Summary
During the 2011 International Pigment Cell Conference (IPCC), the Vitiligo European Taskforce (VETF) convened a consensus conference on issues of global importance for vitiligo clinical research. As suggested by an international panel of experts, the conference focused on four topics: classification and nomenclature; definition of stable disease; definition of Koebner’s phenomenon (KP); and ‘autoimmune vitiligo’. These topics were discussed in seven working groups representing different geographical regions. A consensus emerged that segmental vitiligo be classified separately from all other forms of vitiligo and that the term ‘vitiligo’ be used as an umbrella term for all non-segmental forms of vitiligo, including ‘mixed vitiligo’ in which segmental and non-segmental vitiligo are combined and which is considered a subgroup of vitiligo. Further, the conference recommends that disease stability be best assessed based on the stability of individual lesions rather than the overall stability of the disease as the latter is difficult to define precisely and reliably. The conference also endorsed the classification of KP for vitiligo as proposed by the VETF (history based, clinical observation based, or experimentally induced). Lastly, the conference agreed that ‘autoimmune vitiligo’ should not be used as a separate classification as published evidence indicates that the pathophysiology of all forms of vitiligo likely involves autoimmune or inflammatory mechanisms.
Keywords: vitiligo, consensus conference
Why an international consensus conference on vitiligo?
Vitiligo, the most common hypopigmentary disorder, is an acquired disease characterized by progressive loss of melanocytes. Vitiligo occurs worldwide with an estimated prevalence of 0.5–1% in most populations. In almost half of patients, vitiligo starts before the age of 20 yr, and males and females are affected with approximately equal frequency (Taïeb and Picardo, 2009). Guidelines for the management of vitiligo have been published (Gawkrodger et al., 2008; Parsad and Gupta, 2008); however, a recent Cochrane review (Whitton et al., 2010) and an international textbook (Taieb and Picardo, 2010) identified significant difficulties regarding definitions, nomenclature, outcome measurements, and disease classification. The Vitiligo European Task Force (VETF) has proposed consensus definitions for the disease (Taïeb and Picardo, 2007), but broader international consensus was lacking. In addition, although vitiligo is not a life-threatening disease, it has been associated with concomitant occurrence of a number of other autoimmune diseases, as well as a wide range of psychosocial difficulties, significantly impacting quality of life (Kostopoulou et al., 2009; Mashayekhi et al., 2010; Wang et al., 2011b). Therefore, an approach for achieving broad international consensus was developed with the goal of guiding both clinical research and optimal patient management.
The Vitiligo Global Issues Consensus Conference
The VETF, which includes medical experts, scientists, and representatives of patient support groups, proposed to hold an international consensus conference on vitiligo at the 2011 International Pigment Cell Conference (IPCC), attended by many physicians and scientists in the vitiligo field. Worldwide experts were canvassed for suggestions, and a preliminary list of issues of global importance for clinical research and patient management was drafted. Four principal topics were selected: classification and nomenclature, definition of stable disease, definition of Koebner’s phenomenon (KP), and significance/individualization of ‘autoimmune vitiligo’.
To prepare the Vitiligo Global Issues Consensus Conference (VGICC), seven regional working groups were established: North and South Africa, North and South/Central America, Europe, Middle East, Continental Asia/Singapore, Japan/Taiwan, and the Pacific (Appendix 1). In January, 2011 each group submitted a preliminary contribution addressing the four selected topics. A first round of discussion of the VGICC was held in Seoul, Korea, in May, 2011, prior to the 22nd World Congress of Dermatology (minutes are available at http://www.espcr.org/vetf/Vitiligo_Seoul_2011.pdf), attended by representatives from all seven regional groups. Following this first meeting, a draft consensus document was prepared and circulated for further comments. The final round of discussion was held in Bordeaux, France, in September 2011 at the 21st IPCC. This report summarizes the consensus recommendations of the VGICC.
Classification and nomenclature
Starting discussion points
There was already broad consensus about the hypopigmentary diseases that should be considered in the differential diagnosis of vitiligo (Table 1) (Taieb and Picardo, 2010; Taïeb and Picardo, 2007, 2009). The classification proposed by Taieb and Picardo (2010) was used as a working template for the VGICC revision of nomenclature (Table 2).
Table 1.
Differential diagnosis of vitiligo
| Inherited or genetically induced hypomelanoses (usually present at birth) |
| Piebaldism |
| Tuberous sclerosis |
| Ito’s hypomelanosis |
| Waardenburg’s syndrome |
| Hermanski-Pudlak syndrome |
| Menkès syndrome |
| Ziprkowski-Margolis syndrome |
| Griscelli’s syndrome |
| Post-inflammatory hypomelanoses |
| Related to an increased epidermal turn over |
| Psoriasis |
| Atopic dermatitis |
| Related to an acute lichenoid/cytotoxic infiltrate with pigment incontinence |
| Lichen planus |
| Toxic drug reactions |
| Para-malignant hypomelanoses |
| Mycosis fungoides |
| Melanoma-associated depigmentation |
| Para-infectious hypopigmentation |
| Pityriasis versicolor |
| Leprosy |
| Leishmaniasis |
| Onchocerciasis |
| Acquired macular hypomelanosis |
| Post-traumatic leucoderma |
| Post-burns |
| Post-scars |
| Melasma |
| Occupational and drug-induced depigmentation |
| Phenolic–catecholic derivatives |
| Systemic drugs (chloroquine, fluphenazine, physostigmine, imatinib) |
| Topical drugs (imiquimod, long-term use of topical corticosteroids) |
Table 2.
2010 Classification (Taieb and Picardo, 2010)
| Type of vitiligo | Subtypes | Remarks |
|---|---|---|
| Non-segmental (NSV) | (Focal)a, mucosal, acrofacial, generalized, universal | Subtyping may not reflect a distinct nature, but useful information for epidemiologic studies |
| Segmental (SV) | Focalb, mucosal, unisegmental, bi- or multisegmental | Further classification according to distribution pattern possible, but not yet standardized |
| Mixed (NSV+SV) | According to severity of SV | Usually the SV part in mixed vitiligo is more severe |
| Unclassified | Focal at onset, multifocal asymmetrical non-segmental, mucosal (one site) | This category is a meant to allow, after a sufficient observation time (and if necessary investigations), to make a definitive classification |
Possible onset of NSV.
See text for discussion.
Vitiligo itself has been classified based on clinical grounds into two major forms, namely, segmental vitiligo (SV) and non-segmental vitiligo (NSV), the latter including several variants (generalized vitiligo, acrofacial vitiligo, universal vitiligo) (Taïeb and Picardo, 2007). Non-segmental vitiligo typically evolves over time, in both distribution and extension patterns. This is the case with focal vitiligo, which may evolve into SV, into NSV, or may remain unclassifiable based on the NSV/SV classification paradigm. For NSV, the disease may be initially classified as acrofacial but will later progress to be better classified as generalized or universal. Conversely, some cases of NSV may spare the extremities (generalized non-acrofacial vitiligo). Some cases of NSV exhibit a flexural distribution, and others a predilection for extensor aspects, suggesting different triggers or etiologies. Therefore, precisely reporting the involved sites is of importance (Taïeb and Picardo, 2007). More recently, mixed vitiligo (MV) has been defined as the combination of initial SV followed by the occurrence of bilateral NSV patches several months or, more rarely, years later (Ezzedine et al., 2011c).
Key discussion points
NSV as an umbrella term for all forms of generalized vitiligo and clinical definition of NSV
Although not fully satisfactory, the term ‘non-segmental vitiligo’ is currently used as an umbrella term for different clinical subtypes of vitiligo that are all clearly distinct from SV including acrofacial, generalized, mucosal (multifocal), and universal vitiligo. The terms ‘bilateral’ versus ‘unilateral’ vitiligo (Falabella, 2005; Hann and Nordlund, 2000) have been used as alternative approaches to classification, but raise possible confusion with MV and multisegmental vitiligo. The VGICC participants agreed that the term ‘vulgaris’, as synonymous with ‘common’, conveys a negative connotation to patients and the general public, and thus should not be used.
Clinically, NSV is characterized by depigmented macules that vary in size from a few to several centimeters in diameter, often involving both sides of the body with tendency toward symmetrical distribution. Transient erythema in depigmented skin may be observed following ultraviolet (UV) irradiation, which can be misleading in a clinical context. During the course of the disease, hyperpigmented lesional borders may sometimes be observed, especially in dark-skinned individuals after UV exposure. Involvement of the scalp and other hair-bearing areas may manifest with localized patches of gray or white hairs. Contrary to SV, in NSV, body hairs are usually spared and remain pigmented, although hair depigmentation may occur with disease progression. To optimize clinical examination and treatment assessment of patients with NSV or SV, Wood’s lamp examination should be performed, especially in individuals of fair skin complexion and in darker individuals to assess the involvement of the palms and soles.
Focal vitiligo
The diagnosis of focal vitiligo should be considered only after having ruled out all other diagnoses, and a biopsy may be helpful to exclude other causes of focal hypopigmentation. (Table 1). Focal vitiligo refers to an acquired, small, isolated hypopigmented lesion that does not fit a typical segmental distribution, and which has not evolved into NSV after a period of 1–2 yr.
Mucosal vitiligo
Mucosal vitiligo typically refers to the involvement of the oral and/or genital mucosae. When presenting in isolation, especially for genital involvement, a differential diagnosis of lichen sclerosus should be addressed by biopsy. Concomitant occurrence of genital lichen sclerosus and vitiligo has been reported (Ortonne, 2000), suggesting the possibility of a causal link between the two conditions. In fair-skinned individuals, the diagnosis of oral mucosa vitiligo is rarely made; it is unclear whether this is attributable to low incidence or low diagnostic accuracy. When mucosal vitiligo occurs in the context of NSV, it is readily classified as NSV. However, when mucosal vitiligo presents in isolation, the VGICC participants agreed that it should be classified as undetermined vitiligo (UnV) (Table 3).
Table 3.
Bordeaux VGICC classification and consensus nomenclature
| Subtypes | |
|---|---|
| Vitiligo/NSV | Acrofacial |
| Mucosal (more than one mucosal site) | |
| Generalized | |
| Universal | |
| Mixed (associated with SV) | |
| Rare variants | |
| Segmental Vitiligo | Uni-, bi-, or plurisegmental |
| Undetermined/unclassified Vitiligo | Focal |
| Mucosal (one site in isolation) |
NSV, non-segmental vitiligo; SV, segmental vitiligo; VGICC, Vitiligo Global Issues Consensus Conference.
Universal vitiligo
Universal vitiligo corresponds to complete or nearly complete depigmentation of the skin. This term is commonly used when NSV gradually progresses to complete depigmentation of the skin and body hair, and sometimes oral/genital mucosae. Scalp hair involvement is also common. However, vitiligo may spare the scalp, pubic, and axillary areas early in the course of disease. Small perifollicular, discrete, or coalescent pigmentation may persist in sun-exposed areas. The distinction between vitiligo universalis (VU) progressing from common NSV versus ‘fulminant’ vitiligoid conditions that target skin and non-skin melanocytes (ear, eyes), especially as part of the rare Vogt-Koyanagi Harada syndrome, is not completely clear. Complete skin depigmentation in NSV patients who have undergone therapeutic depigmentation should be excluded from the diagnosis of VU.
Other unclassified or poorly classified generalized vitiligoid conditions
Several conditions that may fit into the general clinical spectrum of vitiligo are difficult to classify into the two classical forms NSV and SV. These forms are illustrated in a glossary (Appendix 2). ‘Punctate vitiligo’ (Falabella et al., 1988) refers to pea-sized depigmented macules that may involve any area of the body. When these lesions coexist with classical vitiligo macules, it is best classified as NSV. If not, the term ‘leukoderma punctata’ should be used. Other distinct conditions that may be difficult to distinguish from vitiligo clinically include idiopathic guttate melanosis and progressive macular hypomelanosis.
Segmental vitiligo
Koga first proposed that SV was to be differentiated from NSV on etiologic grounds (Koga and Tango, 1988). Recent clinical reports of mixed SV and NSV have challenged the concept that these two forms of vitiligo are distinct (Ezzedine et al., 2011b,c; Gauthier et al., 2003; van Geel et al., 2011a; Mulekar et al., 2006; Schallreuter et al., 2007, 2008). Furthermore, evidence of inflammation has been demonstrated in some cases of early SV, suggesting that NSV and SV may both involve inflammatory or immune-related etiology (van Geel et al., 2010). In addition to its limited, segmental distribution, SV has other distinguishing characteristics as compared to NSV. SV typically has earlier age of onset than NSV (Gauthier et al., 2003; Hann and Lee, 1996; Hann et al., 1997a,b, 2000). Segmental vitiligo typically has a rapidly progressive but limited course, depigmentation spreads within the segment over a period of 6–24 months and then stop; further extension is rare. In addition, in contrast to NSV, SV has early involvement of melanocytes of hair follicles, with up to 50% of SV patients exhibiting poliosis in affected areas. In addition, melanocyte autografts typically yield good results in SV patients, with stable repigmentation. The pathophysiology of the segmental distribution remains highly controversial (van Geel et al., 2010; Taïeb et al., 2008).
Mixed vitiligo
The coexistence of SV and NSV was first reported in a pediatric NSV patient treated with UVB, which left a recalcitrant segmental lesion suggestive of preexisting SV (Gauthier et al., 2003). Additional cases were subsequently reported, with the term ‘mixed vitiligo’ proposed to designate this form of the disease (Mulekar et al., 2006), and a case series subsequently proposed definition criteria (Ezzedine et al., 2011c). This association may be viewed as an example of a superimposed segmental manifestation of a generalized polygenic disorder, in which segmental involvement precedes disease generalization and is more resistant to therapy (Happle, 2001, 2007). The presence of halo nevi and leukotrichia at onset may be risk factors for developing MV in patients with SV (Ezzedine et al., 2011b).
Occupational/contact vitiligo
The terms ‘contact’ or ‘occupational vitiligo’(CV) have been used to describe a distinct form of vitiligo induced by exposure to certain chemicals in the workplace or at home, principally aromatic or aliphatic derivatives of phenols and catechols (Boissy, 2010; Boissy and Manga, 2004; Boissy and Nordlund, 1995; Lerner, 1971). However, the precise definition of CV is unclear. Indeed, although the cutaneous depigmentation may be limited to the areas exposed to chemicals, it may extend progressively from the initial site of chemical contact to the whole body, leading to typical NSV (Cummings and Nordlund, 1995). Thus, such chemical agents may serve as uncommon environmental triggers or haptens for the induction of what in fact is typical vitiligo. The opinion of the VGICC participants was that CV requires clearer definition by both case studies and epidemiological investigation in at-risk populations exposed to these causative chemicals, investigation of potential predisposing factors, time between exposure and onset of depigmentation of exposed areas, and time between first depigmentation and onset of generalized vitiligo. As such, at this time, CV should not be included in the classification of vitiligo as a separate entity.
Prognosis-oriented classification
For SV, prediction of the final (theoretical) size of the involved segment is an important clinical problem. On the basis of the observations of the Hann’s group, other collections/registers of cases should be obtained to reach an international consensus on segmentation (Kim et al., 2011). For NSV, the importance of the remaining melanocyte reservoir should be evaluated in addition to the descriptive classification. The VETF grading system that scores follicular reservoir involvement (Taïeb and Picardo, 2007) is an option which needs to be tested in clinical trials.
Summary of the final discussion
Separating SV from other types of vitiligo is the most important consensus position, principally because of its prognostic implications. While the term ‘non-segmental vitiligo’ has been used as an umbrella term to include all other multifocal, usually symmetrical forms of vitiligo not occurring in a segmental pattern, the VGICC participants preferred an alternative nomenclature, because it was considered optimal to define a disease by its most common presentation, rather than by what it is not. The terms ‘common vitiligo’, ‘general vitiligo’, ‘generalized vitiligo’ and ‘vitiligo’ were discussed, and after extensive consideration the term ‘Vitiligo’ was agreed on, for the sake of simplicity and taking into account the current nomenclature for psoriasis. To avoid confusion, based on post-conference correspondence, it was suggested that during the transition period, vitiligo/NSV should be used.
For SV, although it was agreed that clinical diagnosis can be made readily by observation of multiple non-confluent cutaneous macules in a mostly unilateral distribution, it was difficult to achieve clear definition of the term ‘segment’. A definition based on lesion size (to differentiate SV from focal vitiligo) was not judged appropriate.
For MV, there was agreement to classify this as a variant of vitiligo/NSV. It was agreed that in MV, based on reported cases, SV usually precedes vitiligo/NSV.
For CV, it was deemed premature to classify this as a distinct entity.
For the group previously termed ‘unclassified’ (Table 2), it was preferred to retain the provisional term ‘unclassified vitiligo’ (Table 3); though, these may be more precisely classified in the future. Pure mucosal and long-lasting focal vitiligo may remain in this category.
For VU, no consensus was achieved on the minimal percentage of body involvement that should define the condition. Nevertheless, precise recording of extent of skin and hair involvement, as well as possible non-cutaneous melanocyte involvement, is recommended to assess clinical evolution.
A glossary with photographs is provided (Appendix 2).
Consensus statements and classification.
The term ‘vitiligo’ (V) is the recommended umbrella term for all non-segmental forms of vitiligo. As a transition, vitiligo/NSV can be used.
Segmental vitiligo refers to a clinically unambiguous segmental distribution of depigmented lesions, typically associated with rapid onset and with leukotrichia. There is no consensus concerning the mechanism underlying lesion distribution in SV.
Mixed vitiligo, being the coexistence of SV + V, is a subgroup of V.
Focal vitiligo, a term that applies to localized macules characterized by loss of melanocytes, is assigned to the category UnV until more definitive classification can be made on clinical grounds (generally after 1–2 yr of follow-up). Cases with long-lasting focal lesions or of pure mucosal vitiligo, if not classified as SV, may remain ‘unclassifiable’.
The VGICC nomenclature/classification is summarized in Table 3.
Definition of stable disease
Starting discussion points
Establishing the disease stability is important for making therapeutic decisions, but the notion of stable disease is subject to interpretation. Clinically, a three-stage scoring method, namely progressive/regressive/stable disease, is often used. However, the clinical definition of ‘stability’ varies greatly. Moellmann et al. (1982) defined active disease as ‘when lesions are enlarging in 6 weeks before examination’; Cui et al. (1993) as the ‘development of new lesions or extension of old lesions in 3 months before examination’; Uda et al. (1984) as the ‘spread without regression within the last half year’; and Falabella et al. (1995) defined stable vitiligo as ‘a condition that has not progressed for at least 2 yr’. Falabella et al. further proposed a list of clinical criteria to define the stability: (i) lack of progression of old lesions within the past 2 yr; (ii) no new lesions developing within the same period; (iii) absence of recent KP either from history or experimentally induced; (iv) spontaneous repigmentation or repigmentation of depigmented areas by medical treatment; and (v) positive minigrafting test and lack of koebnerization at donor site (Falabella et al., 1995). However, these criteria may be challenged by clinical observations in which KP and minigraft testing are discordant. Data obtained from minigraft testing in case series suggest that the minigraft test provides a reflection of the stability of defined individual lesions, which does not necessarily reflect global stability of the disease (Dupré and Christol, 1978; Lahiri et al., 2004a,b; Malakar et al., 2000).
One of the striking features of vitiligo, compared to other chronic skin conditions, is the absence of clinical symptoms and overt signs of inflammation. However, histological studies indicate that an inflammatory response can be detected at the progressing edge of depigmented lesions (Attili and Attili, 2008; Hann et al., 1992; Le Poole and Das, 1997; Sharquie et al., 2004; van den Wijngaard et al., 2000; Yagi et al., 1997). Absence/presence of inflammatory infiltrate at a lesion margin might be helpful for clinical staging of individual lesions. Indeed, the observation of a microinflammatory border in active vitiligo, and/or of other local markers of disease activity, might become useful to make appropriate therapeutic decisions.
Key discussion points
Is a biopsy needed as an objective marker of disease activity?
Skin biopsy in vitiligo is currently not recommended to make therapeutic decisions. Digitized photographic assessment is useful for individual lesions, especially when surgery is considered. For systemic treatments, reliable histological markers of disease extent and stability are important, whereas the utility of biomarkers such as enumeration of T cells or other markers has not been established and requires additional evaluation.
Which tools are adequate for grading stability?
When assessed carefully, it is not rare to document a variable course of individual lesions in a patient, with different lesions regressing, being stable, or progressing simultaneously. ‘Overall’ stability is thus not a reliable assessment, and careful analysis of individual areas (including taking into account the patient’s own observations) is preferable. The VETF system allows such regional follow-up (Taïeb and Picardo, 2007), as does the Vitiligo Area Scoring Index (VASI) (Hamzavi et al., 2004). When available, serial digital imaging should be employed. Ideally, stability should be assessed using the combined criteria of a clinical scoring system (VASI or VETF), patient self-reporting, and serial digital imaging of specific lesions over at least 12 months.
Is there a minimal duration to define stability for surgical purposes?
Local stability is easily defined in SV but is far more difficult to assess in vitiligo/NSV. A 12-month period of photographically assessed stability is deemed appropriate to consider a lesion ‘stable’ for the purpose of surgical treatment.
Summary of the final discussion
The need to perform biopsies to assess stability was not deemed mandatory until more reliable markers of disease progression are validated and readily available. Except for SV, stability is difficult to predict. More uniform tools for assessment of stability are needed.
Consensus statements.
Assessment of ‘overall’ stability is inaccurate and unreliable, whereas individual lesion stability is more reliable, especially when used in the context of surgical intervention.
There is no current agreement on the reliability of a skin biopsy for assessing lesion stability.
Assessment of stability should include a combination of history, serial digitized photographs, and clinical scoring such as the VASI and the ‘spreading’ item as included in the VETF scoring system.
Stability scoring using the aforementioned tools has been arbitrarily set to 12 months.
Definition of the Koebner phenomenon
Starting discussion points
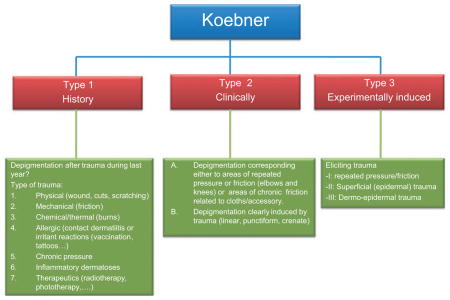
Koebner phenomenon, also called the ‘isomorphic response’, has been defined as ‘the development of lesions at sites of specifically traumatized uninvolved skin of patients with cutaneous diseases’ (Koebner, 1876; Miller, 1982). In vitiligo, KP is responsible for the onset of so-called isomorphic depigmented lesions. These depigmented macules may correspond to traumatized areas (scratches) and are easily recognizable by their shape (Dupré and Christol, 1978). In some cases, KP has been described with a border of intermediate pigmentation, also termed ‘trichrome vitiligo’ (Gauthier and Benzekri, 2010). It has long been known that vitiligo is more prominent in skin areas exposed to physical trauma compared with adjacent, more protected areas, for example, metacarpophalangeal joints (versus between the joints), the nose tip and alae (versus nasal sulci), axilla and elbows. Furthermore, it has been suggested that the location of vitiligo lesions may be related to areas of repeated friction that occurs during daily living activities, such as washing, dressing, personal care, sports and occupational activity, or continuous pressure from clothing or other items (Cario-André et al., 2007; Gauthier, 1995; Gauthier and Benzekri, 2010). Such stimuli are termed chronic koebnerization factors, and these may be responsible for the chronicity of vitiligo. Nevertheless, scientific evidence to support this hypothesis remains lacking. The incidence of KP in NSV has been reported to be from 15 to 70% (El-Nasr and El-Hefnawi, 1963; Ormsby and Montgomery, 1948; Ortonne, 1983; Schallreuter et al., 1994; Sweet, 1978). The occurrence of KP in SV is controversial; in SV patients, whether KP is present or not inside or even outside the related segment is still a matter of debate. Indeed, some authors report no KP in SV (Koga and Tango, 1988; Njoo et al., 1999), whereas others found KP to be present, although at a lower prevalence than in NSV (Barona et al., 1995; Ezzedine et al., 2011a; Hann et al., 1997a). Koebner phenomenon can be assessed by history, by clinical examination, or by assessment of experimentally induced KP. Gopinathan reported depigmentation of clinically non-involved skin in 70% of patients with vitiligo after performing scarification procedures, but in none after tape stripping, suggesting that deep trauma involving the dermis may be necessary (Gopinathan, 1965). Njoo et al. (1999) studied experimentally induced KP after epidermodermal damage to non-depigmented skin by 2-mm punch biopsy, observing KP in 61% of generalized vitiligo (GV) patients but in no patients with SV.
The VETF has recently published a position paper on KP in vitiligo, including the classification into three types of KP, that is, by history, by clinical examination, and by experimental induction (van Geel et al., 2012); though, as noted above, it can be difficult to separate KP and stability (Falabella, 2005; Falabella et al., 1995). The presence or absence of KP is useful for guiding therapeutic intervention, especially surgery (Falabella, 2005).
From a pathophysiological viewpoint, KP may result from melanocyte damage via different pathways, as in psoriasis and lichen planus (Gregorio et al., 2010), such as mechanical trauma, and UV damage. So-called photosensitive vitiligo may reflect Koebnerization linked to sunburn rather than an underlying lupus diathesis.
Key discussion points
Is there a link between KP and disease stability?
There is a general impression that KP and disease stability are related, but objective data are lacking. The VETF position paper (van Geel et al., 2011b) provides definitions and grading that may help to guide future studies.
Is there an association between UV, KP, and systemic lupus erythematosus?
Report of worsening of vitiligo following phototherapy or sun exposure was mentioned by one expert at the Seoul meeting. It was noted in the discussion that the aggravation of vitiligo during phototherapy could be multifactorial, the most frequent being just absence of control of disease with phototherapy alone, koebnerization in case of too large increments of UV radiation, and possible absence of photoadaptation (Hamzavi et al., 2004). The association of true vitiligo with systemic lupus erythematosus is considered as a rare event by experts.
Final discussion summary
For history-based diagnosis of KP, caution is recommended about attributing vitiligo to daily ‘koebnerization’ from friction, clothes, dressing, pressure, etc., as scientific evidence to support this assertion remains lacking. A topic that has not yet been investigated is whether occurrence of KP is more frequent during periods of clinical exacerbation of vitiligo, and whether there is any correlation with markers of local or general inflammation/autoimmunity.
Consensus statement.
The VGICC unanimously endorses the VETF KP classification system (history based, clinical observation based, and experimentally induced) (van Geel et al., 2011b) (Appendix 3).
Definition of autoimmune vitiligo
Starting discussion points
The term ‘autoimmune vitiligo’ has been used in many publications, with no established defining criteria. In fact, it appears that vitiligo may always be, at least in part, a systemic autoimmune disorder (Le Poole and Luiten, 2008; Le Poole et al., 1993; Schallreuter et al., 1994; Spritz, 2012), though predisposing factors and triggers are not completely known. However, for SV, evidence of a general autoimmune context is less convincing. A recent study of SV associated with halo nevi reported an increased number of T cells that recognize melanocyte antigens in SV lesional skin compared with non-lesional skin and blood (van Geel et al., 2010), suggesting a CD8+ melanocyte-specific T cell-mediated immune response, as has been observed in generalized vitiligo. Similar studies of MV might shed additional light on the role of inflammation and autoimmunity in the pathogenesis of both SV and vitiligo (Ezzedine et al., 2011b,c).
Key discussion points
Despite the considerable congruent data on the topic, opinions regarding consistent autoimmune involvement were not yet fully in favor of a consensus prior to the Bordeaux meeting (Table 4). However, there was consensus on the need for additional research, especially by analyzing skin samples in cases of progressive disease. In particular, when performing a biopsy of a vitiligo lesion, it would be worthwhile to include adjacent perilesional skin to assess disease progression in the biopsied area as immune infiltrates are most often present in a defined, narrow margin of actively depigmenting vitiligo skin (Wang et al., 2011a).
Table 4.
Definition of ‘autoimmune’ vitiligo: summary of regional contributions
| Pacific group | Whatever the type of vitiligo, autoimmune mechanisms are important in the pathogenesis of the disease |
| Japan-Taiwan group | Genetic and environmental factors are involved in the development of vitiligo The roles of humoral and cellular immunity remain unclear |
| Latin-American group | Use ‘vitiligo associated with autoimmune diseases’ instead of ‘autoimmune’ vitiligo |
| European group | No consensus position Grading of autoimmunity phenomena associated with vitiligo suggested |
| North American group | Autoimmune vitiligo is an unnecessary term as all vitiligo cases are autoimmune. T cell-mediated autoimmunity predominates although circulating lymphocytes do not necessarily reflect what occurs to the skin |
| North/South Africa group | Association of vitiligo with autoimmune diseases is not synonymous for ‘autoimmune’ vitiligo. Stigma of local immune-mediated inflammation should be preferred to those of generalized autoimmunity for assessing autoimmunity in vitiligo |
Perhaps of greatest importance, it is essential to differentiate between vitiligo/NSV and SV with regard to the association with markers of autoimmunity, including personal and familial history, detection of melanocyte and anti-DNA/anti-histone autoantibodies, and immune cell infiltrates at lesional margins. At present, these are thought to be less often present in SV; though, this may largely reflect a relative lack of data on this topic.
The VGICC stressed the importance of recognizing vitiligo as an autoimmune disease, as this directly impacts optimal treatment strategies, which almost certainly will require controlling the autoimmune process to facilitate melanocyte proliferation and migration to achieve skin repigmentation. Nevertheless, understanding the role of immune mechanisms in progressive depigmentation does not negate the importance of identifying precipitating environmental triggers of melanocyte-directed autoimmunity, as these may be exploited to permit both disease prevention and improved treatment of existing disease.
Consensus statements.
The term ‘autoimmune vitiligo’ should not be included in the consensus classification, as emerging evidence supports autoimmune/autoinflammatory involvement in every type of vitiligo; thus, the term ‘autoimmune’ is redundant.
Vitiligo/NSV is driven by autoimmune/autoinflammatory mechanisms specifically targeting melanocytes within the skin. There should be a clear distinction between specific autoimmune responses against melanocytes (autoantibodies or T cells) and a context of autoimmunity, as defined by either personal/family history of autoimmunity or non-specific autoantibodies.
Finally, vitiligo patients are at elevated risk of developing other concomitant autoimmune diseases, particularly autoimmune thyroid disease, and should be screened for these other diseases on a periodic basis. At present, it remains unclear what environmental factors may trigger vitiligo, and how these can be modified to either treat or prevent disease.
Conclusions
The VGICC nomenclature and recommendations regarding related issues is an important first step, but highlighted several still-unmet needs. The consensus on classification, definition of stability, and KP should facilitate clinical research and patient management worldwide. Compelling evidence exists for inflammatory or immune-mediated mechanisms in vitiligo/NSV. There is an obvious need for a pathophysiology-based classification, which may lead to better treatment approaches, but more work is needed to achieve this level of knowledge. At present, improved clinical definitions may allow investigators to better define patient subgroups for scientific investigation (e.g., immune or inflammatory versus non-immune; halo nevus associated versus non-halo nevus associated, etc.). There is also a need for a prognosis-based classification. For this purpose, case registries with careful documentation of disease presentation, progression, and treatment responses should be established. Approaches to assess the remaining melanocyte reservoir would be valuable, both to improve descriptive classification and to ascertain most appropriate treatment modality. The VETF grading system, which includes severity (Taïeb and Picardo, 2007), may be of predictive utility but requires prospective validation.
Significance.
There is a need for more homogenous reporting in vitiligo research. A broad international consensus was implemented on nomenclature and related issues (definition of stability, Koebner’s phenomenon, and discussion of ‘autoimmune vitiligo’ as an autonomous entity).
Acknowledgments
The authors gratefully acknowledge the dedicated efforts and support of the Associazione Ricerca Informazione per la Vitiligine (ARIV).
Appendix 1: Regional working groups for Vitiligo Global Issues Consensus Conference
| Africa | |
| Laila Benzekri | Africa, Rabat, Morocco |
| Noufal Raboobee | Africa, Durban, South Africa |
| America (Coordinators Henry Lim and Caio de Castro) | |
| Henry Lim | North America, Detroit, USA |
| John Harris | Worcester, Massachussets, USA |
| Pearl Grimes | North America, Los Angeles, USA |
| Richard Spritz | North America, Denver, USA |
| Harvey Lui | North America, Vancouver, Canada |
| Yowen Zhou | North America, Vancouver, Canada |
| Maria Lucia Barona | South and Latin America, Colombia |
| Rafael Falabella | South and Latin America, Colombia |
| Olivério Welsh | South and Latin America, Mexico |
| Juan Pablo | South and Latin America, Mexico |
| Castanedo Cazeres | |
| Carlos D’Apparecida | South and Latin America, Brazil |
| Santos Machado Filho | |
| Paulo Roberto Machado | South and Latin America, Brazil |
| Europe (Coordinators Alain Taieb and Mauro Picardo) | |
| Alain Taieb | Europe, Bordeaux, France |
| Khaled Ezzedine | Europe, Bordeaux, France |
| Yvon Gauthier | Europe, Bordeaux, France |
| Thierry Passeron | Europe, Nice, France |
| Mauro Picardo | Europe, Roma, Italy |
| Giovanni Leone | Europe, Roma, Italy |
| Silvia Moretti | Europe, Florence, Italy |
| David Gawkrodger | Europe, Sheffield, UK |
| Karin Schallreuter | Europe, Bradford, UK |
| Viktoria Eleftheriadou | Europe, Nottingham, UK |
| Matts Olson | Europe, Upssala, Sweden |
| Adrian Tanew | Europe, Vienna, Austria |
| Adriana Alomar | Europe, Barcelona, Spain |
| Jan Peter Van Der Veen | Europe, Amsterdam, Netherlands |
| Rosalie Luiten | Europe, Amsterdam, Netherlands |
| Inka Nieuweboer | Europe, Amsterdam, Netherlands |
| Wiete Westerhof | Europe, Munster, Germany |
| Markus Bohm | Europe, Munster, Germany |
| Nanja Van Geel | Europe, Gent, Belgium |
| Middle East (coordinator Tag Anbar) | |
| Medhat El-Mofty | Middle East, Cairo, Egypt |
| Mahmoud Abdallah | Middle East, Cairo, Egypt |
| Marwa Abdallah | Middle East, Cairo, Egypt |
| Samia Esmat | Middle East, Cairo, Egypt |
| Hamza Abdel-Raouf | Middle East, Al-Minya, Egypt |
| Amal Abdel-Rahman | Middle East, Al-Minya, Egypt |
| Mohamed El-Khayyat | Middle East, Al-Minya, Egypt |
| Continental Asia/Singapure (coordinator D Parsad (India) and BK Goh (Singapore) | |
| Boon Kee Goh | Continental Asia, Singapore |
| Davinder Parsad | Continental Asia, India |
| Hema Jerajani | Continental Asia, India |
| Seung-Kyung Hann | Continental Asia, South Korea |
| Ai-Young Lee | Continental Asia, South Korea |
| Japan/Taiwan (coordinator E Lan (Taiwan) and I Katayama (Japan)) | |
| Ichiro Katayama | Japan/Taiwan, Osaka, Japan |
| Kazuyoshi Fukai | Japan/Taiwan, Osaka, Japan |
| Atsushi Tanemura | Japan/Taiwan, Osaka, Japan |
| Naoki Oiso | Japan/Taiwan, Kindai, Japan |
| Tamio Suzuki | Japan/Taiwan, Yamagata, Japan |
| Cheng che Eric Lan | Japan/Taiwan, Kaoshiung, Taiwan |
| Pacific (coordinator P Kumarasinghe) | |
| Prasad Kumarasinghe | Pacific, Australia |
| Richard Wittal | Pacific, Australia |
Appendix 2: Glossary
Vitiligo/non-segmental vitiligo
Common vitiligo (formerly referred to as vitiligo vulgaris)
This most common form of vitiligo is characterized by asymptomatic, well-circumscribed, milky-white macules involving multiple parts of the body, usually a symmetrical pattern. The disease can start at any site of the body but the fingers, hands, and face are frequently the initial sites.
Acrofacial vitiligo
In acrofacial vitiligo, the involved sites are usually limited to face, head, hands, and feet. A distinctive feature is depigmentation of the distal fingers and facial orifices. It may later include other body sites, resulting in typical generalized vitiligo.
Vitiligo universalis
Vitiligo universalis is the most extensive form of the disease and generally occurs in adulthood. ‘Universalis’ is generally used when depigmentation is virtually universal (80–90% of body surface), but some pigmentation may be still present. Hairs may also be partially spared. Whereas this diagnosis is facile in dark-skinned individuals, it may be difficult in very fair-skinned individuals. Usually, VU is preceded by generalized vitiligo that gradually evolves to complete or near complete depigmentation of the skin and hair.
Mucosal vitiligo
Mucosal vitiligo typically refers to the involvement of the oral and/or genital mucosae. It may present as part of generalized vitiligo when associated with other sites of skin involvement, or as an isolated condition, which in the VGICC nomenclature, remains in the ‘unclassified’ category at least 2 yr of follow-up.
Mixed vitiligo
Mixed vitiligo refers to concomitant occurrence of SV (Figure 1A) and vitiligo/NSV (Figure 1B). Usually, SV precedes vitiligo/NSV, though not always. Criteria proposed for mixed vitiligo (Ezzedine et al., 2011c) are as follows:
Figure 1.
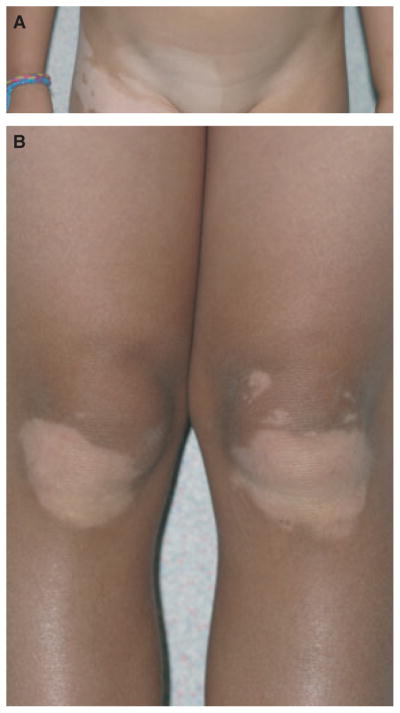
Mixed vitiligo in a 9-yr-old patient with patient (A) segmental and (B) non-segmental involvement.
Absence of depigmented areas in a segmental distribution at birth and in the first year of life, and Wood’s lamp examination excluding nevus depigmentosus.
SV followed by vitiligo/NSV with a delay of at least 6 months.
SV affecting at least 20% of the theoretical distribution of a dermatomal segment or presenting a definite Blaschko linear distribution.
Response to narrow-band UVB treatment in between SV (poor response) and vitiligo/NSV (good response).
Focal vitiligo
Focal vitiligo refers to a small isolated patch that does not fit a segmental distribution, and which has not evolved into vitiligo/NSV after a period of at least 2 yr. This form of vitiligo may evolve into either SV or vitiligo/NSV.
Other unclassified conditions
Vitiligo punctata
Lesions present as sharply demarcated depigmented punctiform 1- to 1.5-mm macules involving any area of the body (Figure 2).
Figure 2.
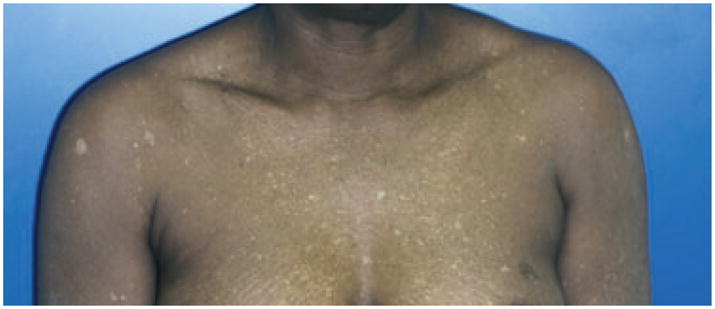
Vitiligo punctata, patient with multiple pea-sized depigmented macules of the trunk, punctate vitiligo.
Vitiligo minor
This form of NSV is rarely reported (Passeron and Ortonne, 2010). The disease seems to be limited to dark-skinned individuals. The term ‘minor’ does not strictly refer to the limitation of the disease to a restricted surface area but rather to the partial defect in pigmentation (Figure 3). The relation to true vitiligo comes from pathology and coexistence with conventional vitiligo macules. The differential diagnosis from early stage cutaneous lymphoma is of primary importance, and repeated biopsies with molecular studies of clonality may be needed.
Figure 3.
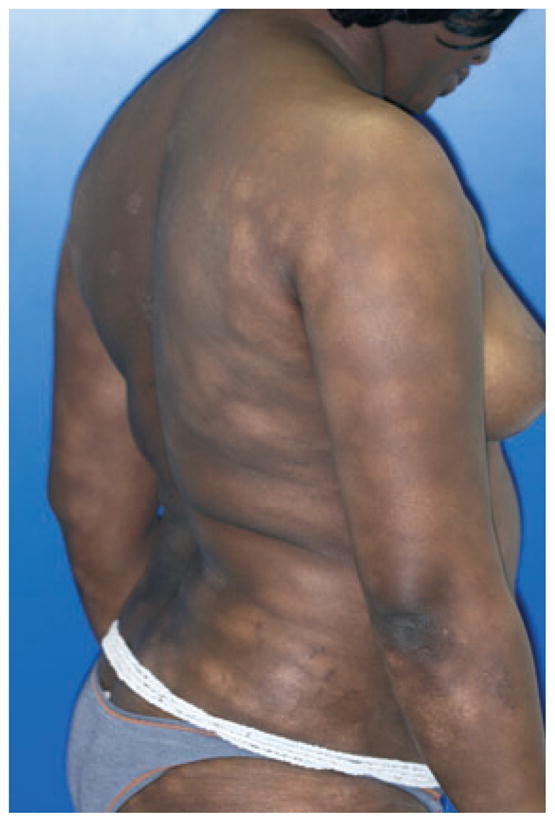
Vitiligo minor, multiple hypopigmented macules in a 27-yr-old dark-skinned woman.
Follicular vitiligo
This refers to a form of generalized vitiligo seen in a young black patient that primary involved the follicular reservoir with limited skin involvement, contrasting with marked generalized hair whitening, and melanocyte loss in hair follicles (Figure 4) (Article in revision, PCMR). Additional cases are required to further assess whether follicular vitiligo constitutes a separate form of vitiligo.
Figure 4.
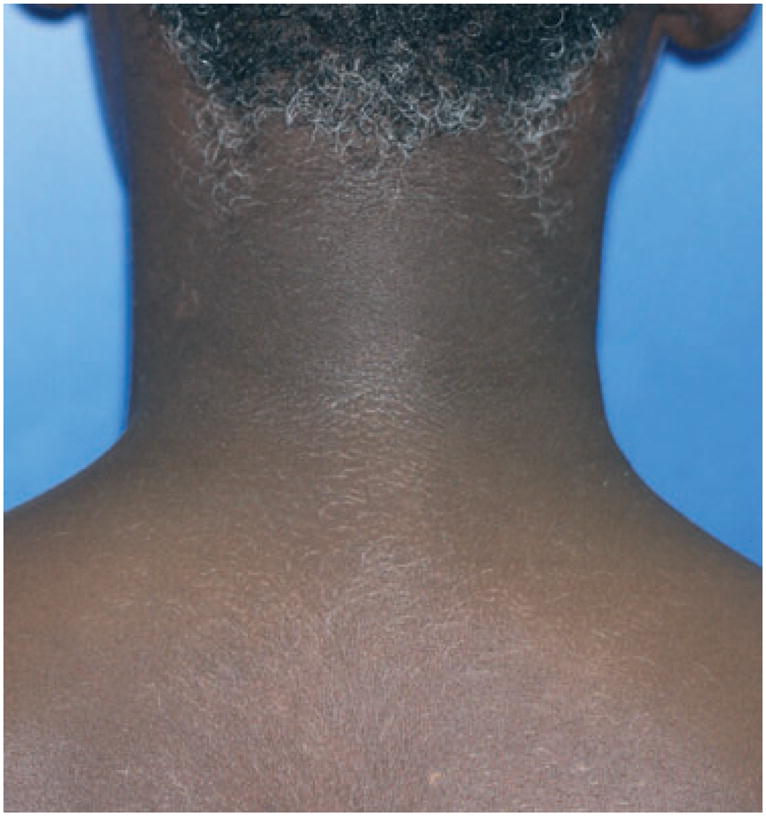
Follicular vitiligo, complete whitening of the body hair (A) associated with depigmented macules of the face and eyelashes whitening (B) in a 12-yr-old boy.
Segmental vitiligo
Mono-segmental vitiligo is the most common form of SV, referring to the presence of one or more white de-pigmented macules distributed on one side of the body (Figure 5), usually respecting the midline (although some lesions may partly cross the midline), early follicular involvement (leukotrichia), and rapid development over a few weeks or months, and overall protracted course.
Figure 5.
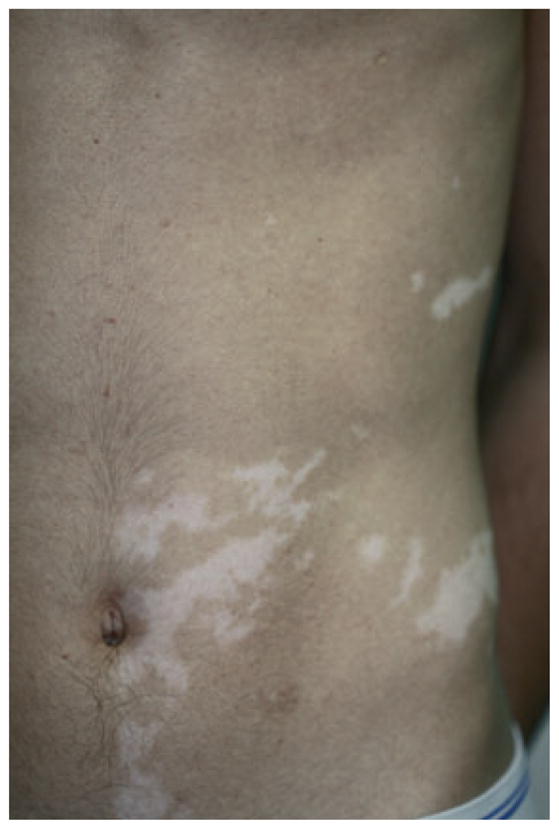
Monosegmental vitiligo, depigmented macules unilaterally distributed with sharp midline demarcation.
Rarely, segmental vitiligo may refer to multiple segmental lesions distributed either unilaterally or bilaterally (Figure 6). The onset may be simultaneous or not. A clear segmental distribution of the lesions with midline demarcation, together with the associated features described in mono-segmental cases (leukotrichia, protracted course), distinguishes this diagnosis versus vitiligo/NSV in bilateral cases.
Figure 6.

Bisegmental vitiligo, bilaterally distributed, depigmented macules of each lesion do not cross the midline.
Appendix 3
Classification of Koebner’s phenomenon as proposed by the Vitiligo European Task Force in 2011 (van Geel et al., 2011b).
References
- Attili VR, Attili SK. Lichenoid inflammation in vitiligo – a clinical and histopathologic review of 210 cases. Int J Dermatol. 2008;47:663–669. doi: 10.1111/j.1365-4632.2008.03672.x. [DOI] [PubMed] [Google Scholar]
- Barona MI, Arrunátegui A, Falabella R, Alzate A. An epidemiologic case-control study in a population with vitiligo. J Am Acad Dermatol. 1995;33:621–625. doi: 10.1016/0190-9622(95)91282-7. [DOI] [PubMed] [Google Scholar]
- Boissy RE. Vitiligo. In: Picardo M, Taieb A, editors. Occupational vitiligo. Heidelberg: Springer Verlag; 2010. pp. 175–180. [Google Scholar]
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208–214. doi: 10.1111/j.1600-0749.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Nordlund JJ. Biology in vitiligo. In: Arndt KA, LeBoit PE, Robinson JK, Wintroub BU, editors. Cutaneous Medicine and Surgery: An Integrated Program in Dermatology. Philadelphia: W. B. Saunders Company; 1995. pp. 1210–1218. [Google Scholar]
- Cario-André M, Pain C, Gauthier Y, Taïeb A. The melanocytorrhagic hypothesis of vitiligo tested on pigmented, stressed, reconstructed epidermis. Pigment Cell Res. 2007;20:385–393. doi: 10.1111/j.1600-0749.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- Cui J, Arita Y, Bystryn JC. Cytolytic antibodies to melanocytes in vitiligo. J Invest Dermatol. 1993;100:812–815. doi: 10.1111/1523-1747.ep12476636. [DOI] [PubMed] [Google Scholar]
- Cummings MP, Nordlund JJ. Chemical leukoderma: fact or fancy. Am J Contact Dermatitis. 1995;6:122–127. [Google Scholar]
- Dupré A, Christol B. Cockade-like vitiligo and linear vitiligo a variant of fitzpatrick’s trichrome vitiligo. Arch Dermatol Res. 1978;262:197–203. doi: 10.1007/BF00455391. [DOI] [PubMed] [Google Scholar]
- El-Nasr HS, El-Hefnawi H. Koebner’s phenomenon in dermatology. A study and report of some unusual stigmata of this phenomenon. J Egypt Med Assoc. 1963;46:1067–1086. [PubMed] [Google Scholar]
- Ezzedine K, Diallo A, Léauté-Labrèze C, et al. Multivariate analysis of factors associated with early-onset segmental and nonsegmental vitiligo: a prospective observational study of 213 patients. Br J Dermatol. 2011a;165:44–49. doi: 10.1111/j.1365-2133.2011.10311.x. [DOI] [PubMed] [Google Scholar]
- Ezzedine K, Diallo A, Léauté-Labrèze C, et al. Halo nevi and leukotrichia are strong predictors of the passage to mixed vitiligo in a subgroup of segmental vitiligo. [accessed 26 December 2011];Br J Dermatol. 2011b doi: 10.1111/j.1365-2133.2011.10709.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22032627. [DOI] [PubMed]
- Ezzedine K, Gauthier Y, Léauté-Labrèze C, Marquez S, Bouchtnei S, Jouary T, Taieb A. Segmental vitiligo associated with generalized vitiligo (mixed vitiligo): a retrospective case series of 19 patients. J Am Acad Dermatol. 2011c;65:965–971. doi: 10.1016/j.jaad.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Falabella R. Surgical approaches for stable vitiligo. Dermatol Surg. 2005;31:1277–1284. doi: 10.1111/j.1524-4725.2005.31203. [DOI] [PubMed] [Google Scholar]
- Falabella R, Escobar CE, Carrascal E, Arroyave JA. Leukoderma punctata. J Am Acad Dermatol. 1988;18:485–494. doi: 10.1016/s0190-9622(88)70071-7. [DOI] [PubMed] [Google Scholar]
- Falabella R, Arrunategui A, Barona MI, Alzate A. The minigrafting test for vitiligo: detection of stable lesions for melanocyte transplantation. J Am Acad Dermatol. 1995;32:228–232. doi: 10.1016/0190-9622(95)90131-0. [DOI] [PubMed] [Google Scholar]
- Gauthier Y. The importance of Koebner’s phenomenon in the induction of vitiligo vulgaris lesions. Eur J Dermatol. 1995;5:704–708. [Google Scholar]
- Gauthier Y, Benzekri L. Vitiligo. In: Picardo M, Taieb A, editors. The Koebner’s phenomenon. Heidelberg: Springer Verlag; 2010. pp. 167–173. [Google Scholar]
- Gauthier Y, Cario Andre M, Taïeb A. A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res. 2003;16:322–332. doi: 10.1034/j.1600-0749.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- Gawkrodger DJ, Ormerod AD, Shaw L, Mauri-Sole I, Whitton ME, Watts MJ, Anstey AV, Ingham J, Young K. Guideline for the diagnosis and management of vitiligo. Br J Dermatol. 2008;159:1051–1076. doi: 10.1111/j.1365-2133.2008.08881.x. [DOI] [PubMed] [Google Scholar]
- van Geel NAC, Mollet IG, De Schepper S, Tjin EPM, Vermaelen K, Clark RA, Kupper TS, Luiten RM, Lambert J. First histopathological and immunophenotypic analysis of early dynamic events in a patient with segmental vitiligo associated with halo nevi. Pigment Cell Melanoma Res. 2010;23:375–384. doi: 10.1111/j.1755-148X.2010.00703.x. [DOI] [PubMed] [Google Scholar]
- van Geel N, De Lille S, Vandenhaute S, Gauthier Y, Mollet I, Brochez L, Lambert J. Different phenotypes of segmental vitiligo based on a clinical observational study. J Eur Acad Dermatol Venereol. 2011a;25:673–678. doi: 10.1111/j.1468-3083.2010.03847.x. [DOI] [PubMed] [Google Scholar]
- van Geel N, Speeckaert R, Taieb A, et al. Koebner’s phenomenon in vitiligo: European position paper. Pigment Cell Melanoma Res. 2011b;24:564–573. doi: 10.1111/j.1755-148X.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- van Geel N, Speeckaert R, Mollet I, De Schepper S, De Wolf J, Tjin EPM, Luiten RM, Lambert J, Brochez L. In vivo vitiligo induction and therapy model: double-blind, randomized clinical trial. Pigment Cell Melanoma Res. 2012;25:57–65. doi: 10.1111/j.1755-148X.2011.00922.x. [DOI] [PubMed] [Google Scholar]
- Gopinathan T. A study of the lesion of vitiligo. Arch Dermatol. 1965;91:397–404. [PubMed] [Google Scholar]
- Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, Arai N, Gallo RL, Digiovanni J, Gilliet M. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzavi I, Jain H, McLean D, Shapiro J, Zeng H, Lui H. Parametric modeling of narrowband UV-B phototherapy for vitiligo using a novel quantitative tool: the Vitiligo Area Scoring Index. Arch Dermatol. 2004;140:677–683. doi: 10.1001/archderm.140.6.677. [DOI] [PubMed] [Google Scholar]
- Hann SK, Lee HJ. Segmental vitiligo: clinical findings in 208 patients. J Am Acad Dermatol. 1996;35:671–674. doi: 10.1016/s0190-9622(96)90718-5. [DOI] [PubMed] [Google Scholar]
- Hann SK, Nordlund JJ. Vitiligo. In: Hann SK, Nordlund JJ, editors. Clinical features of generalized vitiligo. Oxford: Blackwell Publishing Ltd; 2000. pp. 81–88. [Google Scholar]
- Hann SK, Park YK, Lee KG, Choi EH, Im S. Epidermal changes in active vitiligo. J Dermatol. 1992;19:217–222. doi: 10.1111/j.1346-8138.1992.tb03211.x. [DOI] [PubMed] [Google Scholar]
- Hann SK, Chun WH, Park YK. Clinical characteristics of progressive vitiligo. Int J Dermatol. 1997a;36:353–355. doi: 10.1046/j.1365-4362.1997.00162.x. [DOI] [PubMed] [Google Scholar]
- Hann SK, Park YK, Chun WH. Clinical features of vitiligo. Clin Dermatol. 1997b;15:891–897. doi: 10.1016/s0738-081x(97)00130-2. [DOI] [PubMed] [Google Scholar]
- Hann SK, Chang JH, Lee HS, Kim SM. The classification of segmental vitiligo on the face. Yonsei Med J. 2000;41:209–212. doi: 10.3349/ymj.2000.41.2.209. [DOI] [PubMed] [Google Scholar]
- Happle R. Segmental type 2 manifestation of autosome dominant skin diseases. Development of a new formal genetic concept. Hautarzt. 2001;52:283–287. doi: 10.1007/s001050051309. [DOI] [PubMed] [Google Scholar]
- Happle R. Superimposed segmental manifestation of polygenic skin disorders. J Am Acad Dermatol. 2007;57:690–699. doi: 10.1016/j.jaad.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Kim DY, Oh SH, Hann SK. Classification of segmental vitiligo on the face: clues for prognosis. Br J Dermatol. 2011;164:1004–1009. doi: 10.1111/j.1365-2133.2010.10202.x. [DOI] [PubMed] [Google Scholar]
- Koebner H. Zur aetiologie der psoriasis. Vrtljscher Dermatol Syphil. 1876;8:559–561. [Google Scholar]
- Koga M, Tango T. Clinical features and course of type A and type B vitiligo. Br J Dermatol. 1988;118:223–228. doi: 10.1111/j.1365-2133.1988.tb01778.x. [DOI] [PubMed] [Google Scholar]
- Kostopoulou P, Jouary T, Quintard B, Ezzedine K, Marques S, Boutchnei S, Taieb A. Objective vs. subjective factors in the psychological impact of vitiligo: the experience from a French referral centre. Br J Dermatol. 2009;161:128–133. doi: 10.1111/j.1365-2133.2009.09077.x. [DOI] [PubMed] [Google Scholar]
- Lahiri K, Malakar S, Sarma N. Herpes-simplex induced lip leucoderma revisited. Dermatology (Basel) 2004a;208:182. doi: 10.1159/000077336. [DOI] [PubMed] [Google Scholar]
- Lahiri K, Malakar S, Sarma N, Banerjee U. Inducing repigmentation by regrafting and phototherapy (311 nm) in punch grafting failure cases of lip vitiligo: a pilot study. Indian J Dermatol Venereol Leprol. 2004b;70:156–158. [PubMed] [Google Scholar]
- Le Poole IC, Das PK. Microscopic changes in vitiligo. Clin Dermatol. 1997;15:863–873. doi: 10.1016/s0738-081x(97)00127-2. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, Luiten RM. Autoimmune etiology of generalized vitiligo. Curr Dir Autoimmun. 2008;10:227–243. doi: 10.1159/000131485. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, Das PK, van den Wijngaard RM, Bos JD, Westerhof W. Review of the etiopathomechanism of vitiligo: a convergence theory. Exp Dermatol. 1993;2:145–153. doi: 10.1111/j.1600-0625.1993.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Lerner AB. On the etiology of vitiligo and gray hair. Am J Med. 1971;51:141–147. doi: 10.1016/0002-9343(71)90232-4. [DOI] [PubMed] [Google Scholar]
- Malakar S, Lahiri K, Malakar RS. How unstable is the concept of stability in surgical repigmentation of vitiligo? Dermatology (Basel) 2000;201:182–183. doi: 10.1159/000018449. [DOI] [PubMed] [Google Scholar]
- Mashayekhi V, Javidi Z, Kiafar B, Manteghi AA, Saadatian V, Esmaeili HA, Hosseinalizadeh S. Quality of life in patients with vitiligo: a descriptive study on 83 patients attending a PUVA therapy unit in Imam Reza Hospital, Mashad. Indian J Dermatol Venereol Leprol. 2010;76:592. doi: 10.4103/0378-6323.69097. [DOI] [PubMed] [Google Scholar]
- Miller RA. The Koebner phenomenon. Int J Dermatol. 1982;21:192–197. doi: 10.1111/j.1365-4362.1982.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Moellmann G, Klein-Angerer S, Scollay DA, Nordlund JJ, Lerner AB. Extracellular granular material and degeneration of keratinocytes in the normally pigmented epidermis of patients with vitiligo. J Invest Dermatol. 1982;79:321–330. doi: 10.1111/1523-1747.ep12500086. [DOI] [PubMed] [Google Scholar]
- Mulekar SV, Al Issa A, Asaad M, Ghwish B, Al Eisa A. Mixed vitiligo. J Cutan Med Surg. 2006;10:104–107. doi: 10.2310/7750.2006.00018. [DOI] [PubMed] [Google Scholar]
- Njoo MD, Das PK, Bos JD, Westerhof W. Association of the Köbner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol. 1999;135:407–413. doi: 10.1001/archderm.135.4.407. [DOI] [PubMed] [Google Scholar]
- Ormsby OS, Montgomery H. Koebner’s Phenomenon in Dermatology: A Study and Report of Some Unusual Stigmata of this Phenomenon. Philadelphia: Lea and Febriger; 1948. p. 306. [Google Scholar]
- Ortonne JP. Vitiligo. In: Ortonne JP, Mosher DB, Fitzpatrick TB, editors. Vitiligo and Other Hypomelanosis of Hair and Skin. New-York: Plenum Medical Books; 1983. pp. 163–310. [Google Scholar]
- Ortonne JP. Depigmentation of hair and mucous membrane. In: Hann SK, Nordlund JJ, editors. Vitiligo. 1. Oxford: Blackwell Publishing Ltd; 2000. pp. 76–80. [Google Scholar]
- Parsad D, Gupta S. Standard guidelines of care for vitiligo surgery. Indian J Dermatol Venereol Leprol. 2008;74(Suppl):S37–S45. [PubMed] [Google Scholar]
- Passeron T, Ortonne JP. Generalized vitiligo. In: Picardo M, Taieb A, editors. Vitiligo. Heidelberg: Springer Verlag; 2010. pp. 35–39. [Google Scholar]
- Schallreuter KU, Lemke R, Brandt O, Schwartz R, Westhofen M, Montz R, Berger J. Vitiligo and other diseases: coexistence or true association? Hamburg study on 321 patients. Dermatology (Basel) 1994;188:269–275. doi: 10.1159/000247164. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Krüger C, Rokos H, Hasse S, Zothner C, Panske A. Basic research confirms coexistence of acquired Blaschkolinear Vitiligo and acrofacial Vitiligo. Arch Dermatol Res. 2007;299:225–230. doi: 10.1007/s00403-007-0748-7. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Krüger C, Würfel BA, Panske A, Wood JM. From basic research to the bedside: efficacy of topical treatment with pseudocatalase PC-KUS in 71 children with vitiligo. Int J Dermatol. 2008;47:743–753. doi: 10.1111/j.1365-4632.2008.03660.x. [DOI] [PubMed] [Google Scholar]
- Sharquie KE, Mehenna SH, Naji AA, Al-Azzawi H. Inflammatory changes in vitiligo: stage I and II depigmentation. Am J Dermatopathol. 2004;26:108–112. doi: 10.1097/00000372-200404000-00004. [DOI] [PubMed] [Google Scholar]
- Spritz RA. Six decades of vitiligo genetics: genome-wide studies provide insights into autoimmune pathogenesis. J Invest Dermatol. 2012;132:268–273. doi: 10.1038/jid.2011.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RD. Vitiligo as a Köbner phenomenon. Br J Dermatol. 1978;99:223–224. doi: 10.1111/j.1365-2133.1978.tb01988.x. [DOI] [PubMed] [Google Scholar]
- Taieb A, Picardo M. Epidemiology, definitions and classification. In: Picardo M, Taieb A, editors. Vitiligo. Heidelberg: Springer Verlag; 2010. pp. 13–24. [Google Scholar]
- Taïeb A, Picardo M. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20:27–35. doi: 10.1111/j.1600-0749.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- Taïeb A, Picardo M. Clinical practice. Vitiligo N Engl J Med. 2009;360:160–169. doi: 10.1056/NEJMcp0804388. [DOI] [PubMed] [Google Scholar]
- Taïeb A, Morice-Picard F, Jouary T, Ezzedine K, Cario-André M, Gauthier Y. Segmental vitiligo as the possible expression of cutaneous somatic mosaicism: implications for common non-segmental vitiligo. Pigment Cell Melanoma Res. 2008;21:646–652. doi: 10.1111/j.1755-148X.2008.00511.x. [DOI] [PubMed] [Google Scholar]
- Uda H, Takei M, Mishima Y. Immunopathology of vitiligo vulgaris, Sutton’s leukoderma and melanoma-associated vitiligo in relation to steroid effects. II The IgG and C3 deposits in the skin. J Cutan Pathol. 1984;11:114–124. doi: 10.1111/j.1600-0560.1984.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Wang CQF, Cruz-Inigo AE, Fuentes-Duculan J, Moussai D, Gulati N, Sullivan-Whalen M, Gilleaudeau P, Cohen JA, Krueger JG. Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS ONE. 2011a;6:e18907. doi: 10.1371/journal.pone.0018907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KY, Wang KH, Zhang ZP. Health-related quality of life and marital quality of vitiligo patients in China. J Eur Acad Dermatol Venereol. 2011b;25:429–435. doi: 10.1111/j.1468-3083.2010.03808.x. [DOI] [PubMed] [Google Scholar]
- Whitton ME, Pinart M, Batchelor J, Lushey C, Leonardi-Bee J, González U. Interventions for vitiligo. Cochrane Database Syst Rev. 2010:CD003263. doi: 10.1002/14651858.CD003263.pub4. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard R, Wankowicz-Kalinska A, Le Poole C, Tigges B, Westerhof W, Das P. Local immune response in skin of generalized vitiligo patients. Destruction of melanocytes is associated with the prominent presence of CLA+ T cells at the perilesional site. Lab Invest. 2000;80:1299–1309. doi: 10.1038/labinvest.3780138. [DOI] [PubMed] [Google Scholar]
- Yagi H, Tokura Y, Furukawa Y, Takigawa M. Vitiligo with raised inflammatory borders: involvement of T cell immunity and keratinocytes expressing MHC class II and ICAM-1 molecules. Eur J Dermatol. 1997;7:19–22.6. [Google Scholar]


