from the emergency department? Annals of emergency medicine. 2002;39:181. doi: 10.1067/mem.2002.121403. [DOI] [PubMed] [Google Scholar]
- 52.Konstam M, Dracup K, Baker D. Clinical Practice Guidelines No 11: heart failure: evaluation and care of patients with left-ventricular systolic dysfunction. Agency for Health Care Policy and Research; 1994. p. 94. [Google Scholar]
- 53.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 54.Lee DS, Stitt A, Austin PC, et al. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012;156:767–75. doi: 10.7326/0003-4819-156-11-201206050-00003. [DOI] [PubMed] [Google Scholar]
- 55.Diercks DB, Peacock WF, Kirk JD, Weber JE. ED patients with heart failure: identification of an observational unit-appropriate cohort. Am J Emerg Med. 2006;24:319–24. doi: 10.1016/j.ajem.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Felker GM, Leimberger JD, Califf RM, et al. Risk stratification after hospitalization for decompensated heart failure. Journal of cardiac failure. 2004;10:460–6. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–15. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 58.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. The New England journal of medicine. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 59.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. The New England journal of medicine. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 60.Collins SP, Peacock WF, Lindsell CJ, et al. S3 detection as a diagnostic and prognostic aid in emergency department patients with acute dyspnea. Annals of emergency medicine. 2009;53:748–57. doi: 10.1016/j.annemergmed.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 61.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. Jama. 2000;284:79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 62.Moons KG, Donders AR, Steyerberg EW, Harrell FE. Penalized maximum likelihood estimation to directly adjust diagnostic and prognostic prediction models for overoptimism: a clinical example. Journal of clinical epidemiology. 2004;57:1262–70. doi: 10.1016/j.jclinepi.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 63.Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group. The New England journal of medicine. 2000;343:94–9. doi: 10.1056/NEJM200007133430203. [DOI] [PubMed] [Google Scholar]
- 64.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. Journal of clinical epidemiology. 2006;59:1087–91. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 65.McCullagh P. Regression models for ordinal data (with discussion) J Royal Statistical Society, Series B. 1980;42:109–42. [Google Scholar]
- 66.Steyerberg EW. Clinical Prediction Models, A Practical Approach to Development, Validation, and Updating. New York: Springer; 2009. [Google Scholar]
- 67.R: A language and environment for statistical computing. 2012 (Accessed at http://www.R-project.org.)
- 68.rms: Regression Modeling Strategies. R package version 3.3-1. 2011 (Accessed at. http://CRAN.R-projet.org/package=rms.)