Abstract
Cyclic AMP- (cAMP) and calcium-dependent agonists stimulate chloride secretion through the coordinated activation of distinct apical and basolateral membrane channels and ion transporters in mucosal epithelial cells. Defects in the regulation of Cl– transport across mucosal surfaces occur with cystic fibrosis and V. cholerae infection and can be life threatening. Here we report that secramine B, a small molecule that inhibits activation of the Rho GTPase Cdc42, reduced cAMP-stimulated chloride secretion in the human intestinal cell line T84. Secramine B interfered with a cAMP-gated and Ba2+-sensitive K+ channel, presumably KCNQ1/KCNE3. This channel is required to maintain the membrane potential that sustains chloride secretion. In contrast, secramine B did not affect the Ca2+-mediated chloride secretion pathway, which requires a separate K+ channel activity from that of cAMP. Pirl1, another small molecule structurally unrelated to secramine B that also inhibits Cdc42 activation in vitro, similarly inhibited cAMP-dependent but not Ca2+-dependent chloride secretion. These results suggest that Rho GTPases may be involved in the regulation of the chloride secretory response and identify secramine B an inhibitor of cAMP-dependent K+ conductance in intestinal epithelial cells.
Keywords: Secramine, Chloride secretion, Cdc42, cAMP, Potassium channel regulation, KCNQ1/KCNE3
1. Introduction
Transepithelial chloride secretion drives water transport across epithelial barriers for the hydration of all mucosal surfaces. It requires the coordinated activity of membrane transporters and channels differentially distributed on the lumenal (apical) and serosal (basolateral) plasma membranes [1]. Chloride ions enter intestinal epithelial cells from the basolateral surface together with sodium and potassium by passing through the basolateral Na+, K+, 2Cl– cotransporter (NKCC) following the inwardly directed Na+ electrochemical gradient established by the basolateral Na+, K+ ATPase. Chloride then exits the opposite apical cell surface by passing through the cAMP-activated cystic fibrosis transmembrane conductance regulator (CFTR) or the Ca2+-activated chloride channel (CaCC). Export of K+ across the basolateral membrane maintains the electrochemical potential required to sustain apical Cl– secretion. In the intestine, this is likely accomplished by the KCNQ1/KCNE3 and the KCNN4 channels, gated open by intracellular cAMP or Ca2+, respectively [2,3]. Although the general features of cAMP and Ca2+-induced chloride secretion are understood, such as the channels required to sustain chloride secretion, little is known about the coordinated activation and regulation of these chloride secretion pathways.
Several years ago, we synthesized a collection of ~2500 small molecules and screened it for inhibition of membrane traffic from the endoplasmic reticulum (ER) to the plasma membrane via the Golgi apparatus [4]. We identified the structurally related compounds secramine A and secramine B as inhibitors of protein export from the Golgi apparatus to the plasma membrane and subsequently found that they inhibit association of the small Rho GTPase Cdc42 with membranes [5]. Rho GTPases cycle between an inactive, GDP-bound conformation, and an active, GTP-bound conformation. They are best known for regulating actin cytoskeleton dynamics [6], but they also participate in membrane traffic, cell–cell contact, cell-cycle progression, and cell polarity [7].
To investigate whether secramines also inhibit membrane traffic in the reverse direction, namely from the Golgi to the ER, we next studied the effect of secramine B on cholera toxin-induced Cl– secretion. Cholera toxin is the major pathogenic factor causing disease in V. cholerae infection. The toxin is an enzyme that acts by entering the cytoplasm of host cells to activate adenylyl cyclase, which converts ATP to cAMP and thereby stimulates Cl– secretion. To access the cytoplasm, cholera toxin binds to receptors on the plasma membrane and traffics to the trans-Golgi network and then to ER [30], where the enzymatically active portion, the A1 chain, unfolds and passes into the cytoplasm by co-opting the endogenous ER retro-translocation machinery [8]. The A1 chain subsequently refolds in the cytoplasm and activates adenylyl cyclase by catalyzing the ADP-ribosylation of the regulatory heterotrimeric G-protein Gsα.
We found that secramine B inhibited Cl– secretion induced by cholera toxin. Secramine B, however, did not appear to act by inhibiting retrograde transport from the Golgi to the ER. Rather it acted downstream of cholera toxin-induced cAMP production by inhibiting K+ efflux through a basolateral K+ channel, likely KCNQ1/KCNE3. In support of this observation, we found that pirl1, a small molecule structurally unrelated to secramine B that also inhibits Rho GTPase function in vitro, also prevented Cl– secretion in T84 cells induced by cAMP agonists [9]. Neither secramine B nor pirl1, however, inhibited Cl– secretion induced by Ca2+-dependent agonists. Thus, these results suggest a novel regulatory role for Rho GTPases in gating KCNQ1/KCNE3 K+ channels and modulating the extent of intestinal Cl– secretion induced by cAMP-agonists.
2. Materials and Methods
2.1. Reagents
Secramine B was synthesized as previously described [5] and stored in 20 mM aliquots in DMSO at –20 °C. Cholera toxin (EMD Biosciences Inc., San Diego, CA), forskolin (Sigma– Aldrich, St. Louis, MO), 8-Br cAMP (Sigma–Aldrich), BaCl2 (Fisher Scientific Co., Hanover Park, IL), amphotericin B (Sigma–Aldrich) were used. Jeffrey Peterson (Fox Chase Cancer Center, Philadelphia, PA) kindly provided pirl1 and its negative control pirl1.6. T84 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured as described [10].
2.2. Electrophysiology assays
Cl– secretion, assessed as short circuit current (Isc), and transepithelial resistance were measured using standard electrophysiological techniques with T84 cell monolayers grown on 0.33 cm2 inserts [10]. Vehicle represents the addition of 0.25% BSA and 0.2% DMSO to the basolateral media. Secramine B, pirl1 and pirl1.6 were only added to the basolateral media and concentrations are reported as the total volume around the cell monolayers (200 μL apical media + 1000 μL basolateral media). A higher percentage of DMSO (above 0.5%) reduces the transepithelial resistance and BSA serves as a carrier for secramine B (data not shown). Where used, forskolin, carbachol, 8Br-cAMP, and barium chloride were added to the basolateral media and cholera toxin was added to the apical media. Data were fitted to a variable slope sigmoidal dose response curve and plotted using GraphPad Prism 3.0.
2.3. Short-circuit current measurement in semi-permeabilized monolayers
T84 cell monolayers were incubated with secramine B or vehicle in buffers containing K+ or Cl– as the sole permeant ions. Basolateral membrane K+ conductances (Isc-blK) were determined in cells permeabilized apically with 20 μM amphotericin B, in the presence of asymmetrical buffers that imposed a basolaterally directed seven-fold K+ gradient (apical solution: 140 mM KGluconate, 1.25 mM CaSO4, 0.4 mM MgSO4, 10 mM HEPES, 5.6 mM d-glucose, pH 7.4; basal solution 20 mM KGluconate, 1.25 mM CaSO4, 0.4 mM MgSO4, 120 mM NMDG, 10 mM HEPES, 5.6 mM d-glucose, pH 7.4) [11]. Transmembrane potential was clamped at 0 mV, and (Isc-blK) was measured before and after 10 mM forskolin or 100 μM carbachol addition to the basolateral media. Apical Cl– conductances (Isc-apCl) were determined in cells permeabilized basolaterally using 100 μM amphotericin B, in the presence of symmetric high Cl– buffer (0.7 mM NaPO4, 0.43 mM KPO4, 1.25 mM CaCl2, 0.4 mM MgSO4, 142.3 mM choline, 10 mM HEPES, 5.6 mM d-glucose, pH 7.4) with transmembrane potential clamped at +10 mV (apical) [12]. For (Isc-blK), transepithelial current–voltage relationships (I–V curves) were defined by measuring transepithelial currents during 1 s voltage clamp periods ranging from 80 to +80 mV and normalized to baseline Isc at rest as described [12]. Baseline I–V curves obtained in the absence of agonist were subtracted from those measured after agonist treatment to calculate agonist-induced currents.
3. Results
3.1. Secramine B inhibits cAMP-induced Cl– secretion from intestinal cells
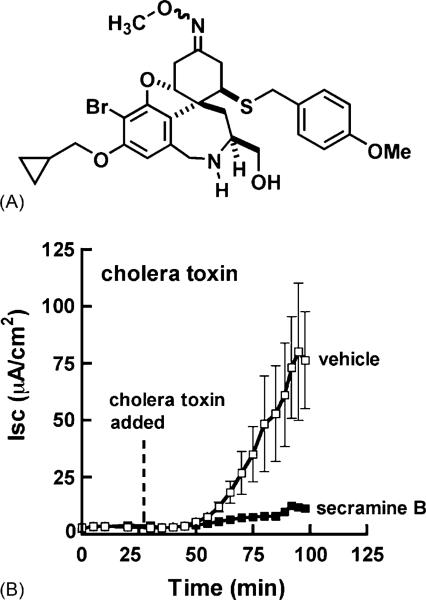
T84 cells reproduce the cAMP- and Ca2+-regulated Cl– secretion observed in native intestinal tissue [13] and increase Cl– secretion in response to cholera toxin, a cAMP agonist. We tested whether secramine B (Fig. 1A) inhibited the cholera toxin-induced increase in Cl– secretion in these cells. Secramine B, as opposed to secramine A, was selected for evaluation because it is more soluble than secramine A and could be used at low vehicle (DMSO) doses that did not perturb transepithelial resistances in polarized T84 epithelial cells.
Fig. 1.
Secramine B inhibits cholera toxin-induced Cl– secretion in T84 cell monolayers. (A) Structure of secramine B. (B) Time course for Cl– secretion (Isc) in T84 monolayers induced by the addition of cholera toxin to the apical media at the indicated time (dotted line) in the presence of 25 μM secramine B (filled squares) or vehicle (0.2% DMSO, 0.25% BSA, open squares) added at 0 min (mean ± S.D., n = 2).
Secramine B (25 μM) strongly inhibited cholera toxin-induced Cl– secretion (Isc) (Fig. 1B) and had no effect on transepithelial resistance (TER) over a 5 h incubation period, indicating that intercellular tight junctions were not affected and the monolayers remained intact (TER = 1080 and 1180 Ω cm2 with 25 μM secramine B and vehicle, respectively, at 5 h).
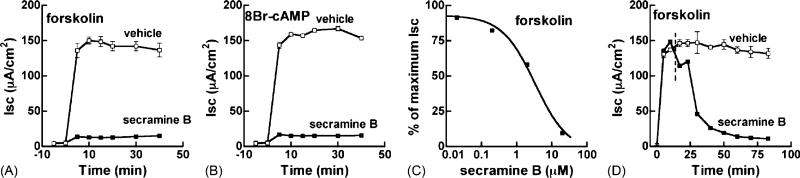
To distinguish whether secramine B inhibited the retrograde traffic of cholera toxin from the Golgi to the ER, or the signaling pathway leading to Cl– secretion, we applied forskolin, a direct activator of adenylyl cyclase, to increase the intracellular levels of cAMP or 8Br-cAMP, a membrane-permeable analog of cAMP. Secramine B inhibited Cl– secretion by >90% in the presence of 10 μM forskolin (Fig. 2A) or 3 mM 8Br-cAMP (Fig. 2B). Secramine B acted with an IC50 of ~3.4 μM (Fig. 2C). When added after the forskolin-induced Isc was maximal, secramine B still potently inhibited Cl– secretion with a half-time of approximately 17 min (Fig. 2D). Thus, the entire inhibitory effect of secramine B on the cholera toxin-induced increase in Cl– secretion can be explained by inhibition of a step(s) downstream of retrograde traffic from Golgi to ER and cAMP production.
Fig. 2.
Secramine B inhibits cAMP-dependent Cl– secretion and acts downstream of cholera toxin transport in T84 cell monolayers. T84 monolayers were incubated with vehicle (open squares) or 20 μM secramine B (filled squares) for 45 min followed by the addition of 10 μM forskolin (A) or 3 mM 8Br-cAMP (B) to the basolateral media at 0 min and the Isc was measured over time (mean ± S.D., n = 2). (C) Dose dependency of secramine B action on Cl– secretory response elicited by 10 μM forskolin (mean ± S.D., n = 2). Secramine B has an IC50 of ~3.4 μM. (D) T84 cells were incubated with 10 μM forskolin (0 min) followed by 20 μM secramine B or vehicle at 15 min (dotted line) (mean ± S.D., n = 2).
3.2. Secramine inhibits the basolateral cAMP-gated K+ efflux
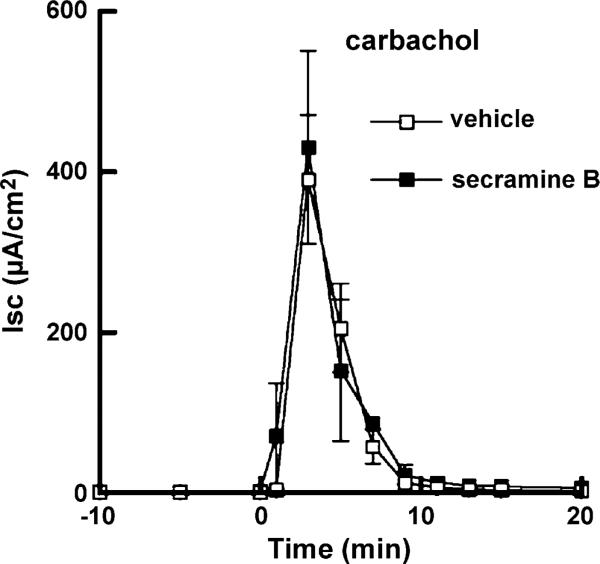
Four distinct membrane events are responsible for the cAMP-elicited vectorial transport of Cl– : (1) increase in the basolateral Na+ efflux by the Na+, K+ ATPase pump; (2) basolateral electroneutral influx of Cl– through the bumetanide-sensitive NKCC transporter; (3) apical passive diffusion of Cl– through the CFTR channel; and (4) increase in the basolateral efflux of K+ through Ba2+-sensitive channels [1]. Both the NKCC transporter and the Na+, K+ ATPase are required for cAMP- and Ca2+-elicited Cl– secretion [14,15]. Therefore, we tested whether secramine might also inhibit Cl– secretion caused by the muscarinic agonist carbachol that acts by inducing an increase in intracellular Ca2+. Secramine B had no detectable effect on the Isc induced by carbachol (Fig. 3). Based on these observations, we reasoned that secramine B inhibited either the apical CFTR or the cAMP-gated basolateral K+ channel.
Fig. 3.
Secramine B does not inhibit Cl– secretion induced by carbachol. T84 cell monolayers were treated with vehicle (open squares) or 20 μM secramine B (filled squares) for 45 min followed by the addition of 100 μM carbachol to the basolateral media at 0 min (mean ± S.D., n = 2).
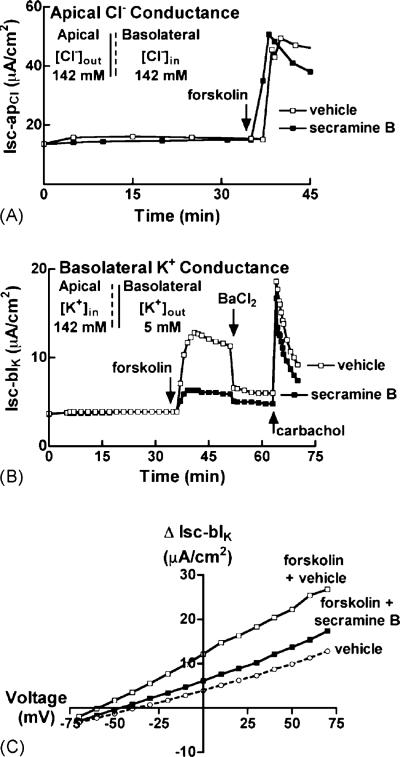
To test whether secramine B inhibited the CFTR channel, we monitored Cl– flux across the apical membranes of T84 monolayers in response to forskolin in the presence of amphotericin B, a polyene that forms channels for monovalent ions and small molecules. Under these conditions, the forskolin-induced transepithelial Isc is only due to Cl– transport across the apical membrane. Confluent monolayers of T84 cells were placed in symmetrical solutions containing only Cl– as the major charge carrying permeant ion. We then selectively permeabilized the basolateral membranes with amphotericin B, and clamped the apical membrane potential at 10 mV [11]. Under these conditions, secramine B had no effect on the Cl– flux stimulated upon addition of 10 μM forskolin (Isc-apCl, Fig. 4A), suggesting that secramine B does not inhibit the cAMP-sensitive CFTR channel.
Fig. 4.
Secramine B selectively inhibits basolateral Ba2+-sensitive K+ conductance in T84 cell monolayers. (A) T84 monolayers were exposed to a symmetrical high Cl– solution and basolaterally permeabilized with 20 μM amphotericin B. At 0 min, 20 μM secramine B or vehicle was added followed by 10 μM forskolin to the basolateral media (arrow). All measurements for the secramine B-treated monolayer were shifted by 8 μA/cm2. Data is representative of two independent experiments. (B) A basolaterally directed K+ gradient was established across T84 monolayers and the apical membrane was permeabilized with 20 μM amphotericin B. At 0 min, 20 μM secramine B or vehicle was added followed by 10 μM forskolin, 3 mM BaCl2, and 100 μM carbachol to the basolateral media (arrows, as indicated). Data is representative of four independent experiments. (C) A basolaterally directed K+ gradient was established across T84 monolayers and the apical membrane was permeabilized with 20 μM amphotericin B. Short circuit currents were recorded at the indicated voltage-clamped test potentials. The ordinate indicates the current difference measured on the same monolayer before and 10 min after 10 μM forskolin stimulation in the presence of 20 μM secramine B or vehicle. Data is representative of two independent experiments.
To test if secramine B affected cAMP-regulated basolateral K+ efflux, we again used amphotericin B, but instead applied it to the apical membrane of T84 cells. We immersed confluent monolayers of T84 cells in asymmetrical solutions containing K+ as the major charge carrying permeant ion, permeabilized the apical membranes with amphotericin B, and clamped the transepithelial voltage at 0 mV [11]. The apical solution had a seven-fold higher concentration of K+. Forskolin (10 μM) induced a strong K+ current across the basolateral membrane (Isc-blK) that was inhibited by 20 μM secramine B (Fig. 4B). Subsequent addition of 3 mM BaCl2 rapidly and strongly inhibited the cAMP-gated K+ channel activity [16]. Barium does not inhibit carbachol-stimulated K+ channel activity [17]. Upon addition of carbachol, we observed transient and strong basolateral K+ efflux in both secramine-treated and untreated monolayers, indicating that secramine B-treated monolayers were still intact and transport-competent and that secramine B had no effect on Ca2+-gated K+ channel activity. Steady-state Isc-blK/V relationships after stimulation of T84 monolayers with forskolin also demonstrated that secramine B inhibited cAMP-induced K+ conductance as evidenced by the lower slope of the current–voltage isotherm (Fig. 4C). These results show that secramine B selectively attenuated K+ efflux through a cAMP-regulated and Ba2+-sensitive basolateral K+ efflux pathway, presumably via the K+ channel KCNQ1/KCNE3 [2].
3.3. Rho GTPase activity and cAMP-dependent Cl– secretion
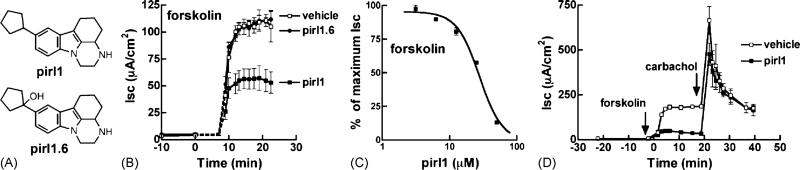
We have shown that secramine B inhibits the Rho GTPase Cdc42 in vitro, and proposed that secramine B also targets Cdc42 in cells because it phenocopies perturbations in membrane traffic resulting from expression of Cdc42 mutants [5]. Pirl1 is a small molecule structurally unrelated to secramine B (Fig. 5A) that also inhibits Cdc42 in vitro in a manner similar to secramine B [9]. Incubation of T84 cells with pirl1, but not its inactive analog pirl1.6 (Fig. 5B, [9]), inhibited forskolin-induced Cl– flux (Fig. 5B–D, IC50 of ~28 μM) and did not inhibit carbachol-induced Cl– flux (Fig. 5D) or effect transepithelial resistance (data not shown). Therefore, two structurally distinct Rho GTPase inhibitors, secramine B and pirl1, both inhibited Cl– secretion induced by cAMP, strongly suggesting that Cl– transport is indeed regulated by Rho GTPases.
Fig. 5.
Pirl1 selectively inhibits cAMP-dependent Cl– secretion in T84 cell monolayers. (A) Structures of pirl1 and pirl1.6. (B) T84 monolayers were incubated with vehicle (open squares), 20 μM pirl1 (filled squares), or pirl1.6 (filled circles) for 45 min followed by the addition of 10 μM forskolin to the basolateral media at 0 min and the Isc was measured over time (mean ± S.D., n = 2). (C) Dose dependency of pirl1 action on Cl– secretory response elicited by 10 μM forskolin (mean ± S.D., n = 2). Pirl1 has an IC50 of ~28 μM. (D) Like (B), with 50 μM pirl1 and the addition of 100 μM carbachol to the basolateral media at 20 min (mean ± S.D., n = 2).
4. Discussion
Here we provide evidence that secramine B and pirl1, two structurally distinct small molecules that inhibit activation and signaling by the Rho GTPase Cdc42, prevented cAMP-induced, but not Ca2+-induced, Cl– transport in polarized T84 epithelial cells. Secramine B acted by inhibiting a basolateral cAMP-dependent K+ channel, likely comprised of the voltage-gated KCNQ1 and its modulatory KCNE3 subunits [2]. This channel, but not the related KCNE1, is expressed in the T84 cells and opens in response to cAMP agonists [2]. Neither the Ca2+-gated K+ channel KCNN4 nor the Cl– channel CFTR were affected by secramine B. These results provide pharmacological evidence that Rho GTPases regulate cAMP-dependent Cl– secretion and basolateral K+ efflux. Precisely how Rho GTPases regulate these processes remains to be determined.
Rho GTPases likely regulate Cl– secretion downstream of cAMP production either through alterations in the organization of the basolateral actin cytoskeleton or transport of channels, channel components, or channel regulators to the basolateral membrane. Elevation of cAMP causes a loss of basement stress fibers and an increase in actin bundles along the lateral membranes of T84 cells [18]. Prevention of this actin redistribution by stabilizing the actin stress fibers with NBD-phallicidin inhibits cAMP-induced Cl– secretion in T84 cells [18]. Both secramine B and pirl1 prevent Cdc42-dependent actin polymerization in cell extracts and pirl1 inhibits PMA-induced actin rearrangements in monkey kidney epithelial BSC-1 cells [5,9]. Like secramine B and pirl1, NBD-phallicidin does not interfere with Ca2+-mediated Cl– secretion induced by carbachol [18]. Among the Rho GTPases, RhoA activation stimulates stress fiber formation and Cdc42 and Rac1 activation stimulate actin-based structures along the cell periphery [6]. Therefore, the cytoskeletal rearrangements downstream of cAMP production are consistent with an inhibition of RhoA and a stimulation of Rac1 and Cdc42. In accordance with these expected changes, PKA, activated upon increases in cAMP, directly phosphorylates RhoA to inhibit its function [19,20] and indirectly activates Cdc42 [21], possibly through phosphorylation of guanine nucleotide exchange factor for Cdc42 [22]. Importantly, although both the secramines and pirl1 inhibit Cdc42 activation, neither inhibits activation of RhoA as monitored by stress fiber stability [5,9]. Thus, the activities of secramine B and pirl1 are consistent with an inhibition of the normal cytoskeletal changes that accompany elevations in cAMP and suggest that cAMP-induced K+ channel activity may depend on these cytoskeletal changes.
Alternatively, cAMP-activated K+ channel activity may require Cdc42-dependent membrane traffic. In polarized epithelial cells, overexpression of dominant inhibitory mutants of Cdc42 disrupts membrane traffic of newly synthesized proteins to the basolateral membrane [23–25] and recycling of basolateral proteins to the basolateral membrane after endocytosis [23]. Therefore, by interfering with Cdc42 activity, secramine B and pirl1 may inhibit the mobilization of membrane-bound components of a fully functional cAMP-activated basolateral K+ channel.
These studies do not rule out the possibility that secramine B and pirl1 inhibit cAMP-gated K+ channel activity independent of an effect on Rho GTPases. Secramine B might bind directly to and inhibit the cAMP-gated K+ conductance. Such a mechanism of inhibition by direct binding to this channel was shown for chromanol 293B, a small molecule that, like secramine B, also does not inhibit Ca2+-induced Cl– flux [26,27]. Furthermore, the KCNQ1 subunit is a promiscuous drug target, having been shown to bind to different small molecules [28]. This possibility, however, seems unlikely since pirl1.6, a small molecule that is highly similar to pirl1 but nevertheless does not inhibit Cdc42 activation, failed to inhibit forskolin-stimulated Cl– transport.
Blockade of the cAMP-gated K+ conductance by secramine B resulted in strong inhibition of the intestinal Cl– secretory response. In addition to identifying a new reagent to dissect critically important K+ channels [29], this study pharmacologically links Rho GTPases to Cl– secretion and thus provides a new avenue to dissect and elucidate the pathway of cAMP-elicited Cl– secretion in intestinal crypt epithelial cells.
Acknowledgements
We thank Paul Rufo and Ramiro Massol for comments on the manuscript, Eli Kern, Heidi Wheeler, Eric Barclay, and Wendy Hamman for technical assistance, and Jeffrey Peterson for providing pirl1 and pirl1.6 prior to publication. This work was supported by NIH NERCE grant 5U54 A1057159, DK48106, and the Harvard Digestive Diseases Center DK34854.
Abbreviations
- CaCC
calcium activated chloride channel
- cAMP
3′,5′-cyclic AMP
- CFTR
cystic fibrosis transmembrane regulator
- ER
endoplasmic reticulum
- Isc
short circuit current
- Isc-blK
basolateral membrane potassium Isc
- Isc-apCl
apical membrane chloride Isc
- I–V
current–voltage
- NKCC
sodium potassium two chloride cotransporter
- TER
transepithelial resistance
REFERENCES
- 1.Barrett KE. Positive and negative regulation of chloride secretion in T84 cells. Am J Physiol. 1993;265(34):C859–68. doi: 10.1152/ajpcell.1993.265.4.C859. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, et al. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–9. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- 3.Warth R, Hamm K, Bleich M, Kunzelmann K, von Hahn T, Schreiber R, et al. Molecular and functional characterization of the small Ca2+-regulated K+ channel (rSK4) of colonic crypts. Pflug Arch. 1999;438(4):437–44. doi: 10.1007/s004249900059. [DOI] [PubMed] [Google Scholar]
- 4.Pelish HE, Westwood NJ, Feng Y, Kirchhausen T, Shair MD. Use of biomimetic diversity-oriented synthesis to discover galanthamine-like molecules with biological properties beyond those of the natural product. J Am Chem Soc. 2001;123(27):6740–1. doi: 10.1021/ja016093h. [DOI] [PubMed] [Google Scholar]
- 5.Pelish HE, Peterson JR, Salvarezza S, Rodriguez-Boulan E, Chen J, Stamnes M, et al. Secramine inhibits Cdc42 functions in cells and Cdc42 activation in vitro. Nat Chem Biol. 2006;2(1):39–46. doi: 10.1038/nchembio751. [DOI] [PubMed] [Google Scholar]
- 6.Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 7.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–55. [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai B, Rodighiero C, Lencer WI, Rapoport T. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell. 2001;104:937–48. doi: 10.1016/s0092-8674(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 9.Peterson JR, Lebensohn AM, Pelish HE, Kirschner MW. Biochemical suppression of small molecule inhibitors: a new strategy to identify inhibitor targets and signaling pathway components. Chem Biol. 2006;13:443–52. doi: 10.1016/j.chembiol.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lencer WI, de Almeida JB, Moe S, Stow JL, Ausiello DA, Madara JL. Entry of cholera toxin into polarized human intestinal epithelial cells. Identification of an early brefeldin A sensitive event required for A1-peptide generation. J Clin Invest. 1993;92:2941–51. doi: 10.1172/JCI116917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rufo PA, Merlin D, Riegler M, Ferguson-Maltzman MH, Dickinson BL, Brugnara C, et al. The antifungal antibiotic, clotrimazole, inhibits chloride secretion by human intestinal T84 cells via blockade of distinct basolateral K+ conductances. J Clin Invest. 1997;100(12):3111–20. doi: 10.1172/JCI119866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merlin D, Jiang L, Strohmeier GR, Nusrat A, Alper SL, Lencer WI, et al. Distinct Ca2+- and cAMP-dependent anion conductances in the apical membrane of polarized T84 cells. Am J Physiol. 1998;275:484–95. doi: 10.1152/ajpcell.1998.275.2.C484. [DOI] [PubMed] [Google Scholar]
- 13.Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984;246:G204–8. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- 14.Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest. 1986;77(2):348–54. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wasserman SI, Barrett KE, Huott PA, Beuerlein G, Kagnoff MF, Dharmsathaphorn K. Immune-related intestinal Cl– secretion. I. Effect of histamine on the T84 cell line. Am J Physiol. 1988;254(1 Pt. 1):C53–62. doi: 10.1152/ajpcell.1988.254.1.C53. [DOI] [PubMed] [Google Scholar]
- 16.Mandel K, McRoberts JA, Beuerlein G, Foster E, Dharmsathaphorn K. Ba inhibition of VIP- and A23187-stimulated Cl secretion by T84 cell monolayers. Am J Physiol. 1986;259:C486–94. doi: 10.1152/ajpcell.1986.250.3.C486. [DOI] [PubMed] [Google Scholar]
- 17.Mayol JM, Hrnjez BJ, Akbarali HI, Song JC, Smith JA, Matthews JB. Ammonia effect on calcium-activated chloride secretion in T84 intestinal epithelial monolayers. Am J Physiol Cell Physiol. 1997;273:C634–42. doi: 10.1152/ajpcell.1997.273.2.C634. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro M, Matthews J, Hecht G, Delp C, Madara JL. Stabilization of F-Actin prevents cAMP-elicited Cl— secretion in T84 cells. J Clin Invest. 1991;87:1903–9. doi: 10.1172/JCI115215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996;15(3):510–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Howe AK. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta. 2004;1692(2–3):159–74. doi: 10.1016/j.bbamcr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Feoktistov I, Goldstein AE, Biaggioni I. Cyclic AMP and protein kinase A stimulate Cdc42: role of A2 adenosine receptors in human mast cells. Mol Pharm. 2000;58:903–10. doi: 10.1124/mol.58.5.903. [DOI] [PubMed] [Google Scholar]
- 22.Chahdi A, Miller B, Sorokin A. Endothelin 1 induces beta 1Pix translocation and Cdc42 activation via protein kinase A-dependent pathway. J Biol Chem. 2005;280(1):578–84. doi: 10.1074/jbc.M411130200. [DOI] [PubMed] [Google Scholar]
- 23.Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- 24.Musch A, Cohen D, Kreitzer G, Rodriguez-Boulan E. Cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. EMBO J. 2001;20(9):2171–9. doi: 10.1093/emboj/20.9.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen D, Musch A, Rodriguez-Boulan E. Selective control of basolateral membrane protein polarity by Cdc42. Traffic. 2001;2:556–64. doi: 10.1034/j.1600-0854.2001.20805.x. [DOI] [PubMed] [Google Scholar]
- 26.Suessbrich H, Bleich M, Ecke D, Rizzo M, Waldegger S, Lang F, et al. Specific blockade of a slowly activating IsK channels by chromanols—impact on the role of IsK channels in epithelial. FEBS Lett. 1996;396:271–5. doi: 10.1016/0014-5793(96)01113-1. [DOI] [PubMed] [Google Scholar]
- 27.Lerche C, Seebohm G, Wagner CI, Scherer CR, Dehmelt L, Abitbol I, et al. Molecular impact of MinK on the enantiospecific block of IKs by chromanols. Br J Pharm. 2000;131:1503–6. doi: 10.1038/sj.bjp.0703734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clancy CE, Kurokawa J, Tateyama M, Wehrens XHT, Kass RS. K+ channel structure-activity relationships and mechanisms of drug-induced QT prolongation. Annu Rev Pharmacol Toxicol. 2003;43:441–61. doi: 10.1146/annurev.pharmtox.43.100901.140245. [DOI] [PubMed] [Google Scholar]
- 29.Wickenden AD. K+ channels as therapeutic drug targets. Pharm Ther. 2002;94:157–82. doi: 10.1016/s0163-7258(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 30.Feng Y, Jadhav AP, Rodighiero C, Fujinaga Y, Kirchhausen T, Lencer WI. Retrograde transport of cholera toxin from the plasma membrane to the endoplasmic reticulum requires the trans-Golgi network but not the Golgi apparatus in Exo2-treated cells. EMBO Rep. 2004;5(6):596–601. doi: 10.1038/sj.embor.7400152. [DOI] [PMC free article] [PubMed] [Google Scholar]